Abstract
Generalized tonic-clonic seizures cause widespread physiological changes throughout the cerebral cortex and subcortical structures in the brain. Using combined blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) at 9.4 T and electroencephalography (EEG) these changes can be characterized with high spatiotemporal resolution. We studied BOLD changes in anesthetized Wistar rats during bicuculline-induced tonic-clonic seizures. Bicuculline, a GABAA receptor antagonist, was injected systemically and seizure activity was observed on EEG as high amplitude, high-frequency polyspike discharges followed by clonic paroxysmal activity of lower frequency, with mean electrographic seizure duration of 349 s. Our aim was to characterize the spatial localization, direction, and timing of BOLD signal changes during the pre-ictal, ictal and post-ictal periods. Group analysis was performed across seizures using paired t-maps of BOLD signal superimposed on high resolution anatomical images. Regional analysis was then performed using volumes of interest to quantify BOLD timecourses. In the pre-ictal period we found focal BOLD increases in specific areas of somatosensory cortex (S1, S2) and thalamus several seconds before seizure onset. During seizures we observed BOLD increases in cortex, brainstem and thalamus and BOLD decreases in the hippocampus. The largest ictal BOLD increases remained in the focal regions of somatosensory cortex showing pre-ictal increases. During the post-ictal period we observed widespread BOLD decreases. These findings support a model in which “generalized” tonic-clonic seizures begin with focal changes before electrographic seizure onset, which progress to non-uniform changes during seizures, possibly shedding light on the etiology and pathophysiology of similar seizures in humans.
Keywords: tonic-clonic seizure, fMRI, cortex, thalamus, bicuculline, cortical focus theory
Introduction
Epilepsy is one of the most common chronic neurological disorders affecting about 50 million people worldwide. The most severe and dangerous type of epileptic seizure is the tonic-clonic seizure, which can occur either in primary generalized epilepsy or in partial epilepsy with secondary generalization. Tonic-clonic seizures are composed of two phases: a tonic phase, characterized by sudden muscle rigidity, followed by a clonic phase, consisting of rapid muscle contractions and relaxations, causing convulsions. Because of this violent motor activity, human studies of generalized tonic-clonic seizures have been limited, and the mechanisms and pathophysiology of these episodes remain poorly understood.
Prior studies of tonic-clonic seizures in both humans and animal models reported contradictory results regarding the distribution and pattern of changes in metabolic activity during seizures. Some studies reported widespread and diffuse increases in neuronal activity throughout the brain (Engel et al., 1982; McCown et al., 1995; Andre et al., 2002) while other studies found a non-uniform pattern of changes in ictal neurophysiology (Ackermann et al., 1986; McIntyre et al., 1991). These studies relied upon techniques for metabolic mapping that suffer from low spatial and temporal resolution, making the interpretation of results difficult.
More recently, several studies have reported focal patterns of altered neurophysiology during so-called “generalized” seizures, including both tonic-clonic and spike wave discharges (SWD). For example, study of WAG/Rij rats, a genetic absence model, has led to the “cortical focus theory,” in which local activity in the peri-oral region of somatosensory cortex feeds corticothalamic networks causing SWD (Meeren et al., 2002; Nersesyan et al., 2004a; Meeren et al., 2005). Chemically-induced tonic-clonic seizures studied with functional magnetic resonance imaging (fMRI) in rats have, likewise, shown some focal features, but have not been fully characterized (Brevard et al., 2006; Schridde et al., 2008). In human patients, single photon emission computerized tomography (SPECT) studies of both spontaneous secondarily-generalized and electroconvulsive therapy (ECT) induced tonic-clonic seizures reported heterogeneous patterns of changes in cerebral blood flow (CBF) during seizures (McNally and Blumenfeld, 2004; Blumenfeld et al., 2009). The main disadvantage of SPECT is its relatively low spatial and temporal resolution, leaving the progression and timecourse of physiological changes during these episodes obscure. Despite the advances made by these studies, our understanding of the spatiotemporal dynamics of tonic-clonic seizures remains incomplete.
The principal aim of this study was to characterize with high anatomical and temporal specificity, the pattern of changes in neuronal activity, as measured through blood oxygen dependent (BOLD) fMRI signals throughout the brain during bicuculline-induced generalized tonic-clonic seizures in Wistar rats. We found that focal areas of somatosensory cortex and thalamus show most intense involvement during seizures, and that these regions show significant focal changes that precede electrographic seizure onset. These findings suggest that so-called “generalized” tonic-clonic seizures may be localized in their onset. Interestingly, the somatosensory cortex has also been implicated in focal onset of absence seizures, suggesting the cortical focus theory could be extended to explain the origin of tonic-clonic seizures as well.
Materials and Methods
Animals and General Procedures
A total of 17 experiments were performed in 13 adult, female, Wistar rats (Charles River Laboratories, Wilmington, MA). Female rats were used due to their smaller body size enabling them to fit more easily into the MRI scanner. All experiments were conducted in accordance with local guidelines, approved by the ethical committee of Yale University School of Medicine Institutional Animal Care and Use Committee and in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rats were anesthetized using intravenous (iv) fentanyl (40 μg/kg, Hospira, Inc., Lake Forest, IL) and intraperitoneal (ip) haloperidol (1 mg/kg, Bedford Labs, Bedford, OH) and paralyzed using repeated injections of iv D-tubocurarine (0.5 mg/kg Sigma-Aldrich, Inc., St. Louis, MO). Rats were tracheotomized, artificially ventilated (70% N2O, 30% O2) and blood gas levels, blood pH, and mean arterial blood pressure (MABP) were monitored and remained in the physiological range throughout experiments: pH = 7.29 ± 0.01, pCO2 = 41.74 ± 1.87 mmHg, pO2 = 123.24 ± 4.23 mmHg, MABP = 128.80 ± 4.70 mmHg (Mean ± SEM). Body temperature was monitored with a rectal thermometer and kept constant at 37°C by a heating pad. An injection of iv bicuculline (0.5 mg/kg; Sigma-Aldrich, Inc.), a gamma-aminobutyric acid (GABA) A receptor antagonist, was administered to induce tonic-clonic seizures.
Recording Paradigm
Experimental data runs were acquired over a time period of 30 min surrounding each bicuculline injection. After 5 min of uninterrupted preinjection recordings, 0.5 mg/kg bicuculline was injected iv to induce generalized tonic-clonic seizures. Recordings were then continued for approximately 25 min after seizure induction. When more than one bicuculline injection was performed in a single rat, we always allowed a stabilization period of at least 30 min between the end of each recording and the next bicuculline injection (ca. 1 h or more between injections). In all experiments, recordings were only initiated after all signals and vital functions reached stable readings in the normal physiological range.
EEG-fMRI Experiments
Images were acquired as described in detail previously (Englot et al., 2008; Schridde et al., 2008). Briefly, the rat was positioned prone in a specially designed plastic holder with the head fixed and bregma positioned at the center of the surface coil. To record simultaneous MRI and EEG signals while minimizing the MRI signal distortion, a pair of carbon-filament electrodes (diameter = 1 mm; WPI, Sarasota, FL) were placed laterally between the scalp and the outside surface of the skull in the right frontal and occipital areas (Nersesyan et al., 2004a). The electrodes were secured using tissue glue. EEG signals between the two electrodes were acquired in differential mode, amplified and filtered (1-100 Hz) using a Grass Data Recording System Model 79D (Grass Instruments Co., Quincy, MA). The EEG signals were digitized and recorded (sampling rate 1000 Hz) using a CED Micro 1401 and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
All MRI experiments were acquired on a 9.4-tesla Bruker horizontal-bore (16-cm-diameter working space) spectrometer (Bruker Avance, Billerica, MA), equipped with passively shielded shim/gradient coils (47.5 G/cm) operating at 400.5 MHz for 1H. The transceiver system consisted of a 1H resonator radio frequency surface coil (15.18 mm diameter) for transmission of radio frequency pulses and receiving. To optimize the homogeneity of the static magnetic field, the system was shimmed before each experiment using either global manual shimming or local FASTMAP (Gruetter, 1993).
High spatial resolution anatomical images for each animal were acquired with 11 interlaced slices in the coronal plane using the fast, low-angle shot (FLASH) sequence with time repetition (TR) 100 ms; time echo (TE) 6 ms; flip angle = 30°; field of view (FOV) 25 × 25 mm; 256 × 256 matrix and in-plane resolution of 98 × 98 μm; and slice thickness 1000 μm. BOLD fMRI data were obtained in the same planes as anatomical images using single-shot spin echo, echo planar imaging with the following parameters: TR = 1000 ms, TE = 25 ms; excitation flip angle 90°; inversion flip angle 180°; FOV = 25 × 25 mm, 64 × 64 matrix, and in-plane resolution of 390 × 390 μm; and slice thickness 1000 μm. The fMRI image acquisitions of 11 slices/s were obtained continuously with 2 s in between image acquisitions, resulting in a total of 600 image acquisitions per 30-min experiment.
Data Analysis and Statistics
All EEG signals from MRI experiments were first processed using Spike 2 software to reduce MRI artifacts as described previously (Nersesyan et al., 2004a). Using post-processed EEG, seizure onset and duration were determined. Seizure onset and offset were first determined from EEG visually by identifying high amplitude, high-frequency oscillations, progressing into clonic paroxysmal discharges of lower frequency toward the end (Schridde et al., 2008).
Quantitative EEG power spectrum analysis was then performed in MATLAB 7.5 (The MathWorks, Inc., Natick, MA). Data were downsampled from 1000 Hz to 50 Hz and passed through a 0.5 Hz high-pass Butterworth filter. The short-time Fourier transform was calculated using a sliding window of 5.12 s and frequency bands from 0 to 12 Hz were analyzed. Signal components faster than 12 Hz were removed during MRI artifact removal. The power spectrum for each seizure was normalized to peak power across time and frequency. Data were temporally aligned to seizure onset, a group average was computed and then similarly renormalized to peak power = 1.0.
MRI images were processed using BioImage Suite (Yale University, New Haven, CT) and our own software running on a MATLAB platform (The MathWorks, Inc., Natick, MA). Although rats were completely paralyzed during experiments, all fMRI series were first screened for movement artifacts using a MOVIE function and center of mass analysis (Sanganahalli et al., 2008). We then used a movement exclusion criterion of 20% of a pixel (78μm) in either plane of the center of mass image. In fact, because all animals were paralyzed throughout experiments, almost no movement occurred and the entire cohort would be eligible for analysis at an even smaller threshold (e.g. 10% of a pixel in either plane of center of mass image). All BOLD fMRI images were then coregistered to a single template using rigid body transformation in BioImage Suite. The template consisted of one rat's fMRI images from within the data set for additional anatomical accuracy when overlaying fMRI data onto the template anatomical image. The likelihood of introducing bias through signal interpolation was minimal as only integer translations were used, in plane rotations were small (<7° in all cases), and have been shown to have minimal quantitative effects on MR signal due to interpolation (Lehmann et al., 1999). After image coregistration, only 9 of the original 11 coronal slices contained overlapping data from all 17 seizures, so only these 9 slices were used for group analysis. The coordinates of the final set of slices relative to bregma were +1.2 mm to -6.8 mm.
Each fMRI time series contained a significant non-linear spatially inhomogeneous component due to MR scanner drift over the 30 m experiment (Smith et al., 1999; Foerster et al., 2005; Benner et al., 2006). This component was removed by subtracting a composite “drift map” obtained by averaging 6 time series obtained under identical conditions in 5 animals that were not given bicuculline injections and did not have tonic-clonic seizures.
Signal-to-noise ratio (SNR) was investigated using a series of 30 baseline, pre-injection images. We used the standard definition for SNR of mean divided by standard deviation for each voxel (μ / σ). We observed that SNR values were within an acceptable range for structures we studied: S1 = 35-40dB, S1BF = 35-38dB, S2/Au1 = 30-39dB, Thalamus = 37-39dB, Hippocampus = 37-41dB, Brainstem = 29-36dB. Meanwhile, more ventral structures had lower SNR: Hypothalamus = 18-21dB, and Amygdala = 24-28dB.
Two different types of analysis were performed on the fMRI data. We first constructed a temporal series of t-maps before, during and after seizures to identify important regions of change for subsequent analysis. We next analyzed the timecourse of fMRI changes in specific anatomical volumes of interest. For both analyses, ten consecutive (30 s) artifact-free images with normal EEG immediately preceding bicuculline injection were chosen as a baseline period to which all analysis periods were compared.
For t-map construction, time series data for the pre-ictal and ictal phases were temporally aligned by electrographic seizure onset, and data for the postictal period were temporally aligned to seizure offset. To create a temporal series of t-maps, we used three image (9 s) non-overlapping time bins. An average image across each time bin (3 images) and an average image across baseline (10 images) were used for t-map comparison. T-maps were calculated for each time bin using a voxelwise two-sample paired t-test compared to baseline, using a height threshold of |t|>2.00 (p<0.05) and extent threshold of 2 mm3 (13 voxels, with voxel dimensions = 98 μm × 98 μm × 1000 μm). Results were overlaid onto a high resolution anatomical template in the coronal plane obtained from within the data set. T-maps using the same thresholds were also overlaid using BioImage Suite as 3-dimensional surface renderings onto template anatomical images from the Karolinska rat atlas (http://mr.imaging-ks.nu/expmr.htm). This texture mapping accelerated rendering mode displays signal in a linearly attenuated fashion as tissue depth increases causing deeper signal changes to appear dimmer than more superficial ones.
Manual segmentation for regional analysis using volumes of interest (VOI) was performed using BioImage Suite (Supplementary Figure 1, online). VOIs were selected based on anatomical regions showing maximal cortical or subcortical fMRI changes on t-maps, corresponding to S1 (primary somatosensory cortex, including hindlimb [S1HL], forelimb [S1FL], dysgranular zone [S1DZ], and trunk [S1Tr], but excluding the barrel field [S1BF]), S2 (secondary somatosensory cortex), anterior portion of Au1 (primary auditory cortex), dorsal hippocampus, thalamus, and brainstem tegmentum. For comparison to the cortical VOIs showing prominent changes on t-maps, we also selected a nearby cortical VOI corresponding to S1BF (primary somatosensory cortex, barrel field), which appeared to show less, or more delayed fMRI increases on t-maps. These 6 VOIs were segmented from 4 of the 9 slices used for the t-map analysis showing the most prominent changes, resulting in 4 slices shown in Figure 1 and Supplementary Figure 1.
Figure 1.
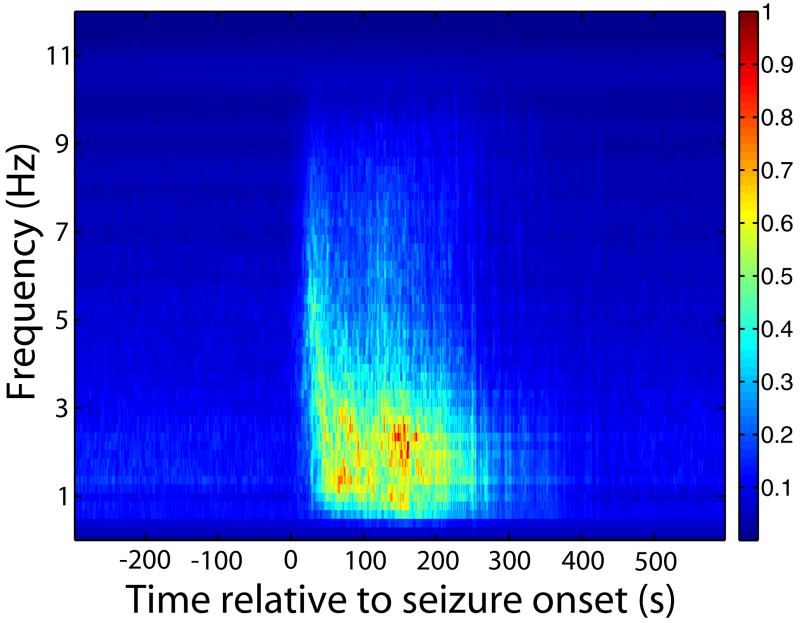
EEG time-frequency analysis across seizures. EEG signal power changes abruptly at seizure onset and offset. Analysis was performed using a short-time Fourier transform. Peak power was normalized to 1.0 before and after averaging. The dominant ictal signal frequency was 1∼3 Hz with higher frequency components up to ∼9-10 Hz occurring earlier and diminishing in power towards the end of seizures.
VOI-based timecourse analysis was performed using MATLAB. Images acquired at 3 s time resolution were first temporally aligned to seizure onset on EEG. BOLD signal at each time point was then spatially averaged over each VOI and compared to the mean signal during the 30 s baseline (prior to bicuculline injection) to calculate fMRI signal (S) percent change as {(St − Sbaseline) / Sbaseline. } × 100%. Statistical analyses were then performed using SPSS 16.0 across seizures for each region on the following parameters: peak amplitude, first time above threshold (|t|>2.00), and rate consant, r, of an exponential fit (cert) of the initial rise (or fall) of the timecourse 30 s prior to seizure onset. These quantifiers were compared across regions using one-way ANOVA followed by Tukey's HSD method for posthoc pairwise comparison. Early changes were also detected by analyzing the 30 s prior to seizure onset using a two-way mixed model ANOVA with time as a repeated factor and region as a non-repeated factor with posthoc pairwise analysis using Tukey's HSD by region. Analyzing group data when there are multiple seizures in some animals has two potential sources of bias: if results are analyzed across seizures (n=number of seizures) results could be biased towards an outlier animal with more seizures; if results are analyzed by first averaging data within animals and then analyzing across animals (n=number of animals) results could be biased towards an outlier animal with fewer seizures. To determine if either form of bias could affect our results, all analyses were performed using both methods. Results from analyses across seizures (shown in figures) were nearly identical and were similarly statistically significant to analyses across animals (data not shown), suggesting these potential sources of bias were not a significant factor. No animal had more than 2 seizures used for analysis.
Results
Timecourse of EEG changes
Changes in EEG signal power began and ended abruptly with seizures (Figure 1). A Fourier Transform was performed to calculate the time-frequency dynamics of the EEG signal during pre-ictal, ictal and post-ictal time periods. Fig. 1 shows a 2D plot of average time-frequency-power of seizures relative to seizure onset. Higher frequency signal components (up to ∼9-10 Hz) were stronger early in seizures and gradually decreased in power towards seizure offset. A mixture of lower frequency, particularly 1-3 Hz power components were also seen throughout seizures. There were no substantial frequency components seen before or after the seizure period.
BOLD t-maps in tonic-clonic seizures
We analyzed 17 seizure events across 13 animals with electrographic seizure duration of 348.71 ± 25.78 s (mean ± SEM). Seizures followed shortly after bicuculline injection (mean latency = 37.06 ± 2.71 s) and appeared on EEG as high-frequency, high-amplitude polyspike discharges followed by lower-frequency clonic paroxysmal activity (see for example Nersesyan et al., 2004a; Schridde et al., 2008). Post-ictal EEG showed typical signal depression with occasional brief paroxysmal episodes.
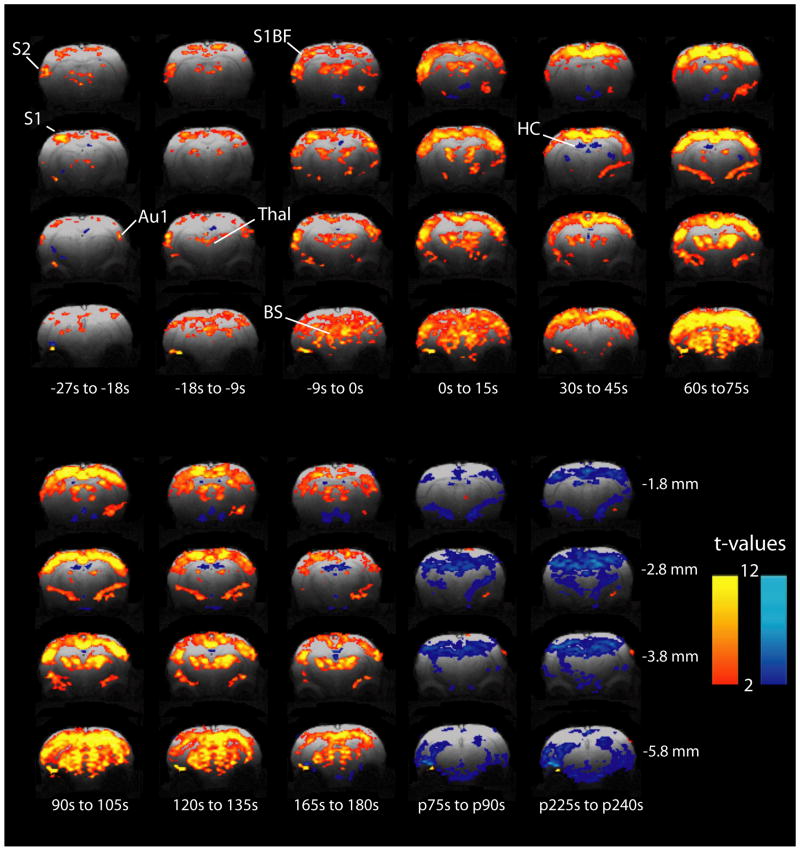
As an initial exploratory analysis to identify regional changes, we calculated paired t-maps comparing various times with 30 s baseline across all seizures. These maps were used to observe the spatiotemporal distribution of BOLD increases and decreases before, during and after seizures (Fig 2). During the period before electrographic seizure onset (pre-ictal), we initially observed significant (|t|>2.00) focal BOLD increases in S1, S2, Au1 and thalamus while other regions were relatively spared (Fig 2). Later in the pre-ictal period, while the signal in these areas continued to increase, increases also appeared in other areas of the cortex such as S1BF, and in additional subcortical regions such as the brainstem tegmentum. As electrographic seizures began, widespread cortical and subcortical increases were observed, still most prominent in focal cortical areas, while fMRI decreases were observed in the hippocampus (Figure 2). In the post-ictal phase, t-maps showed widespread BOLD decreases in cortical and subcortical regions that persisted for several minutes. The most significant postictal BOLD decreases appeared in the hippocampus.
Figure 2.
Focal fMRI increases begin in the preictal period and remain relatively increased after tonic-clonic seizure onset. Two-sample paired t-maps comparing BOLD signal of the specified time frame relative to electrographic seizure onset, to 30 s baseline signal. Times preceded by “p” indicate post-ictal and are relative to seizure offset. T-maps have been overlaid onto high resolution FLASH anatomical images with height threshold t>2.00 (p<0.05) and extent threshold of 13 voxels (2 mm3). Although images were acquired from +1.2 to -6.8 mm relative to bregma, representative slices are shown at -1.8, -2.8, -3.8 and -5.8 mm which encompass all volumes of interest. Similarly, representative timepoints only are shown during and after seizures. S1 = Primary somatosensory cortex subregions including hindlimb (S1HL), forelimb (S1FL), dysgranular zone (S1DZ), and trunk (S1Tr); S1BF = Primary somatosensory cortex, barrel field; S2 = Secondary somatosensory cortex; Au1 = Primary auditory cortex; Thal = Thalamus; HC = Hippocampus; BS = Brainstem. Warm colors represent fMRI increases and cool colors decreases.
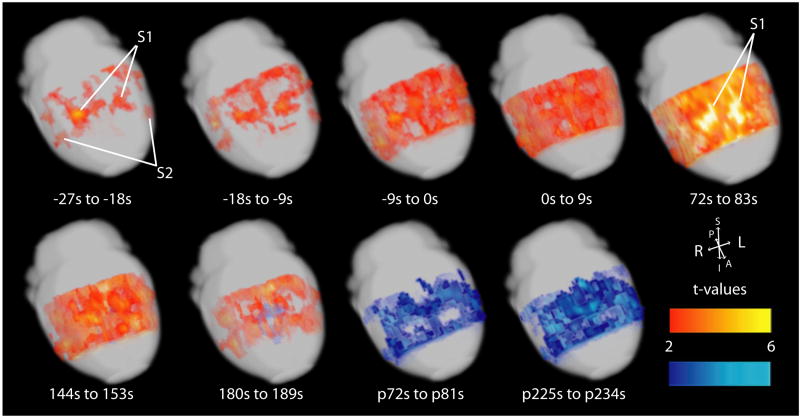
3-dimensional surface maps of fMRI changes similarly showed early focal fMRI increases in S1, S2 and Au1 in the preictal period (Figure 3). During seizures, widespread increases were seen, however, increases remained most prominent in focal cortical areas, particularly in S1. In the postictal period, cortical increases gave way to widespread fMRI decreases.
Figure 3.
Evolution of focal fMRI increases before and during tonic-clonic seizures displayed on brain surface. Two-sample paired t-maps comparing BOLD signal of the specified time frame, relative to electrographic seizure onset, to 30 s baseline signal. Representative timepoints only are shown during and after seizures. Times preceded by “p” indicate post-ictal and are relative to seizure offset. T-maps have been overlaid onto template anatomical images from the Karolinska rat atlas (http://mr.imaging-ks.nu/expmr.htm) with height threshold t>2.00 (p<0.05) and extent threshold of 250 voxels (2 mm3) and are displayed as 3-D cortical surface renderings. S1 = Primary somatosensory cortex subregions including hindlimb (S1HL), forelimb (S1FL), dysgranular zone (S1DZ), and trunk (S1Tr); S2 = Secondary somatosensory cortex. Warm colors represent fMRI increases and cool colors decreases.
VOI BOLD percent change timecourses
To further quantify the pattern of BOLD signal changes observed in the t-maps, we next examined timecourses of percent change of BOLD signal compared to 30 s baseline in specific volumes of interest (VOIs) that showed dramatic changes in t-maps (see Supplementary Figure 1, online).
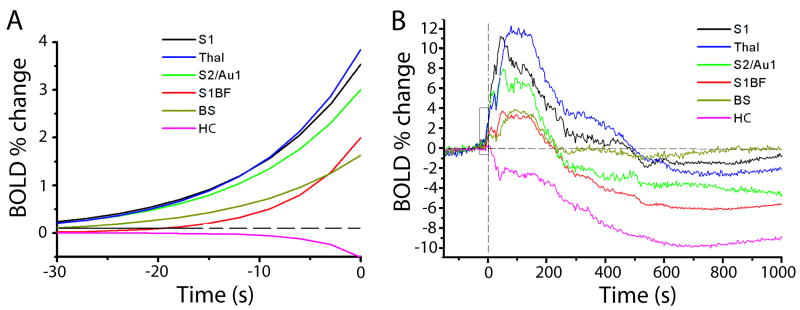
As observed in the t-maps, significant (|t|>2.00) changes in BOLD signal appeared in several regions prior to electrographic seizure onset, and certain focal cortical regions showed increases before others (Figure 4). To determine which regions showed significant increases before others, we performed analyses using one-way ANOVA with post-hoc pairwise comparisons between different regions. Using this approach, the first time above threshold (|t|>2.00) for the different VOIs yielded significant differences (F=24.98, P<0.001, df=5, 96), with the following 4 groupings (P<0.05 for all pairwise comparisons between each group) from earliest to latest: S1 and S2/Au1 (mean=-26.23 s), thalamus (-15.58 s), S1BF and brainstem (-3.56 s), and hippocampus (28.96 s). Similarly, analysis of the rate constant, r, of an exponential fit (cert) of the initial rise (or fall) of timecourses 30 s prior to seizure onset led to significant differences (F=10.75, P<0.01, df =5, 96) and the following 3 groupings from fastest to slowest: S1, S2/Au1 and thalamus (mean=0.16 s-1); S1BF and brainstem (0.05 s-1); and hippocampus (∼0.01 s-1) (Figure 4 A).
Figure 4.
Timecourses of percent change in BOLD signal compared to 30 s baseline signal in various regions. Times are relative to seizure onset. A. Exponential fits to mean timecourses 30s before seizure onset (fit to data from times in box, B). ANOVA across seizures and regions (F=10.75, P<0.01, df =5, 96) with post-hoc pairwise comparisons reveals 3 groupings from fastest to slowest: S1, S2/Au1 and thalamus (mean=0.16 s-1); S1BF and brainstem (0.05 s-1); and hippocampus (∼0.01 s-1). B. Full timecourses of mean percent changes in BOLD reveal different peak amplitudes in different regions. ANOVA across seizures and regions (F= 24.34, P<0.001, df= 5, 96) with post-hoc comparisons demonstrated the following 4 groups from largest increase to largest decrease: S1 and thalamus (mean=12.35%); S2/Au1 (8.56%); S1BF and brainstem (4.21%); and finally hippocampus (-10.14%). S1= Primary somatosensory cortex excluding S1BF, but including hindlimb (S1HL), forelimb (S1FL), dysgranular zone (S1DZ), and trunk (S1Tr); S1BF = Primary somatosensory cortex, barrel field; S2 = Secondary somatosensory cortex; Au1 = Primary auditory cortex; Thal = Thalamus; HC = Hippocampus; BS = Brainstem.
In agreement with these analyses, when we analyzed early changes during the 30 s prior to seizure onset across time and region with two-way mixed model ANOVA, we found significant increases in S1 and S2/Au1 at -24 s followed by increases in thalamus at -15 s (F=15.35, P<0.005, df= 5, 96). Overall, these analyses indicate significant early fMRI increases in S1 and S2/Au1 preceding other brain regions.
To analyze the direction and amplitude of fMRI changes in the group data, we performed a one-way ANOVA on peak signal amplitudes (Figure 4 B). This demonstrated significant difference across regions (F= 24.34, P<0.001, df= 5, 96) with the following 4 groups resulting from post-hoc comparisons (P<0.05 for all between-group pairs) from largest increase to largest decrease: S1 and thalamus (mean=12.35%); S2/Au1 (8.56%); S1BF and brainstem (4.21%); and finally hippocampus, which showed decreases (-10.14%).
Discussion
Rather than seeing a uniform pattern of changes in brain physiology during tonic-clonic seizures, we observed early focal changes in specific cortical and subcortical areas that were more intense than those in other regions. The results of our experiments demonstrate that not only do specific networks show dramatic changes in BOLD signal during tonic-clonic seizures while others are relatively spared, but that focal areas show significant neurophysiological changes nearly 30 s prior to electrographic seizure onset. Specifically, early BOLD increases in primary and secondary somatosensory cortices suggest that tonic-clonic seizures may arise from focal changes in cortical excitatory activity. These changes are quickly joined by thalamic increases, suggesting that activity spreads into corticothalamic networks, and are later accompanied by BOLD increases in brainstem and decreases in hippocampus.
Because BOLD signal directly reflects blood oxygen content, it is influenced by cerebral blood volume (CBV), cerebral blood flow (CBF) and neuronal activity, specifically cerebral metabolic rate of oxygen consumption (CMRO2) (Shulman et al., 2002). Although we did not measure electrophysiological changes, blood flow or volume in all the regions investigated in this study, our prior work has shown that in the rat cerebral cortex, fMRI signals are closely related to neuronal activity during seizures in terms of intensity and anatomical localization (Nersesyan et al., 2004b; Nersesyan et al., 2004a; Englot et al., 2008; Schridde et al., 2008). Nevertheless, fMRI results should be interpreted with caution, since it has been shown that BOLD signals and neuronal activity can become decoupled during seizures, particularly in some regions such as the hippocampus (Shulman et al., 2007; Schridde et al., 2008; Motelow and Blumenfeld, 2009). Power spectral analysis of the EEG signals did not detect early changes at the time of initial fMRI increases. However, the EEG measurements were limited since they did not directly record from the local cortical regions in which early fMRI changes occurred. It would be of interest to perform such measurements with microelectrodes in future studies.
Based on the t-map and VOI timecourse analyses, the areas showing most intense involvement during tonic-clonic seizures and significant focal BOLD increases prior to seizure onset are the medial primary somatosensory cortex (S1), secondary somatosensory cortex (S2), anterior primary auditory cortex (Au1) and thalamus. Cortical increases first arise focally in S1 and S2/Au1 and spread throughout the cortex as the seizure progresses, but these areas remain among the most strongly activated during seizures. Analysis of various timecourse quantifiers suggests that BOLD increases occur first in S1 (excluding S1BF) and S2/Au1 (-27 s) which are soon accompanied by thalamus (-15 s) and are later joined by S1BF (-3 s). Thalamus and S1 also had the greatest peak BOLD increases (∼12%) followed by S2/Au1 (∼8%) with S1BF much lower (∼4%). Previous work in this model has demonstrated increased electrophysiological activity and CBF in primary somatosensory cortex, coupled with a compensatory increase in CBF leading to locally increased blood oxygenation and increased BOLD signal (Schridde et al., 2008). Similar BOLD increases in corticothalamic networks have been implicated in pentylenetetrazol(PTZ)-induced tonic-clonic seizures in rats (Brevard et al., 2006). However, previous studies have not included group analyses or statistical analyses of fMRI timecourses in multiple regions as in the present work.
Regions of the primary somatosensory cortex also showed the most significant changes in BOLD signal during spike-wave discharges in the WAG/Rij rodent absence model (Nersesyan et al., 2004a) as well as in GAERS (David et al., 2008). Detailed study of EEG during SWD in WAG/Rij rats has implicated a region of somatosensory cortex as the driver of corticothalamic networks (Meeren et al., 2002), giving rise to the cortical focus theory for SWD generation (Meeren et al., 2005). Further support for the cortical focus theory has come with the demonstration of focal ion channel changes and altered dendritic morphology (Klein et al., 2004; Strauss et al., 2004; Karpova et al., 2005). A recent study also showed abnormal white matter connections between these somatosensory cortex regions based on diffusion tensor imaging (DTI) (Chahboune et al., 2009). The present findings suggest that the cortical focus theory may be applicable to other so-called “generalized” epilepsies. Thus, the rodent somatosensory cortex may be particularly sensitive to generalized seizures, including both spike-wave and tonic-clonic epileptiform activity. Further investigation is warranted to determine the mechanisms for the focal activations we observed in the somatosensory cortex before and during tonic-clonic seizures. Possibilities include focal alterations in intrinsic cortical excitability, network changes, alterations in ion channels, excitatory and inhibitory neurotransmission, local pharmacological sensitivity to the GABAA receptor antagonist bicuculline, neuronal morphology, or synaptic connections. Altered GABAA receptor function associated with various mutations occurring in several types of generalized epilepsy has been reported in human patients (Dibbens et al., 2009; Macdonald and Kang, 2009).
Application of the concept that focal cortical regions are most intensely involved in tonic-clonic seizures has important implications for understanding and treating this disorder in human patients. Indeed, studies of secondarily generalized seizures in human patients based on ictal SPECT (Blumenfeld et al., 2003a; Blumenfeld et al., 2003b; Enev et al., 2007; Blumenfeld et al., 2009; Varghese et al., 2009) and intracranial EEG (Schindler et al., 2007) suggest that focal cortical regions are intensely involved while others are relatively spared. Further investigation of focal networks crucial for the initiation and maintenance of tonic-clonic seizures may ultimately enable targeted therapies, including neurostimulation, disconnection procedures, and novel pharmacological treatments directed at these structures.
We also observed ictal BOLD increases in the brainstem tegmentum. This was not surprising as increased neuronal activity in the brainstem has been demonstrated in tonic-clonic seizures in both humans (McNally and Blumenfeld, 2004; Blumenfeld et al., 2009) and animal models (Browning, 1985; Gale, 1992; Faingold, 1999; Nersesyan et al., 2004a; Brevard et al., 2006). The hippocampus showed significant ictal and post-ictal BOLD decreases. In a previous study from our group on this same model, we observed no change or a decrease in hippocampal BOLD signal despite a large increase in electrical activity during tonic-clonic seizures suggesting a local dissociation between neuronal activity and BOLD signal in this region (Schridde et al., 2008). The present study confirms and extends this finding by demonstrating overall hippocampal BOLD signal decreases based on group analysis. One possible mechanism for BOLD decreases amid increased neuronal activity is that the intense local increase in cerebral metabolic rate of oxygen consumption (CMRO2) had increased beyond the accompanying increase that was observed in CBF, leading to increased deoxygenated hemoglobin and decreased BOLD signal in the hippocampus (Schridde et al., 2008).
The current study has several limitations that should be addressed through further investigation. While the fMRI changes suggest that early focal changes in neuronal activity occur prior to onset of chemically-induced tonic-clonic seizures, this should be confirmed using direct multi-site recordings of electrical neuronal activity, ideally during spontaneously occurring tonic-clonic seizures in awake-behaving animals. As with many fMRI studies, slow signal drift can serve as a confounding factor. Many of the methods typically used to remove these effects were not appropriate in this study. For example, the use of a high pass filter or removal of a polynomial fit of the entire timecourse would not isolate and remove drift due to the length and magnitude of the seizure event. We believe our approach (subtraction analysis using animals under the same conditions but without seizures) allows for removal of drift signal while maintaining the physiological changes due to the seizure. Additionally, the principal focus of this study is on the novelty of the preictal changes which occur early on in the scan and would be much less affected by any potential confounding slow drift signals. In addition, although a large portion of the rat brain was included in our current study, we cannot exclude possibly important changes in more anterior or posterior brain regions, which may be included in future studies with continually improving fMRI coil technology. Specifically, ventral structures where lower signal-to-noise ratio was observed would be better studied with a modified coil design.
In conclusion, we measured the timecourse and spatial distribution of BOLD signal changes during bicuculline-induced tonic-clonic seizures and observed early and intense changes in focal brain regions. Specific areas of cortex, S1 and S2/Au1, along with thalamus showed the strongest and earliest BOLD increases. We observed moderate BOLD increases in S1BF and brainstem tegmentum as well that came later. BOLD decreases were observed ictally and post-ictally in the hippocampus. These results support the conclusion that so-called “generalized” tonic-clonic seizures do not affect the entire brain homogeneously and implicate focal cortical regions, similar to those involved in absence seizure generation, as the origin of seizure activity. These findings will play a key role in elucidating the causes and mechanisms of similar seizure disorders in humans, and in guiding further therapeutic interventions.
Supplementary Material
Supplemental Figure 1. A) Volumes of interest (VOIs) for timecourse analysis, shown in coronal cross sections superimposed on the fMRI template data set. Slices are indicated in millimeters relative to bregma. B) Corresponding coronal cross sections reproduced with permission from Paxinos and Watson (Paxinos, 1998). S1= Primary somatosensory cortex (excluding S1BF), including hindlimb (S1HL), forelimb (S1FL), dysgranular zone (S1DZ), and trunk (S1Tr); S1BF = Primary somatosensory cortex, barrel field; S2 = Secondary somatosensory cortex; Au1 = Primary auditory cortex; Thal = Thalamus; HC = Hippocampus; BS = Brainstem tegmentum.
Acknowledgments
This work was supported by NIH R01 NS049307 (HB), P30 NS052519 (FH), Epilepsy Foundation Award ID: 123505 (AMM), and by the Betsy and Jonathan Blattmachr family. We thank Dario Englot, Xiaoxiao Bai and Nathan Danielson for helpful comments on the manuscript. We thank Dr. Bei Wang for technical assistance in animal preparation, the engineering staff of the Magnetic Resonance Research Center (http://mrrc.yale.edu) and Quantitative Neuroscience with Magnetic Resonance (http://qnmr.yale.edu) for hardware maintenance and radio frequency probe construction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann RF, Engel J, Jr, Baxter L. Positron emission tomography and autoradiographic studies of glucose utilization following electroconvulsive seizures in humans and rats. Annals of the New York Academy of Sciences. 1986;462:263–269. doi: 10.1111/j.1749-6632.1986.tb51260.x. [DOI] [PubMed] [Google Scholar]
- Andre V, Henry D, Nehlig A. Dynamic variations of local cerebral blood flow in maximal electroshock seizures in the rat. Epilepsia. 2002;43:1120–1128. doi: 10.1046/j.1528-1157.2002.17702.x. [DOI] [PubMed] [Google Scholar]
- Benner T, van der Kouwe AJ, Kirsch JE, Sorensen AG. Real-time RF pulse adjustment for B0 drift correction. Magn Reson Med. 2006;56:204–209. doi: 10.1002/mrm.20936. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Ostroff RB, Zubal IG. Targeted prefrontal cortical activation with bifrontal ECT. Psychiatry Res. 2003a;123:165–170. doi: 10.1016/s0925-4927(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, Uranga P, Tanhehco T, Smith A, Seibyl JP, Stokking R, Studholme C, Spencer SS, Zubal IG. Selective frontal, parietal and temporal network activation in generalized seizures. Neuroimage. 2003b;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Brevard ME, Kulkarni P, King JA, Ferris CF. Imaging the neural substrates involved in the genesis of pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–754. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Browning RA. Role of the brain-stem reticular formation in tonic-clonic seizures: lesion and pharmacological studies. FASEB. 1985;44:2425–2431. [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, Hyder F, Blumenfeld H. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Harkin LA, Richards M, Hodgson BL, Clarke AL, Petrou S, Scheffer IE, Berkovic SF, Mulley JC. The role of neuronal GABA(A) receptor subunit mutations in idiopathic generalized epilepsies. Neurosci Lett. 2009;453:162–165. doi: 10.1016/j.neulet.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Enev M, McNally KA, Varghese G, Zubal IG, Ostroff RB, Blumenfeld H. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–244. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Kuhl DE, Phelps ME. Patterns of Human Local Cerebral Glucose Metabolism During Epileptic Seizures. Science. 1982;218:64–66. doi: 10.1126/science.6981843. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL. Neuronal networks in the genetically epilepsy-prone rat. Advances in Neurology. 1999;79:311–321. [PubMed] [Google Scholar]
- Foerster BU, Tomasi D, Caparelli EC. Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magn Reson Med. 2005;54:1261–1267. doi: 10.1002/mrm.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K. Subcortical structures and pathways involved in convulsive seizure generation. Journal of Clinical Neurophysiology. 1992;9:264–277. doi: 10.1097/00004691-199204010-00007. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Karpova AV, Bikbaev AF, Coenen AM, van Luijtelaar G. Morphometric Golgi study of cortical locations in WAG/Rij rats: the cortical focus theory. Neurosci Res. 2005;51:119–128. doi: 10.1016/j.neures.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Lehmann TM, Gonner C, Spitzer K. Survey: interpolation methods in medical image processing. IEEE Trans Med Imaging. 1999;18:1049–1075. doi: 10.1109/42.816070. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ. Molecular Pathology of Genetic Epilepsies Associated with GABA(A) Receptor Subunit Mutations. Epilepsy Curr. 2009;9:18–23. doi: 10.1111/j.1535-7511.2008.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Duncan GE, Johnson KB, Breese GR. Metabolic and functional mapping of the neural network subserving inferior collicular seizure generalization. Brain Res. 1995;701:117–128. doi: 10.1016/0006-8993(95)00970-x. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Don JC, Edson N. Distribution of [14C]2-deoxyglucose after various forms and durations of status epilepticus induced by stimulation of a kindled amygdala focus in rats. Epilepsy Res. 1991;10:119–133. doi: 10.1016/0920-1211(91)90004-y. [DOI] [PubMed] [Google Scholar]
- McNally KA, Blumenfeld H. Focal network involvement in generalized seizures: new insights from electroconvulsive therapy. Epilepsy & Behavior. 2004;5:3–12. doi: 10.1016/j.yebeh.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. Journal of Neuroscience. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelow JE, Blumenfeld H. Functional neuroimaging of spike-wave seizures. Methods Mol Biol. 2009;489:189–209. doi: 10.1007/978-1-59745-543-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004a;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004b;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Waston C. The rat brain in stereotaxic coordinates. 4th. San Diego: Academic Press; 1998. [Google Scholar]
- Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21:410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Leung H, Lehnertz K, Elger CE. How generalised are secondarily “generalised” tonic clonic seizures? J Neurol Neurosurg Psychiatry. 2007;78:993–996. doi: 10.1136/jnnp.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Biophysical basis of brain activity: implications for neuroimaging. Q Rev Biophys. 2002;35:287–325. doi: 10.1017/s0033583502003803. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Lewis BK, Ruttimann UE, Ye FQ, Sinnwell TM, Yang Y, Duyn JH, Frank JA. Investigation of low frequency drift in fMRI signal. Neuroimage. 1999;9:526–533. doi: 10.1006/nimg.1999.0435. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Paige AL, Zubal IG, Spencer SS, Blumenfeld H. Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain. 2009;132:2102–2113. doi: 10.1093/brain/awp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A) Volumes of interest (VOIs) for timecourse analysis, shown in coronal cross sections superimposed on the fMRI template data set. Slices are indicated in millimeters relative to bregma. B) Corresponding coronal cross sections reproduced with permission from Paxinos and Watson (Paxinos, 1998). S1= Primary somatosensory cortex (excluding S1BF), including hindlimb (S1HL), forelimb (S1FL), dysgranular zone (S1DZ), and trunk (S1Tr); S1BF = Primary somatosensory cortex, barrel field; S2 = Secondary somatosensory cortex; Au1 = Primary auditory cortex; Thal = Thalamus; HC = Hippocampus; BS = Brainstem tegmentum.