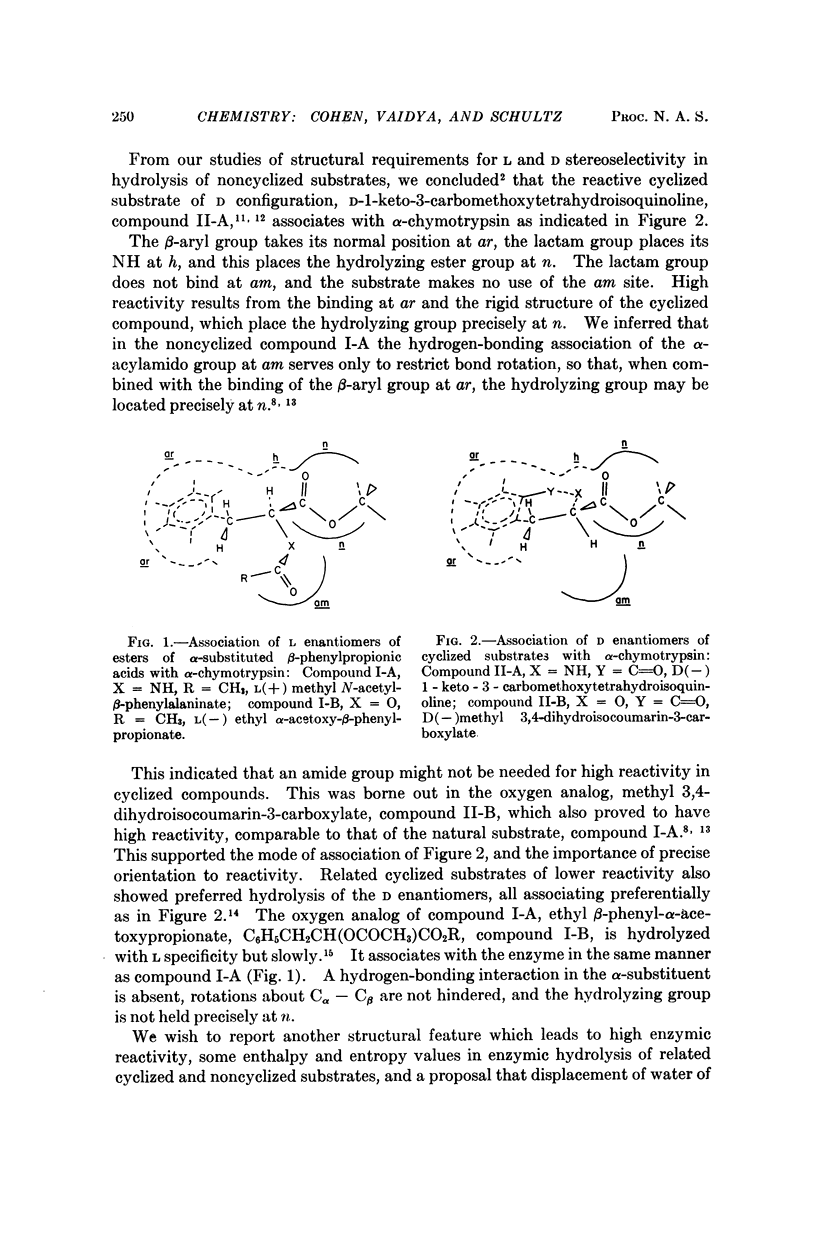
Abstract
High reactivity toward α-chymotrypsin is observed for derivatives of β-arylpropionic acids of varied structure—L-α-acylamido compounds, D-cyclized compounds, and, now, L-glycolamide esters. Compensating enthalpy and entropy effects are observed which appear to be caused by changes in water of solvation. High reactivity with varied structure, and physical evidence, appear to rule out induced fit and distortion as important for this enzyme. The high reactivity results from precise fit of the hydrolyzing group at the critical serine-imidazole junction, resulting from binding of the aryl group and restriction of rotation. Part of the energy of binding is used to desolvate the reactant groups of substrate and enzyme, decreasing activation energies by several kilocalories and raising reactivity by 103 or more. Solvation by water stabilizes many compounds, allowing them to be present in solution in biological systems. Their reactions may occur as their reactivity is increased when they are desolvated and brought from solution into association with reactive groups in enzymes, membranes, and structured particles.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLS A. K., JANSEN E. F. Stoichiometric inhibition of chymotrypsin. Adv Enzymol Relat Subj Biochem. 1952;13:321–343. doi: 10.1002/9780470122587.ch8. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Matthews B. W., Blow D. M. Atomic co-ordinates for tosyl-alpha-chymotrypsin. Biochem Biophys Res Commun. 1969 Jul 7;36(1):131–137. doi: 10.1016/0006-291x(69)90659-7. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. G., CROSSLEY J., KHEDOURI E. ACTION OF ALPHA-CHYMOTRYPSIN ON DIETHYL N-ACETYLASPARTATE AND ON DIETHYL N-METHYL-N-ACETYLASPARTATE. Biochemistry. 1963 Jul-Aug;2:820–823. doi: 10.1021/bi00904a034. [DOI] [PubMed] [Google Scholar]
- Cohen S. G., Klee L. H., Weinstein S. Y. Action of alpha-chymotrypsin on the diethyl esters of fumaric, maleic, and acetylenedicarboxylic acids. J Am Chem Soc. 1966 Nov 20;88(22):5302–5305. doi: 10.1021/ja00974a050. [DOI] [PubMed] [Google Scholar]
- Cohen S. G., Milovanović A., Schultz R. M., Weinstein S. Y. On the active site of alpha-chymotrypsin. Absolute configurations and kinetics of hydrolysis of cyclized and noncyclized substrates. J Biol Chem. 1969 May 25;244(10):2664–2674. [PubMed] [Google Scholar]
- Cohen S. G., Neuwirth Z., Weinstein S. Y. Association of substrates with alpha-chymotrypsin, diethyl alpha-acetoxysuccinate, and diethyl malate. J Am Chem Soc. 1966 Nov 20;88(22):5306–5315. doi: 10.1021/ja00974a051. [DOI] [PubMed] [Google Scholar]
- Cohen S. G., Schultz R. M. THE ACTIVE SITE IN alpha-CHYMOTRYPSIN: METHYL 3,4-DIHYDROISOCOUMARIN-3-CARBOXYLATE I. Proc Natl Acad Sci U S A. 1967 Feb;57(2):243–249. doi: 10.1073/pnas.57.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. G., Schultz R. M. The active site in alpha-chymotrypsin. Absolute configuration and kinetics of hydrolysis of methyl 3,4-dihydroisocoumarin-3-carboxylate. J Biol Chem. 1968 May 25;243(10):2607–2617. [PubMed] [Google Scholar]
- Gerig J. T. Nuclear magnetic resonance studies of the interaction of tryptophan with alpha-chymotrypsin. J Am Chem Soc. 1968 May 8;90(10):2681–2686. doi: 10.1021/ja01012a038. [DOI] [PubMed] [Google Scholar]
- HEIN G., NIEMANN C. An interpretation of the kinetic behavior of model substrates of alpha-chymotrypsin. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1341–1355. doi: 10.1073/pnas.47.9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles D. W., Knowles J. R. The stereospecificity of alpha-chymotrypsin. Biochem J. 1968 Jul;108(4):561–569. doi: 10.1042/bj1080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc Natl Acad Sci U S A. 1958 Feb;44(2):98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- Parker L., Wang J. H. On the mechanism of action at the acylation step of the alpha-chymotrypsin-catalyzed hydrolysis of anilides. J Biol Chem. 1968 Jul 10;243(13):3729–3734. [PubMed] [Google Scholar]
- Riepe M. E., Wang J. H. Infrared studies on the mechanism of action of carbonic anhydrase. J Biol Chem. 1968 May 25;243(10):2779–2787. [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Ludwig M. L., Quiocho F. A., Lipscomb W. N. The structure of carboxypepidase A. V. Studies of enzyme-substrate and enzyme-inhibitor complexes at 6 A resolution. J Biol Chem. 1967 Oct 25;242(20):4662–4668. [PubMed] [Google Scholar]
- Wang J. H. Facilitated proton transfer in enzyme catalysis. It may have a crucial role in determining the efficiency and specificity of enzymes. Science. 1968 Jul 26;161(3839):328–334. doi: 10.1126/science.161.3839.328. [DOI] [PubMed] [Google Scholar]



