Abstract
Purpose
The inherited bone marrow failure syndromes (IBMFS) are a heterogeneous group of genetic disorders that share the inability of the bone marrow to produce an adequate number of blood cells. The four most frequent syndromes are Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anemia (DBA) and Shwachman-Diamond syndrome (SDS). All four syndromes have been associated with various physical abnormalities. As part of a genotype/phenotype/cancer susceptibility study, we determined the prevalence of ophthalmic manifestations in these four syndromes.
Design
Cross-sectional study of a patient cohort.
Participants
Seventy-five patients with an IBMFS and 121 of their first degree relatives were seen in the National Eye Institute, National Institutes of Health from 2001 to 2007. The patient group included 22 FA, 28 DC, 19 DBA and six SDS.
Methods
Every participant underwent a complete ophthalmic evaluation, as well as digital facial photography with an adhesive paper ruler on the patient's forehead for an internal measure of scale. Interpupillary distance (IPD), inner canthal distance (ICD), outer canthal distance (OCD), palpebral fissure length (PFL) and corneal diameter (CD) were measured. Thirteen of the 22 patients with FA underwent axial length (AL) measurements by A-scan ultrasonography.
Main Outcome Measures
Type and prevalence of ophthalmic manifestations.
Results
Ninety-five percent of patients with FA had at least one abnormal parameter, and 25% at least four abnormal parameters. Eighty-two percent had small palpebral fissures, 69% simple microphthalmia, 64% small OCD, 55% microcornea, 28% ptosis, and six percent epicanthal folds. In patients with DC, abnormalities of the lacrimal drainage system (29%) were the most prevalent findings, followed by retinal abnormalities (pigmentary changes, retinal neovascularization, retinal detachment, exudative retinopathy) in 21%, cicatricial entropion with trichiasis and blepharitis in seven percent each, sparse eyelashes and congenital cataract in three and a half percent each. No significant ophthalmic abnormalities were seen in patients with DBA or SDS.
Conclusions
Syndrome-specific ocular findings are associated with FA and DC and may antedate diagnosis of the specific syndrome. Early recognition of these abnormalities is important for optimal management.
The inherited bone marrow failure syndromes (IBMFS) are a heterogeneous group of genetic disorders that share inability of the bone marrow to produce an adequate number of blood cells1-3. Approximately 10-25% of patients who present with aplastic anemia at any age have an inherited etiology.1, 3, 4 The precise incidence of these syndromes remains unclear. The bone marrow failure can involve all lineages or a single cell line, and the age of initial presentation ranges from early childhood to adulthood. The four most common of these syndromes are Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anemia (DBA) and Shwachman-Diamond syndrome (SDS).
Fanconi anemia (FA)
FA is a rare, genetically and phenotypically heterogeneous primarily autosomal recessive (rarely X-linked) DNA repair disorder, characterized by progressive bone marrow failure and a very high risk of cancer (leukemia, and head and neck, gynecologic, and anogenital squamous cell carcinomas). FA is associated with a high frequency of various birth defects including cardiac, renal, eye, ear and limb malformations (radial aplasia, thumb aplasia/hypoplasia, or duplicated thumb) as well as dermal pigmentary changes.
Dyskeratosis Congenita (DC)
DC is a disease of defective telomere biology,5-7 classically diagnosed by the presence of at least two of the three features of the diagnostic triad: dysplastic fingernails and/or toenails, lacy reticular pigmentation of the skin, and oral leukoplakia. Multiple other manifestations have been associated with the disease, including potential involvement of dental, gastrointestinal, skeletal, neurological, immunological, ophthalmic, genitourinary, and pulmonary tissues, as well as bone marrow aplasia.
Diamond-Blackfan anemia (DBA)
DBA, or congenital pure red cell aplasia, characteristically presents in early infancy as a steroid responsive macrocytic anemia. Accompanying physical abnormalities may be subtle and include thumb abnormalities, flat thenar muscles and short stature.
Shwachman-Diamond syndrome (SDS)
SDS is an autosomal recessive disorder (MIM 260400) characterized by exocrine pancreatic insufficiency, bone marrow failure (primarily neutropenia) and somatic abnormalities such as metaphyseal dysplasia (in 50%).
As a component of an ongoing genotype/phenotype/cancer susceptibility study at the National Cancer Institute, we examined ophthalmic parameters in patients with IBMFS and their first degree relatives.
PATIENTS AND METHODS
Patients
Seventy-five patients with an IBMFS and 121 of their first degree relatives were seen at the National Eye Institute, National Institutes of Health clinic from 2001 to 2007. All participants were enrolled in protocol 02-C-0052 “Etiologic Investigation of Cancer Susceptibility in Inherited Bone Marrow Failure Syndromes: A Natural History Study”, approved by the Institutional Review Board of the National Cancer Institute (www.clinicaltrials.gov, trial NCT00056121, URL last accessed July 16, 2009). Written informed consent was obtained from each participant and/or his/her parent or guardian; written assent was obtained from those between 11 and 18 years of age.
The diagnoses and ages of the participants are shown in Table 1. There were 23 families with FA, including 22 patients (one had died), 33 parents (obligate carriers) and 18 siblings (nine normal, two carriers and seven unknown). The DC group included 18 families with 28 patients. Inheritance was autosomal dominant in nine families, X-linked in three, and the pattern of inheritance was unclear from the pedigree in six. The DBA group included 13 families with 19 patients. Parents and siblings of probands with DC or DBA were included as patients if they too had those diagnoses; “parents” and “siblings” thus are unaffected family members. There were six families with six patients with SDS.
Table 1.
Characteristics of Participants
| Number | Male:Female | Median Age, Years (Range) | |
|---|---|---|---|
| Fanconi anemia | |||
| Total group | 73 | ||
| Patients | 22 | 7:15 | 21 (5-42) |
| Siblings | 18 | 10:8 | 14 (3-43) |
| Parents | 33 | 16:17 | 51 (37-76) |
| Dyskeratosis congenita | |||
| Total group | 58 | ||
| Patients | 28 | 22:6 | 15 (1.5-43) |
| Siblings | 9 | 3:6 | 29 (4-34) |
| Parents | 21 | 8:13 | 41 (30-63) |
| Diamond-Blackfan anemia | |||
| Total group | 47 | ||
| Patients | 19 | 14:5 | 11 (3-44) |
| Siblings | 13 | 7:6 | 7 (1-34) |
| Parents | 16 | 7:9 | 41 (33-64) |
| Shwachman-Diamond syndrome | |||
| Total group | 17 | ||
| Patients | 6 | 2:4 | 13 (8-42) |
| Siblings | 3 | 3:0 | 10 (10-11) |
| Parents |
8 |
2:6 |
47 (30-63) |
| Total Participants | 196 | 101:95 | 1.5-76 |
While many patients with inherited bone marrow failure syndromes have physical appearances and/or laboratory findings that are specific to each syndrome, there is a subset of patients who may be harder to classify. The diagnosis of each inherited bone marrow failure syndrome was initially based on the syndrome-specific clinical findings and supported by specific laboratory tests.4 The diagnosis of FA was confirmed by increased chromosomal breakage in the peripheral blood or skin fibroblasts in the presence of clastogenic agents such as mitomycin C or diepoxybutane. DC was strongly suspected by the presence of extremely short telomeres (less than the first percentile for age) in peripheral blood leukocyte subsets; this test is used as a diagnostic marker for DC.8 The diagnosis of DBA was supported by an increased red blood celladenosine deaminase (ADA).9 SDS was diagnosed if there were very low serum levels of pancreatic enzymes trypsinogen and isoamylase, plus neutropenia.10 Where possible, diagnoses were confirmed in cases where mutations were found in the appropriate genes.
Ophthalmologic Examination
All participants underwent a complete ophthalmologic evaluation, including best corrected visual acuity with manifest refraction, slit-lamp biomicroscopy, and dilated ophthalmoscopic examination after instillation of phenylephrine 2.5% and tropicamide 1%. Cycloplegia was not used for children. Visual acuity was assessed using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts. External facial photographs were obtained with a handheld digital camera (FinePix SR2 by Fuji, Tokyo, Japan with a Nikon, Tokyo, Japan 105mm lens and flash), using a technique similar to one previously described.11 The photographs were taken with a 30 mm adhesive paper ruler on the patient's forehead for an internal measure of scale. Interpupillary distance (IPD), inner canthal distance (ICD), outer canthal distance (OCD), palpebral fissure length (PFL) and corneal diameter (CD) measurements were calculated from the facial photographs with the absolute digimatic solar caliper by Mitutoyo, Tokyo, Japan. IPD, ICD, OCD, PFL and CD were compared with previously established normal values for age and gender performed by direct measurement12, 13. Margin reflex distance (MRD) was also calculated for patients with obvious ptosis. Levator function was not evaluated for any of the patients. Using the published normal values, the Z-score was calculated for IPD, ICD, OCD and PFL for each participant based on the equation:
The Z-score provides an age- and gender-corrected comparison of each parameter to published normal values. Z-scores below or equal to minus two and equal to or above two were abnormal for the calculated parameter. Corneal diameter was considered to be abnormal if it was smaller than 11 mm. Corneal, anterior segment and fundus photographs were obtained if abnormalities were noticed. Since microcornea and microphthalmia were previously reported in FA, the last 13 patients with FA underwent A-scan ultrasonography for axial length (AL), which also included anterior chamber length (ACL) and lens thickness (LT) measurements in five patients. AL, ACL and LT were compared with established normal values for age.14-16
Validation of Photographic Measurements
In order to validate our method of photographic measurements of the ocular and orbital parameters, 11 normal volunteers underwent digital photography and measurements of the ICD, IPD, OCD, PFL and CD according to the above method, as well as direct measurements using a ruler. In addition, a subgroup of patients (10 FA, 22 DC, 12 DBA and five SDS) also underwent direct measurements of IPD, ICD, OCD and PFL by one of the authors (CM). To maintain consistency, the data used for this report were those obtained by the analyses of digital photographs, since all patients had these measurements.
Statistics
Statistical analyses were performed using Microsoft Excel and STATA10 (StataCorp LP. Stata Statistical Software: Release 10. 2007. College Station, TX.: Stata Corporation). P-values less than or equal to 0.05 were considered statistically significant.
RESULTS
Validation of photographic measurements
Photographic measurements in the normal volunteers were often larger than direct measurements, suggesting that the photographic method tended to overestimate the true value. In the subgroup of patients (approximately 30%) that underwent direct and photographic measurements, about 60% of the patients had larger IPD and ICD by photographic measurements, consistent with an underestimation of the numbers of patients with small eye dimensions.
Fanconi anemia (FA)
Two of the 22 patients with FA (9%) had epicanthus palpebralis, six (28%) ptosis (mean MRD for those six patients 1.1 mm, range 0-1.7 mm), six (28%) limbal neovascularization and, one (4.5%) posterior embryotoxon. Three patients developed cataracts; all three had received stem cell transplants: one 11 year-old developed bilateral posterior subcapsular cataracts six years after receiving 4.5 Gy total body irradiation and steroids; one nine year-old had bilateral small nuclear opacities not affecting visual acuity one year after transplant without total body irradiation; and one patient had undergone bilateral cataract extraction at 20 years of age complicated by retinal detachment and repair in the postoperative period. This patient had undergone bone marrow transplantation at the age of 10 years, without irradiation. No further information is available regarding the type of cataract.
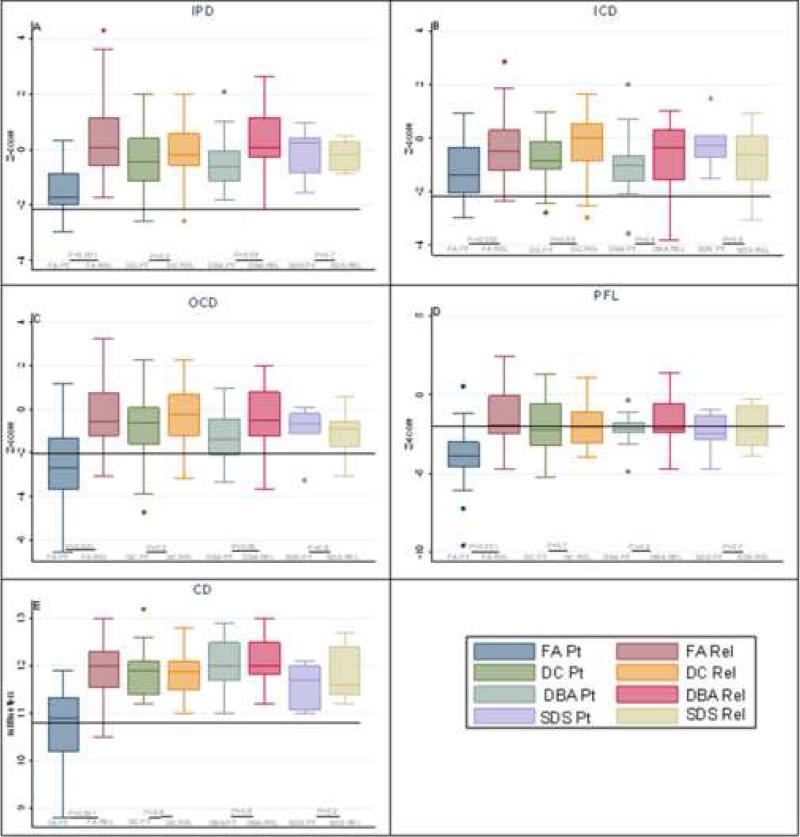
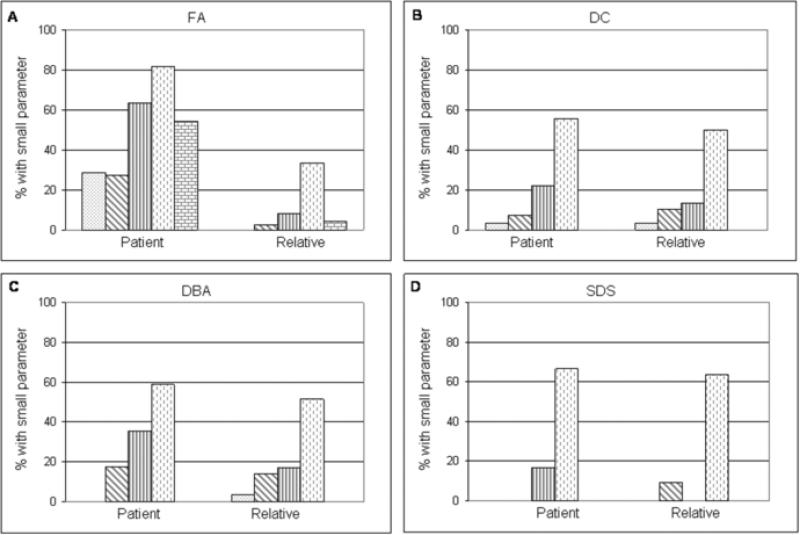
Digital photography showed that all orbital and ocular measurements were statistically significantly smaller (p<0.01) in patients with FA compared with their unaffected relatives (Figure 1). Patients with FA also had significantly smaller IPD, OCD and CD than patients with any other IBMFS (p<0.01) (Figure 1A, C and E). PFL was significantly smaller when compared with DC and DBA patients (p<0.01) but not when compared with SDS patients (Figure 1D). Twenty-nine percent of the patients with FA had abnormally small IPD, 27% small ICD, 64% small OCD, 82% small PFL and 55% microcornea (small CD) compared with age- and gender-matched published normal values (Figure 2A). In general, more patients with FA had small orbital and ocular measurements compared with their unaffected relatives or patients with other disorders (Figure 2). Ninety-five percent of the patients with FA had at least one abnormally small parameter, and 25% had four or five abnormal parameters. Seven patients with FA had microcephaly. All seven patients with microcephaly had small OCD and PFL; however, only seven out of 15 patients without microcephaly had small OCD and PFL (p=0.02), indicating that small orbital measurements strongly associated with small head size; however, small measurements were present in some patients with normal head size.
Figure 1.
Comparison of Z-scores for ophthalmic parameters among patients and their relatives: A) Interpupillary distance (IPD); B) Inner canthal distance (ICD); C) Outer canthal distance (OCD); and D) Palpebral fissure length (PFL); E) Corneal diameter (CD)*. Each box represents 25th - 75th percentile. The whiskers are between 5th - 95th percentiles; outliers are individual observations beyond the whiskers; horizontal line through each box is the median. Any observation at or below the solid black line (≤-2 Z-score) is an abnormally low value. *CD is in millimeters; normal is at or above 11 millimeters. PT, patient. REL, relative. Relatives are unaffected siblings and parents.
Figure 2.
Frequency of small eye parameters in patients and relatives: A) Fanconi anemia (FA); B) Dyskeratosis congenita (DC); C) Diamond-Blackfan anemia (DBA); D) Shwachman-Diamond syndrome (SDS). Black dots, Interpupillary distance (IPD). Diagonal lines, Inner canthal distance (ICD). Vertical lines, Outer canthal distance (OCD). Interrupted vertical lines, Palpebral fissure length (PFL). Small blocks, Corneal diameter (CD).
Thirteen patients with FA (ages nine-42 years) underwent A-scan ultrasonography. The mean AL was 21.1 mm (normal for an adult 23.15 mm ± 0.76 mm). Nine patients (69%) had microphthalmia (defined as an axial length smaller than the mean for age minus two standard deviations and a structurally normal eye). Five of the 13 patients also had ACL and LT measured; the mean ACL was 3.3 mm and mean LT 3.6 mm; both these measurements were within normal limits for all patients.
Seven of the 20 (35%) patients who underwent refraction had clinically significant myopia (defined as a spherical equivalent of −1.00 D or less in either eye) and four (20%) had clinically significant astigmatism (defined as a cylinder of 1.0 D or greater in either eye). No patient had clinically important hyperopia (defined as a spherical equivalent of +3.00 D and above). The frequency of myopia was similar to numbers seen in the general population but the frequency of astigmatism was slightly lower, possibly because of the small number of patients in our study.17
Dyskeratosis Congenita (DC)
Eight of the 28 patients with DC (28.6%) were noted to have obstruction of the lacrimal drainage system: seven had absence of puncti and one had a history of nasolacrimal duct obstruction and had undergone dacryorhinocystostomy. Two patients had cicatricial entropion and trichiasis, one had sparse eyelashes and two had episodes of keratoconjunctivitis. One had a history of prior cataract extraction but information about the type of cataract was unavailable. Fundoscopic changes were seen in 21% of the patients. The most frequent funduscopic findings were granular retinal pigment epithelial changes in three patients, retina neovascularization in three patients, retinal detachment in two patients, vascular sheathing in one patient (Figure 3B and 3C), and exudative retinopathy in one patient. Some of the patients had a combination of two or more of the above fundoscopic findings. Twenty-seven patients with DC, 21 parents and nine siblings had digital facial photos. Overall, no significant abnormalities were noted for any of the measurements (Figure 2B). Twenty-two percent of the patients had small OCD and 55% small PFL; these percentages were not significantly different from those in the unaffected family members. In DC, there was no correlation of small eyes with microcephaly.
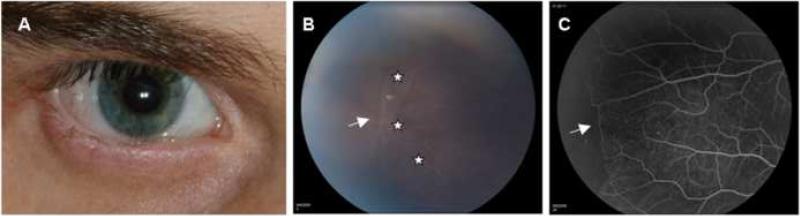
Figure 3.
Ophthalmic manifestations in Dyskeratosis congenita (DC): A) External photograph of the right eye of a 21 year-old patient with cicatricial entropion and trichiasis; B) Funduscopic photograph, and C) Fluorescein angiography of the periphery of the retina of a 12 year-old patient with areas of perivascular sheathing (B, white stars) and non-perfusion (C, white arrows).
Diamond-Blackfan anemia (DBA)
Two of the 19 patients with DBA had epicanthus palbebralis, two had posterior embryotoxon, and one patient had a history of congenital glaucoma. Seventeen DBA patients, 13 siblings and 16 parents underwent digital facial photography. The patient group had lower IPD compared with the relatives (p=0.05; Figure 1A). OCD tended to be lower than unaffected relatives (p=0.06), but the difference was not statistically significant (Figure 1C). Seventeen percent of the patients were noted to have abnormally small measurements for ICD, 35% for OCD and 59% for PFL, but none of these frequencies were significantly different than in unaffected relatives (Figure 2C). There was no correlation between small eye parameters and microcephaly.
Shwachman-Diamond syndrome (SDS)
No significant ophthalmic abnormalities were noted in patients with SDS. Facial digital photography was done in six patients, three siblings and eight parents. Overall, all the measurements in the patients and their unaffected relatives were similar (Figure 1). One patient had abnormally small OCD and four had abnormally small PFL; the frequencies were not significantly different from those in the relatives (Figure 2D). Similar to the findings in DC and DBA, there was no association between small eye measurements and microcephaly.
DISCUSSION
We compared photographic ophthalmic measurements with normal values for the same parameters obtained by direct methods, because there are no normal values in the literature using digital photography. We found that the photographic method overestimated the length of most parameters in the majority of our normal volunteers, thus potentially underestimating the true incidence of microcornea and small orbital parameters in our patient population. In fact, the photographic measurements were larger than the digital ones in about half of our patients. A limitation of the photographic method is that the measurement is made on a two-dimensional photograph. Strengths, however, are that the measurement is performed using an internal scale from an included ruler, and a digimatic caliper. A limitation of the direct method is that a non-digital tape measure is used and the method is subjective, operator-dependent, and requires patient cooperation. For these reasons, we selected digital photography as our standard, recognizing that some patients with small eyes might be misclassified as normal.
Despite numerous case reports describing ophthalmic manifestations in patients with an IBMFS, the prevalence of these findings is not clear from the literature. Based on our study and previous reported cases, both FA and DC are associated with significant ophthalmic abnormalities.
Fanconi anemia (FA)
Ocular abnormalities have been reported in up to 38% of patients and include microcornea with or without microphthalmia,18 strabismus, epicanthal folds, ptosis, cataract,19, 20 increased crystalline lens thickness and phacomorphic glaucoma,21 congenital glaucoma22 and retinal vasculopathy leading to ischemia and retinal neovascularization.19, 23, 24
Our series of FA patients had a higher incidence of ophthalmic abnormalities than previously reported, as well as several new findings. We found microcornea in 55% and microphthalmia in 69%, higher than previously reported in similar series of patients.18 In addition, 82% of our patients with FA had small PFL, possibly the result of decreased stimulation of palpebral fissure growth by smaller eyes, and 64% had small OCD. Ptosis, epicanthal folds and cataracts were also seen in FA, as described by others, and cataract was either part of the natural history or a result of radiation and/or steroids associated with hematopoietic stem cell transplantation. A novel finding in our patients was limbal neovascularization in six patients; this did not correlate with the presence or absence of anemia, contact lens use or stem cell transplantation. Limbal neovascularization was recently reported in a patient with DC due to presumed corneal limbal insufficiency possibly from telomere deficiency and defective DNA repair.25 The same mechanism could result in limbal stem cell insufficiency and neovascularization in patients with FA. None of our patients had glaucoma, although it was previously described in two siblings by Elgohary et al, who also had short anterior chamber length and increased lens thickness.21 . However, all five patients that we tested had normal anterior chamber length and lens thickness. The cause of microcornea and microphthalmia in FA remains unknown, but is consistent with the growth retardation and general underdevelopment in this disorder. Although none of our patients has developed glaucoma to date, microphthalmic eyes are at increased risk for angle closure glaucoma, and thus it is important that ophthalmologists be aware of the high incidence of microphthalmia in FA, and include axial length measurements in the evaluation of their patients. FA should be considered in the differential diagnosis of patients with microphthalmia and/or microcornea. Patients should be cautioned about the possibility of angle closure glaucoma, and routine eye examinations should be advised.
Dyskeratosis Congenita (DC)
Ophthalmic manifestations were reported in 40% of patients through 1991.26 Abnormalities of the adnexa and anterior segment include punctal atresia and nasolacrimal duct obstruction,26-32 conjunctivitis,33 trichiasis,28, 33 entropion, ectropion, loss of eye lashes,33 corneal scarring,33, 34 ulceration and perforation,28 and cataracts. Optic nerve atrophy,27, 29, 35 retinal vascular sheathing, retinal hemorrhages and neovascularization29, 34, 36-39 and exudative retinopathy40, 41 have been described involving the posterior segment. Revesz syndrome, a severe variant of DC, is associated with exudative retinopathy, aplastic anemia, and central nervous system abnormalities.38-41
The most common findings in patients with DC were obliteration of the lacrimal drainage system, entropion and trichiasis; these may be secondary to epithelial abnormalities in ocular skin and mucous membranes. These complications can lead to recurrent blepharitis, conjunctivitis and keratitis, corneal scarring, and eventually significant decrease in visual acuity if left untreated. Early recognition can lead to optimal management and better outcomes. Although only a small proportion of our patients (three of 28) had retinal vascular changes, early recognition and treatment of this problem prior to the development of retinal neovascularization, exudative retinopathy, and retinal detachment is critical. As stated above, exudative retinopathy is specific to Revesz syndrome.38-41 In our experience, retinal neovascularization was associated with another severe subset, Hoyeraal-Hreidarsson syndrome.42 A baseline ophthalmic evaluation should be part of the initial evaluation of every patient with DC, and subsequent periodic examinations should be part of their routine care.
In addition to the above syndrome specific manifestations, radiation and steroid use for stem cell transplantation in FA and DC patients may lead to glaucoma, cataract formation and radiation retinopathy. A detailed eye examination with possible treatment of any abnormality definitely deserves emphasis prior to the transplant.
Diamond-Blackfan anemia (DBA)
Ophthalmic manifestations have been reported in a small number of patients with DBA and include hypertelorism, strabismus, epicanthal folds, ptosis, blue sclera, congenital cataract and glaucoma.43-47 Epicanthal folds, posterior embryotoxon and congenital glaucoma were seen in a small percentage of our patients.
Shwachman-Diamond syndrome (SDS)
No significant ophthalmic morbidity was seen in our patients with SDS, nor did we find any reports in the literature.
In summary, both FA and DC (and not DBA or SDS) are associated with several ophthalmic manifestations. General ophthalmologists should be aware of these findings, because these rare conditions are often unrecognized.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute and the National Eye Institute, of the National Institutes of Health. This research was also was supported through contracts N02-CP-11019, N02-CP-65504, and N02-65501 with Westat, Inc, Rockville, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Manuscript will be presented in part as a poster at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Fort Lauderdale, Florida in May, 2009.
REFERENCES
- 1.Alter BP. Bone marrow failure syndromes in children. Pediatr Clin North Am. 2002;49:973–88. doi: 10.1016/s0031-3955(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 2.Alter BP. Bone marrow failure syndromes. Clin Lab Med. 1999;19:113–33. [PubMed] [Google Scholar]
- 3.Dokal I, Vulliamy T. Inherited aplastic anaemias/bone marrow failure syndromes. Blood Rev. 2008;22:141–53. doi: 10.1016/j.blre.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Alter BP. Diagnosis, genetics, and management of inherited bone marrow failure syndromes. Hematology Am Soc Hematol Educ Program. 2007:29–39. doi: 10.1182/asheducation-2007.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mech Ageing Dev. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–7. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 7.Vulliamy TJ, Dokal I. Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2008;90:122–30. doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–47. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glader BE, Backer K. Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases. Br J Haematol. 1988;68:165–8. doi: 10.1111/j.1365-2141.1988.tb06184.x. [DOI] [PubMed] [Google Scholar]
- 10.Rothbaum R, Perrault J, Vlachos A, et al. Shwachman-Diamond syndrome: report from an international conference. J Pediatr. 2002;141:266–70. doi: 10.1067/mpd.2002.125850. [DOI] [PubMed] [Google Scholar]
- 11.Avner M, Henning P, Koren G, Nulman I. Validation of the facial photographic method in fetal alcohol spectrum disorder screening and diagnosis. [August 4, 2009];J FAS Int [serial online] 2006 4:e20. Available at: http://www.motherisk.org/JFAS_documents/JFAS_6009-e20_10_10_06.pdf.
- 12.Hall JG, Froster-Iskenius UG, Allanson JE. Handbook of Normal Physical Measurements. Oxford University Press; Oxford: 1989. pp. 132–157. [Google Scholar]
- 13.Farkas L. Anthropometry of the Head and Face. 2nd ed. Raven Press; New York: 1994. pp. 272–84. [Google Scholar]
- 14.Ramani KK, Mani B, Ronnie G, et al. Gender variation in ocular biometry and ultrasound biomicroscopy of primary angle closure suspects and normal eyes. J Glaucoma. 2007;16:122–8. doi: 10.1097/01.ijg.0000212285.55174.f5. [DOI] [PubMed] [Google Scholar]
- 15.Zadnik K, Mutti DO, Mitchell GL, et al. Normal eye growth in emmetropic schoolchildren. Optom Vis Sci. 2004;81:819–28. doi: 10.1097/01.opx.0000145028.53923.67. [DOI] [PubMed] [Google Scholar]
- 16.Larsen JS. The sagittal growth of the eye. 1. Ultrasonic measurement of the depth of the anterior chamber from birth to puberty. Acta Ophthalmol (Copenh) 1971;49:239–62. doi: 10.1111/j.1755-3768.1971.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 17.Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111–9. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdougall LG, Greeff MC, Rosendorff J, Bernstein R. Fanconi anemia in black African children. Am J Med Genet. 1990;36:408–13. doi: 10.1002/ajmg.1320360408. [DOI] [PubMed] [Google Scholar]
- 19.Bahar I, Weinberger D, Kramer M, Axer-Siegel R. Retinal vasculopathy in Fanconi anemia: a case report. Retina. 2005;25:799–800. doi: 10.1097/00006982-200509000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Merriman M, Mora J, McGaughran J. Fanconi anemia and primary cataracts: first case. Ophthalmic Genet. 2002;23:253–5. doi: 10.1076/opge.23.4.253.13880. [DOI] [PubMed] [Google Scholar]
- 21.Elgohary MA, Lim KS, Siriwardena D, et al. Increased crystalline lens thickness and phacomorphic glaucoma in patients with Fanconi anemia. J Cataract Refract Surg. 2006;32:1771–4. doi: 10.1016/j.jcrs.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Aslan D, Ozdogan S, Onol M, et al. An unusual ocular manifestation in Fanconi anemia: congenital glaucoma. Am J Hematol. 2005;78:64–6. doi: 10.1002/ajh.20232. [DOI] [PubMed] [Google Scholar]
- 23.Yahia SB, Touffahi SA, Zeghidi H, et al. Ocular neovascularization in a patient with Fanconi anemia. Can J Ophthalmol. 2006;41:778–9. doi: 10.3129/i06-078. [DOI] [PubMed] [Google Scholar]
- 24.Gayatri NA, Hughes MI, Lloyd IC, Wynn RF. Association of the congenital bone marrow failure syndromes with retinopathy, intracerebral calcification and progressive neurological impairment. Eur J Paediatr Neurol. 2002;6:125–8. doi: 10.1053/ejpn.2001.0559. [DOI] [PubMed] [Google Scholar]
- 25.Aslan D, Ozdek S, Camurdan O, et al. Dyskeratosis congenita with corneal limbal insufficiency. Pediatr Blood Cancer. 2009;53:95–7. doi: 10.1002/pbc.21960. [DOI] [PubMed] [Google Scholar]
- 26.Drachtman RA, Alter BP. Dyskeratosis congenita: clinical and genetic heterogeneity. Report of a new case and review of the literature. Am J Pediatr Hematol Oncol. 1992;14:297–304. [PubMed] [Google Scholar]
- 27.Chambers JK, Salinas CF. Ocular findings in dyskeratosis congenita. Birth Defects Orig Artic Ser. 1982;18:167–74. [PubMed] [Google Scholar]
- 28.Zagorski Z, Biziorek B, Rakowska E, Jedrzejewski D. Zinsser-Engman-Cole syndrome (dyskeratosis congenita) with severe sicca syndrome, panuveitis and corneal perforation--a case report [in German]. Klin Monatsbl Augenheilkd. 2001;218:455–8. doi: 10.1055/s-2001-16262. [DOI] [PubMed] [Google Scholar]
- 29.Sirinavin C, Trowbridge AA. Dyskeratosis congenita: clinical features and genetic aspects. Report of a family and review of the literature. J Med Genet. 1975;12:339–54. doi: 10.1136/jmg.12.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson HR, Connor JM. Dyskeratosis congenita. J Med Genet. 1988;25:843–6. doi: 10.1136/jmg.25.12.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor JM, Teague RH. Dyskeratosis congenita: report of a large kindred. Br J Dermatol. 1981;105:321–5. doi: 10.1111/j.1365-2133.1981.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 32.Reich H. Eye involvement in dyskeratosis congenita (Zinsser-Cole-Engman syndrome) [in German]. Klin Monatsbl Augenheilkd. 1972;161:288–92. [PubMed] [Google Scholar]
- 33.Merchant A, Zhao TZ, Foster CS. Chronic keratoconjunctivitis associated with congenital dyskeratosis and erythrokeratodermia variablis: two rare genodermatoses. Ophthalmology. 1998;105:1286–91. doi: 10.1016/S0161-6420(98)97035-X. [DOI] [PubMed] [Google Scholar]
- 34.Womer R, Clark JE, Wood P, et al. Dyskeratosis congenita: two examples of this multisystem disorder. Pediatrics. 1983;71:603–9. [PubMed] [Google Scholar]
- 35.Kawaguchi K, Sakamaki H, Onozawa Y, Koike M. Dyskeratosis congenita (Zinsser-Cole-Engman syndrome): an autopsy case presenting with rectal carcinoma, non-cirrhotic portal hypertension, and Pneumocystis carinii pneumonia. Virchows Arch A Pathol Anat Histopathol. 1990;417:247–53. doi: 10.1007/BF01600141. [DOI] [PubMed] [Google Scholar]
- 36.Roth K, Lange CE. Fundus changes in Zinsser-Engman-Cole syndrome [in German]. Klin Monatsbl Augenheilkd. 1975;166:695–8. [PubMed] [Google Scholar]
- 37.Nazir S, Sayani N, Phillips PH. Retinal hemorrhages in a patient with dyskeratosis congenita. J AAPOS. 2008;12:415–7. doi: 10.1016/j.jaapos.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Scheinfeld MH, Lui YW, Kolb EA, et al. The neuroradiological findings in a case of Revesz syndrome. Pediatr Radiol. 2007;37:1166–70. doi: 10.1007/s00247-007-0592-0. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira LF, Shields CL, Marr B, et al. Bilateral retinal vasculopathy in a patient with dyskeratosis congenita. Arch Ophthalmol. 2008;126:134–5. doi: 10.1001/archophthalmol.2007.4. [DOI] [PubMed] [Google Scholar]
- 40.Revesz T, Fletcher S, al-Gazali LI, DeBuse P. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? J Med Genet. 1992;29:673–5. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajtar P, Mehes K. Bilateral Coats retinopathy associated with aplastic anaemia and mild dyskeratotic signs. Am J Med Genet. 1994;49:374–7. doi: 10.1002/ajmg.1320490404. [DOI] [PubMed] [Google Scholar]
- 42.ason JO, III, Yunker JJ, et al. Proliferative retinopathy as a complication of dyskeratosis congenita. Retin Cases Brief Rep. 2009;3:259–62. doi: 10.1097/01.ICB.0000315662.87050.bf. [DOI] [PubMed] [Google Scholar]
- 43.Willig TN, Niemeyer CM, Leblanc T, et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. Pediatr Res. 1999;46:553–61. doi: 10.1203/00006450-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Ball SE, McGuckin CP, Jenkins G, Gordon-Smith EC. Diamond-Blackfan anaemia in the U.K.: analysis of 80 cases from a 20-year birth cohort. Br J Haematol. 1996;94:645–53. doi: 10.1046/j.1365-2141.1996.d01-1839.x. [DOI] [PubMed] [Google Scholar]
- 45.Young TL, Schaffer DB, Cohen AR. Infantile glaucoma associated with the Diamond-Blackfan syndrome. J Pediatr Ophthalmol Strabismus. 1992;29:55–8. doi: 10.3928/0191-3913-19920101-11. [DOI] [PubMed] [Google Scholar]
- 46.Orfali KA, Ohene-Abuakwa Y, Ball SE. Diamond Blackfan anaemia in the UK: clinical and genetic heterogeneity. Br J Haematol. 2004;125:243–52. doi: 10.1111/j.1365-2141.2004.04890.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuze M, Matsubara H, Uji Y. Ocular hypertelorism and exotropia as presenting signs in Diamond-Blackfan anemia [letter]. Jpn J Ophthalmol. 2009;53:67–8. doi: 10.1007/s10384-008-0610-2. [DOI] [PubMed] [Google Scholar]