Abstract
Purpose
To evaluate the clinical outcomes of cyclosporine treatment for non-infectious ocular inflammation
Design
Retrospective cohort study
Participants
Three hundred seventy-three patients with non-infectious ocular inflammation managed at four tertiary ocular inflammation clinics in the United States observed to use cyclosporine as a single non-corticosteroid immunosuppressive agent to their treatment regimen, between 1979-2007 inclusive.
Methods
Participants were identified from the Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Demographic and clinical characteristics, including dosage of cyclosporine and main outcome measures were obtained for every eye of every patient at every visit via medical record review by trained expert reviewers. Main Outcome Measures: Control of inflammation, sustained control after reducing corticosteroid dosages, and discontinuation of therapy because of toxicity.
Results
Of the 373 patients (681 eyes) initiating cyclosporine monotherapy, 33.4% by six months and 51.9% by one year gained sustained, complete control of inflammation over at least two visits spanning at least 28 days. Approximately 25% more improved to a level of slight inflammatory activity by each of these time points. Corticosteroid-sparing success (completely controlled inflammation for at least 28 days with prednisone 10 mg/day or less) was achieved by 22.1% by six months and 36.1% within one year. Toxicity led to discontinuation of therapy within one year by 10.7% of the population. Patients over 55 years of age were over 3-fold more likely to discontinue therapy because of toxicity than patients ages 18-39 years. Doses of 151-250 mg/day tended to be more successful than lower doses, and were not associated with a higher discontinuation for toxicity rate; higher doses did not appear to offer a therapeutic advantage.
Conclusion
Cyclosporine, with corticosteroid therapy as indicated, was modestly effective for controlling ocular inflammation. Our data support a preference for cyclosporine adult dosing between 151-250 mg/day. While cyclosporine was tolerated by the majority of patients, toxicity was much more frequent with increasing age; alternative agents may be preferred for patients over 55 years of age.
Cyclosporine (Cyclosporine A) is the most commonly used agent in the T-cell inhibitor class of immunosuppressive drugs.1, 2 Cyclosporine A preferentially inhibits antigen-triggered signal transduction in T lymphocytes, blunting the expression of many lymphokines—including interleukin-2 (IL-2)—and the expression of antiapoptotic proteins.3 This pharmacologic action is mediated by binding of cyclosporine to its immunophilin, cyclophilin, which inhibits the phosphatase calcineurin, thereby preventing the generation of the potent nuclear factor of activated T cells (NF-AT).4 This calcineurin-mediated step is essential for the up-regulation of the mRNA of various cytokines, particularly IL-2—which is necessary for the proliferation and maturation of T cells—and interferon gamma (IFN-γ), which is critical for the activation of macrophages.
Nussenblatt et al reported first the effects of cyclosporine in experimental ocular inflammatory disease in animals5 and conducted the first clinical trials in humans.6 Patients with endogenous (autoimmune) ocular inflammatory disease responded favorably to cyclosporine therapy. However, prolonged treatment with high doses of cyclosporine was not advised because of nephrotoxicity.7-9 Successful cyclosporine treatment has been reported in small to medium-sized series for a variety of challenging posterior uveitis cases of various etiologies-including Behçet's disease,10-14 Vogt-Kayanagi-Harada syndrome,15, 16 birdshot retinochoroidopathy,17-21 serpiginous choroiditis,22, 23 multifocal choroiditis and panuveitis,24 and intermediate uveitis.15, 25-27 Four double-masked, randomized controlled clinical trials for endogenous uveitis and chronic idiopathic uveitis also have been reported where cyclosporine was compared to prednisone alone, chlorambucil, and colchicine, and was found to have more favorable outcomes than the comparison medications.10, 12, 27, 28 Cyclosporine has become one of the immunosuppressive drugs widely used for the treatment of ocular inflammation.1 Cyclosporine can be administered intravenously or orally. In ophthalmology, oral preparations typically are used, available in two forms: oil-based gelatin capsules (Sandimmune®) and a microemulsion with a greater and more consistent bioavailability (Neoral®, Gengraf®).2
To evaluate better the benefits of cyclosporine for ocular inflammation, here we report the outcomes of a large series of 373 patients followed from the point of initiation of cyclosporine as a single non-corticosteroid immunosuppressive therapy at four ocular inflammation referral centers in the United States.
Methods
Study population
The methods of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) cohort study have been reported previously.29 For this report, all patients with non-infectious ocular inflammation seen since the inception of the center and treated with cyclosporine were identified at three academic subspecialty centers, plus a ∼40% random sample of such patients from a fourth center. Patients from a fifth center participating in the study were excluded from this analysis because the center's co-management approach to treatment, wherein partner centers not included in the study conducted most visit, biased ascertainment of the time-to-treatment success to substantially higher values The patients identified at the other four centers who initiated cyclosporine therapy during follow-up—without simultaneous use of other T-cell inhibitors, antimetabolites, alkylating agents, biologic response modifiers, or dapsone—constitute the study population reported here. All patients were seen between 1979-2007 inclusive. The study was performed with the approval of all the participating centers’ Institutional Review Boards and was conducted in accordance with the Declaration of Helsinki.
Data collection
Information on every eye of every patient at every visit was entered into a custom database (Access, Microsoft Corporation, Redmond, WA) by trained expert reviewers, using a computer-based standardized data entry system with a variety of real-time embedded quality controls such as range checks.29 Data collected relevant to this analysis included: demographic characteristics, the presence or absence of systemic diseases, prior use of immunosuppressive drugs, site of ocular inflammation, ophthalmologic examination findings at every visit, all medications (including corticosteroids and cyclosporine) that patients were receiving at each clinic visit (including dose and route of administration), and (when applicable) reasons for discontinuation of cyclosporine.
Main outcome measures
For the analyses reported herein, patients were followed beginning from the time they started cyclosporine and ending at the time they either stopped cyclosporine, started an additional immunosuppressive drug, stopped attending the study site or when the end of data collection was reached (whichever came first). Inflammatory status was categorized as “active” (anterior chamber cells of 1+ or higher, vitreous haze of 1+ or more, or described by terms such as “active,” “worsening inflammation,” or “disease progression”), “slightly active” (activity that is minimally present, described also by terms such as mild, few or trace cells), or “inactive” (as noted by terms such as quiet, quiescent, no cells, and no active inflammation) for every eye at every visit based on the clinician's documentation at each visit. “Corticosteroid-sparing success” was evaluated based on time-to-reduction of the prednisone (or prednisone-equivalent3) dose to 10mg/day or less, 5mg/day or less, or 0mg with sustained control of the ocular inflammation observed over a period of at least 28 days, among those not meeting the respective criterion for success at the outset. Dates of discontinuation of cyclosporine and the reasons for discontinuation were noted.
Statistical analysis
Frequencies of demographic and clinical characteristics at enrollment were tabulated for the study population using SAS version 9.1 (SAS Corporation, Cary, NC). A time-to-success approach was used to evaluate the benefits of therapy, whereas time-to-discontinuation of therapy was used to assess treatment failure. Only outcomes that were sustained over at least 2 visits spanning at least 28 days were counted as successes in the primary analysis, to avoid counting transient improvements and brief interruptions of therapy as successes or failures. The time-to-success analyses were repeated omitting the requirement that success be sustained, and also including improvement from “active” to a “slightly active” or better inflammation status as sensitivity analyses (to evaluate the extent to which success estimates changed when these definitions changed). A time-to-failure approach was used to evaluate discontinuation of cyclosporine, in relation to reasons given for such discontinuation. The outcomes were summarized using incidence rates and Kaplan-Meier estimates. Multiple regression analysis with the Cox proportional hazards model30 was used to adjust for potentially confounding variables such as demographic characteristics, the anatomic site of inflammation, cyclosporine dosage, and prior usage of immunosuppressive therapies.
Results
Demographic and clinical characteristics of the study population (373 patients who had 681 eyes diagnosed with ocular inflammation) at the time of starting cyclosporine are summarized in Table 1. Their median age was 36.1 years (range 5.5-81.5). The median follow-up time on treatment was 0.9 years; the total person-time under treatment with cyclosporine included in the analysis was 689.1 person-years. The majority (62.7%) of subjects was female and 73.7% were Caucasian. Primary inflammatory diagnoses included anterior uveitis (75 patients, 20.1%), intermediate uveitis (99 patients, 26.5%), posterior or panuveitis (171 patients, 45.8%), scleritis (15 patients, 4.0%), ocular mucous membrane pemphigoid (6 patients, 1.6%), and other forms of ocular inflammation (7 patients, 1.9%) including lichen planus of conjunctiva, peripheral ulcerative keratitis, and idiopathic orbital pseudotumor.
Table 1.
Characteristics of Patients Initiating Cyclosporine Monotherapy
| Characteristic | Anterior Uveitis | Intermediate Uveitis | Posterior or Panuveitis | Scleritis | Mucous Membrane Pemphigoid | Othera | Total |
|---|---|---|---|---|---|---|---|
| Person Specific Characteristics | |||||||
| Patient Number (%) | 75 (20.1) | 99 (26.5) | 171 (45.8) | 15 (4.0) | 6 (1.6) | 7 (1.9) | 373 |
| Age: less than 18 years (%) | 11 (14.7) | 11 (11.1) | 13 (7.6) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 36 (9.7) |
| Age: 18-39 years (%) | 30 (40.0) | 57 (57.6) | 94 (55.0) | 3 (20.0) | 1 (16.7) | 3 (42.9) | 188 (50.4) |
| Age: 40-54 years (%) | 21 (28.0) | 24 (24.2) | 41 (24.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 88 (23.6) |
| Age: 55-64 years (%) | 10 (13.3) | 6 (6.1) | 12 (7.0) | 4 (26.7) | 1 (16.7) | 2 (28.6) | 35 (9.4) |
| Age: 65 years and older (%) | 3 (4.0) | 1 (1.0) | 11 (6.4) | 5 (33.3) | 4 (66.7) | 2 (28.6) | 26 (7.0) |
| Gender, Female (%) | 56 (74.7) | 60 (60.6) | 98 (57.3) | 12 (80.0) | 2 (33.3) | 6 (85.7) | 234 (62.7) |
| Race, Caucasian (%) | 48 (64.0) | 84 (84.8) | 121 (70.8) | 11 (73.3) | 5 (83.3) | 6 (85.7) | 275 (73.7) |
| Race, Black (%) | 21 (28.0) | 8 (8.1) | 36 (21.1 | 1 (6.7) | 0 (0.0) | 1 (14.3) | 67 (18.0) |
| Race, Other (%) | 6 (8.0) | 7 (7.1) | 14 (8.2) | 3 (20.0) | 1 (16.7) | 0 (0.0) | 31 (8.3) |
| Duration of Inflammation (years) | 4.3 (0.1 - 28.3) | 3.2 (0.0 – 25.2) | 1.8 (0.0 - 29.0) | 1.4 (0.0 - 11.5) | 1.5 (0.5 - 1.8) | 5.8 (0.0 - 13.8) | 2.6 (0.0 – 29.0) |
| Systemic (Extraocular) Autoimmune Disease [N (%)] | 37 (49.3) | 15 (15.2) | 47 (27.5) | 6 (40.0) | 5 (83.3) | 3 (42.9) | 113 (30.3) |
| Bilateral Inflammation (%) | 58 (77.3) | 88 (88.9) | 145 (84.8) | 8 (53.3) | 5 (83.3) | 4 (57.1) | 308 (82.6) |
| Associated Systemic Inflammatory Disease (%) | 58 (77.3) | 88 (88.9) | 145 (84.8) | 8 (53.3) | 5 (83.3) | 4 (57.1) | 308 (82.6) |
| Eye Specific Characteristics | |||||||
| Number of affected eyes (%) | 133 (19.5) | 187 (27.5) | 316 (46.4) | 23 (3.4) | 11 (1.6) | 11 (1.6) | 681 |
| 20/50 or Worse [N (%)] | 74 (55.6) | 105 (56.1) | 171 (54.1) | 7 (30.4) | 3 (27.3) | 3 (27.3) | 363 (53.3) |
| 20/200 or Worse [N (%)] | 47 (35.3) | 32 (17.1) | 83 (26.3) | 2 (8.7) | 3 (27.3) | 2 (18.2) | 169 (24.8) |
| Ocular complicationsb, affected eyes [N (%)] | 78 (58.6) | 107 (57.2) | 151 (47.8) | 10 (43.5) | 4 (36.4) | 6 (54.5) | 356 (52.3) |
| Overall Activity – Inactive (%) | 78 (58.6) | 111 (59.4) | 184 (58.2) | 13 (56.5) | 3 (27.3) | 7 (63.6) | 396 (58.1) |
| Overall Activity - Slightly Active (%) | 27 (20.3) | 50 (26.7) | 62 (19.6) | 2 (8.7) | 4 (36.4) | 2 (18.2) | 147 (21.6) |
| Overall Activity – Active (%) | 28 (21.1) | 26 (13.9) | 70 (22.2) | 8 (34.8) | 3 (27.3) | 2 (18.2) | 137 (20.1) |
| Overall Activity – Missing (%) | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (0.1) |
Lichen planus of conjunctiva, Peripheral ulcerative keratitis, Idiopathic orbital pseudotumor
Ocular hypertension, Hypotony, Band keratopathy, Macular edema, Epiretinal membrane, Exudative retinal detachment, Retinal neovascularization, Choroidal neovascularization, Cataract, Glaucoma
Table 2 displays the outcome of cyclosporine therapy. Complete, sustained control of inflammation at six months, defined as no activity among those initially active or slightly active, was 33.4% (95% CI 28.0-39.5). However when success was defined as a transition from “active” to “inactive or slightly active,” 60.8% (95% CI 53.7-67.9) met this success definition. By site of inflammation, complete sustained control of inflammation was achieved by six months in 30.4%, 39.3%, 29.2%, 62.3%, and 20.0% of patients with anterior uveitis, intermediate uveitis, posterior/panuveitis, scleritis and ocular pemphigoid respectively. When the definition of “success” was broadened to “inactive or slightly active” inflammation, “success” was achieved much more frequently (52.8%, 73.7%, 56.6%, 56.8%, and 20.0% respectively), except for scleritis patients—where the success rate turned out to be slightly lower because the number of patients “at risk” of improvement differed—and ocular pemphigoid patients.
Table 2.
Outcomes of Cyclosporine Therapy by Patient
| Outcome | Anterior | Intermediate | Posterior/Panuveitis | Scleritis | Mucous Membrane Pemphigoid | Other | Total |
|---|---|---|---|---|---|---|---|
| Outcomes, by Patient | |||||||
| Used as only immunosuppressive drug therapy | 75 | 99 | 171 | 15 | 6 | 7 | 373 |
| Treatment success within six months | |||||||
| Controlled inflammation - no activity at 6 months [% (95% CI)] | 30.4 (19.6-45.3) | 39.3 (29.3-51-3) | 29.2 (22.0-38.0) | 62.3 (29.6 - 93.3) | 20.0 (3.1 - 79.6) | 33.3 (5.9 - 94.6) | 33.4 (28.0-39.5) |
| Controlled inflammation - no activity or slightly active at 6 months [% (95% CI)] | 52.8 (35.8- 72.0) | 73.7 (61.3- 84.7) | 56.6 (46.7- 67.0) | 56.8 (23.9 - 92.4) | 20.0 (3.1 - 79.6) | 100.0 (. - .) | 60.8 (53.7- 67.9) |
| Controlled inflammation and prednisone ≤10mg/day at 6 months [% (95% CI)] | 28.5 (18.4- 42.7) | 24.1 (16.2- 34.9) | 16.2 (10.9- 23.7) | 52.8 (27.9 - 82.2) | 20.0 (3.1 - 79.6) | 0.00 (. - .) | 22.1 (17.7- 27.3) |
| Controlled inflammation and prednisone ≤ 5mg/day at 6 months [% (95% CI)] | 26.9 (17.0- 41.0) | 19.0 (12.1- 29.2) | 12.7 (8.1- 19.4) | 40.8 (18.8 - 73.1) | 20.0 (3.1 - 79.6) | 0.00 (. - .) | 18.1 (14.2- 23.0) |
| Controlled inflammation without systemic steroids at 6 months [% (95% CI)] | 8.8 (3.7- 20.0) | 3.7 (1.2- 11.2) | 0.7 (0.1- 4.6) | 16.7 (4.4 - 51.8) | 0.00 (. - .) | 0.00 (. - .) | 3.6 (2.0- 6.4) |
| Treatment success within 12 months | |||||||
| Controlled inflammation - no activity at 12 months [% (95%CI)] | 54.3 (40.0 - 69.9) | 51.8 (40.4 - 64.2) | 51.7 (42.6 - 61.6) | 62.3 (29.6 - 93.3) | 20.0 (3.1- 79.6) | 33.3 (5.5 - 94.6) | 51.9 (45.5 - 58.5) |
| Controlled inflammation - no activity or slightly active at 12 months [% (95% CI)] | 85.8 (68.5 - 96.3) | 80.2 (68.3 - 89.9) | 75.2 (64.8 - 84.5) | 56.8 (23.9 - 92.4) | 20.0 (3.1- 79.6) | 100.00 (. - .) | 76.4 (69.5 - 82.7) |
| Controlled inflammation and prednisone ≤ 10mg/day at 12 months [% (95% CI)] | 42.4 (29.9 - 57.5) | 38.0 (27.9 - 50.2 | 32.3 (24.4 - 41.9) | 52.8 (27.9 - 82.2) | 20.0 (3.1 - 79.6) | 0.00 (. - .) | 36.1 (30.5 - 42.4) |
| Controlled inflammation and prednisone ≤ 5mg/day at 12 months [% (95% CI)] | 40.4 (28.2 - 55.4) | 32.5 (23.1 - 44.4) | 23.6 (17.0 - 32.3) | 50.6 (26.0 - 80.8) | 20.0 (3.1 - 79.6) | 0.00 (. - .) | 30.0 (24.8 - 35.9) |
| Controlled inflammation without systemic steroids at 12 months [% (95% CI)] | 14.9 (7.7 - 27.7) | 9.2 (4.5 - 18.5) | 3.9 (1.6 - 9.2) | 25.0 (8.8 - 59.2) | 0.00 (. - .) | 0.00 (. - .) | 8.2 (5.5 - 12.1) |
CI=Confidence interval
Corticosteroid-sparing success—defined as complete inactivity of inflammation sustained over at least two visits spanning at least 28 days after having tapered prednisone to 10 mg/day or less—was achieved within 6 months overall for 22.1% of the patients (95% CI 17.7%-27.3%) and within 12 months for 36.1% (95% CI 30.5%-42.2%). By anatomic site, corticosteroid-sparing success within six months was observed for 28.5% of patients with anterior uveitis, 24.1% of patients with intermediate uveitis, 16.2% of patients with posterior/panuveitis, 52.8% of patients with scleritis, and 20.0% of patients with ocular pemphigoid (Table 2). Control of inflammation with prednisone tapered to 5 mg or less was seen in 26.9% of patients with anterior uveitis and in 40.8% of patients with scleritis. Only 3.6% and 8.2% of the total patient population were able to completely discontinue corticosteroids while maintaining sustained control of inflammation within six months and one year of therapy respectively. Patients with scleritis were the most likely to maintain suppression of inflammation after stopping corticosteroid within six months (16.7%), followed by the anterior uveitis group (8.8%); whereas patients with intermediate and posterior/panuveitis treated with cyclosporine seldom maintained control of inflammation after discontinuing prednisone (<4%). At 12 months success was somewhat more frequent for all the success measures studied for the anterior uveitis, intermediate uveitis, and posterior/panuveitis groups (Table 2).
Sensitivity analyses evaluating success in controlling inflammation and corticosteroid-sparing outcomes also were performed without requiring the 28 day period of controlled inflammation to allow better comparisons to prior reports of outcomes with various immunosuppressive drugs. Results appeared substantially better with this approach, with complete suppression of inflammation at some visit before 6 months in 56.9% of all patients (95% CI 50.7%-63.2%) and in 62.8%, 63.2%, 49.7%, 84.4%, and 20% for anterior uveitis, intermediate uveitis, posterior/panuveitis, scleritis, and ocular pemphigoid respectively. Corticosteroid-sparing success (prednisone ≤10 mg/day) was achieved at one or more visits by 6 months in 37.6% of all patients (95% CI 32.1%-43.7%) and among 52.5%, 41.4%, 28.2%, 78.2%, and 20% for the same groups of patients respectively.
Multiple regression analysis was performed to evaluate whether demographic or clinical characteristics predicted response to therapy (Table 3). Demographic characteristics and prior use of cyclosporine or of other immunosuppressive drugs were not consistently associated with statistically significant differences in subsequent treatment success. Cyclosporine doses of 151-250 mg/day were associated with an increased likelihood of control of inflammation (adjusted relative risk (RR)=1.89, CI 1.15-3.09) with respect to 150 mg/day or less, but the likelihood of corticosteroid-sparing success was similar across all dosage groups. Doses higher than 250 mg/day were not associated with further therapeutic advantage. Similar patterns of association with the covariates were observed in multiple regression analyses evaluating corticosteroid-sparing to the level of 5 mg or discontinuation of prednisone, where the number of events was lower. Likewise, patterns of covariate associations were similar when reduction of inflammation from “active” to either “inactive” or “slightly active” was used as the standard of success, where the proportion achieving success was slightly higher.
Table 3.
Factors Associated with Successful Outcome of Cyclosporine Treatment*
| Characteristic Name | Crude RR for complete control of Inflammation RR (95%CI) |
Adjusted RR Complete control of Inflammation RR (95%CI) |
Crude RR, corticosteroid-sparing success (prednisone ≤10 mg) RR (95%CI) |
Adjusted RR, corticosteroid-sparing success (prednisone ≤10 mg) RR (95%CI) |
|
|---|---|---|---|---|---|
| Gender | Male | 0.93 (0.68 - 1.25) | 1.14 (0.83 - 1.57) | 0.74 (0.52 – 1.04) | 0.82 (0.56 – 1.20) |
| Race | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.96 (0.69 - 1.32) | 0.97 (0.67 - 1.40) | 0.82 (0.57 – 1.19) | 0.79 (0.52 – 1.20) | |
| Other | 1.01 (0.64 - 1.58) | 0.98 (0.60 - 1.61) | 1.14 (0.69 – 1.88) | 1.15 (0.65 – 2.03) | |
| Age | Less than 18 years | 0.90 (0.57 - 1.42) | 0.79 (0.47 - 1.34) | 1.13 (0.70 – 1.83) | 1.00 (0.60 - 1.68) |
| 18-39 years | 1.00 | 1.00 | 1.00 | 1.00 | |
| 40-54 years | 1.39 (0.96 - 2.03) | 1.47 (0.97 - 2.22) | 1.33 (0.88 - 2.01) | 1.22 (0.79 - 1.90) | |
| 55-64 years | 1.72 (1.11 - 2.67) | 1.72 (1.11 - 2.65) | 1.46 (0.84 - 2.53) | 1.30 (0.71 - 2.38) | |
| 65 years or more | 1.24 (0.61 - 2.50) | 1.07 (0.44 - 2.57) | 1.84 (0.88 - 3.89) | 1.91 (0.74 - 4.90) | |
| Type of inflammation | Scleritis | 1.00 | 1.00 | 1.00 | 1.00 |
| Anterior | 0.62 (0.25 - 1.55) | 0.73 (0.26 - 2.05) | 0.77 (0.28 - 2.08) | 1.17 (0.32 - 4.21) | |
| Intermediate | 0.54 (0.22 - 1.33) | 0.67 (0.24 - 1.85) | 0.57 (0.22 - 1.49) | 0.86 (0.25 - 2.93) | |
| Posterior/Panuveitis | 0.58 (0.23 - 1.41) | 0.69 (0.26 - 1.84) | 0.55 (0.21 - 1.42) | 0.83 (0.25 - 2.69) | |
| Mucous Membrane Pemphigoid | 0.23 (0.04 - 1.23) | 0.23 (0.04 - 1.24) | 0.36 (0.06 - 2.08) | 0.33 (0.05 - 2.13) | |
| Other | 1.33 (0.42 - 4.19) | 1.31 (0.38 - 4.47) | 0.69 (0.09 - 5.20) | 0.79 (0.11 - 5.42) | |
| Systemic (extraocular) autoimmune disease | Yes | 0.90 (0.67 - 1.21) | 0.83 (0.60 - 1.14) | 0.86 (0.60 - 1.23) | 0.86 (0.59 - 1.26) |
| Previous Cyclosporine | Yes | 1.04 (0.45 - 2.43) | 0.74 (0.23 - 2.46) | 1.23 (0.56 - 2.71) | 1.07 (0.34 - 3.42) |
| Antimetabolite(s) prior to treatment | Yes | 1.24 (0.74 - 2.06) | 1.55 (0.84 - 2.85) | 1.31 (0.77 - 2.25) | 1.31 (0.69 - 2.47) |
| Alkylating agent(s) prior to treatment | Yes | 1.14 (0.53 - 2.45) | 1.34 (0.65 - 2.75) | 0.82 (0.32 - 2.06) | 0.80 (0.27 - 2.32) |
| Dosage (mg/day) | 150 or less | 1.00 | 1.00 | 1.00 | 1.00 |
| 151 - 250 | 1.77 (1.07 - 2.91) | 1.89 (1.15 - 3.09) | 1.10 (0.68 - 1.78) | 1.24 (0.76 - 2.01) | |
| 251 - 350 | 1.36 (0.81 - 2.30) | 1.40 (0.80 - 2.46) | 0.88 (0.53 - 1.47) | 0.99 (0.58 - 1.67) | |
| 351 or more | 0.91 (0.56 - 1.47) | 0.96 (0.58 - 1.59) | 0.67 (0.42 - 1.10) | 0.85 (0.50 - 1.42) |
Counts of the numbers of patients for each level of the covariates indicated here are given in Table 1, with the exception of Dosage, which was not a presenting characteristic. Dosage ranges represent round-number approximations of quartiles of the distribution. Exact counts in each Dosage range vary over time, because this variable was analyzed in a time-updated fashion.
RR=Relative risk; CI=Confidence interval
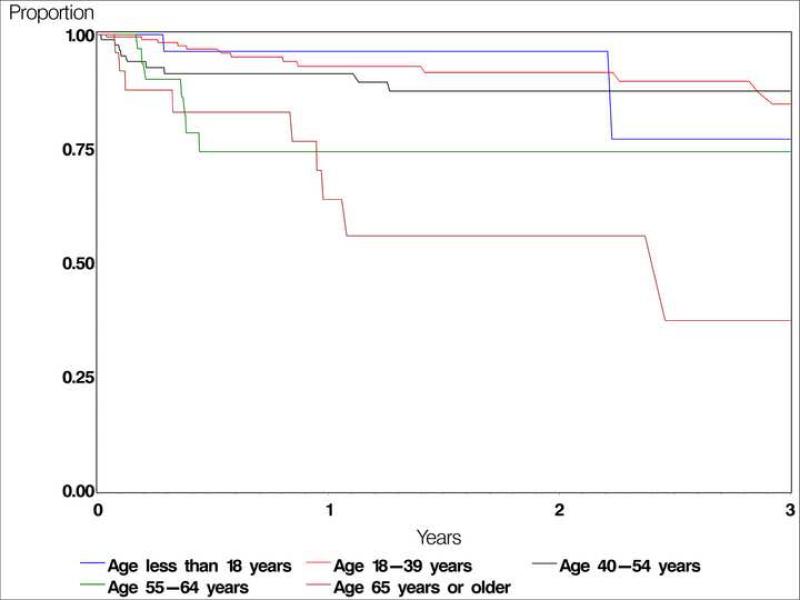
Cyclosporine was discontinued by 182 patients (48.8%) during follow-up (Table 4). Side effects attributed to cyclosporine led to discontinuation within the first year for 47 (10.7%, 95% CI 7.6%-15.1%) of these patients. An additional 12.4% stopped treatment within one year for unknown/missing reasons. Renal toxicity and hypertension were the most frequently observed side-effects leading to cessation of therapy, contributing to 4.3% and 3.2% of drug discontinuation respectively by one year of therapy. Surprisingly, discontinuation due to side-effects was significantly lower among the patients taking cyclosporine at the dose of 151mg to 250mg compared to lower doses (adjusted RR=0.34, CI 0.16-0.73, p=0.005), although the reasons for selecting a particular starting dose are unknown (Table 5). Compared with patients ages 18-39, discontinuation for toxicity was progressively more frequent with increasing age (see Figure 1), particularly among patients between 55 years and 64 years of age (adjusted RR=3.25, CI 1.54-6.88) and patients over 65 years (adjusted RR=5.66, CI 2.14-14.98, p=0.0005). After the first year of therapy had elapsed, most discontinuation occurred on the basis of remission of disease; discontinuation for side-effects or ineffectiveness was uncommon after one year. Fifty-five patients stopped taking cyclosporine due to remission of the disease, at the rate of 0.08/person-year (PY), most of them after ≥1 year of initiating therapy. About half of patients continued taking cyclosporine throughout the available follow-up, with 65 (17%) subsequently starting another immunosuppressive drug along with cyclosporine, and 126 patients (34%) continuing cyclosporine as the only non-corticosteroid immunosuppressive drug for the remainder of (variable) follow-up.
Table 4.
Reasons for Discontinuation of Cyclosporine*
| Reason | No. of affected patients | Discontinuation rate per person-year (95% CI) | KM estimate, proportion discontinuing cyclosporine by 1 year, % (95% CI) |
|---|---|---|---|
| Favorable Reasons | |||
| For remission | 55 (15%) | 0.08 (0.06 , 0.1) | 6.3 (3.9 - 10.2) |
| Unfavorable Reasons | |||
| For adverse events | 47 (13%) | 0.07 (0.05 , 0.09) | 10.7 (7.6 - 15.1) |
| Hypertension | 12 (3.2%) | 0.02 (0.009 , 0.03) | 3.2 (1.6 - 6.4) |
| Renal toxicity | 16 (4.3%) | 0.02 (0.01 , 0.04) | 2.5 (1.2 - 5.1) |
| Gingival hyperplasia | 3 (0.8%) | 0.004 (0.0009 , 0.01) | 1.4 (0.5 - 4.4) |
| Elevated liver enzymes | 4 (1.1%) | 0.006 (0.002 , 0.01) | 1.1 (0.4 - 3.6) |
| Hirsutism | 2 (0.5%) | 0.003 (0.0004 , 0.01) | 0.7 (0.2 - 3.0) |
| Opportunistic infection | 2 (0.5%) | 0.003 (0.0004 , 0.01) | 0.7 (0.2 - 2.6) |
| Malaise | 3 (0.8%) | 0.004 (0.0009 , 0.01) | 1.0 (0.3 - 3.0) |
| Bone marrow suppression | 1 (0.3%) | 0.001 (0.0 , 0.008) | 0.0 (0.0 - 0.0) |
| Other adverse events | 8 (2.1%) | 0.01 (0.005 , 0.02) | 1.7 (0.8 - 3.8) |
| For ineffectiveness | 25 (6.7%) | 0.04 (0.02 , 0.05) | 5.6 (3.3 - 9.2) |
| Reasons Unknown | 61 (16%) | 0.09 (0.07 , 0.11) | 12.4 (9.1 - 16.7) |
| Total Stopping Cyclosporine for Any Reason | 182 (49%) | 0.26 (0.23 , 0.31) | 29.8 (25.0 - 35.3) |
More than one reason may have contributed to discontinuation. Others for discontinuation of cyclosporine include: Non-specific intolerance (2), suspicion of mycobacterial infection (not judged to be opportunistic), choroidal infiltrate, polyarthralgia, skin rash, diagnosis of cutaneous melanoma, respiratory difficulties.
CI=Confidence interval; KM=Kaplan-Meier
Table 5.
Factors Influencing Discontinuation of Cyclosporine
| Characteristic Name | Crude RR Discontinuation Due to Side Effects, RR (95% CI) | Adjusted RR Discontinuation Due to Side Effects, RR (95% CI) | |
|---|---|---|---|
| Age | Less than 18 years | 0.88 (0.31 - 2.47) | 0.80 (0.29 - 2.23) |
| Age | 18-39 years | 1.00 | 1.00 |
| Age | 40-54 years | 1.81 (1.00 - 3.28) | 1.69 (0.90 - 3.18) |
| Age | 55-64 years | 3.35 (1.63 - 6.88) | 3.25 (1.54 - 6.88) |
| Age | 65 years or more | 4.43 (2.09 - 9.36) | 5.66 (2.14 - 14.98) |
| Type of inflammation | Anterior | 1.00 | 1.00 |
| Type of inflammation | Intermediate | 0.39 (0.18 - 0.83) | 0.49 (0.22 - 1.09) |
| Type of inflammation | Posterior/Panuveitis | 0.68 (0.38 - 1.21) | 0.73 (0.38 - 1.42) |
| Type of inflammation | Scleritis | 2.30 (0.93 - 5.67) | 2.01 (0.55 - 7.40) |
| Type of inflammation | Mucous Membrane Pemphigoid | 0.90 (0.14 - 5.75) | 0.41 (0.05 - 3.36) |
| Dosage | 150 mg/day or less | 1.00 | 1.00 |
| Dosage | 151-250 mg/day | 0.34 (0.17 - 0.69) | 0.34 (0.16 - 0.73) |
| Dosage | 251-350 mg/day | 0.53 (0.27 - 1.04) | 0.59 (0.29 - 1.20) |
| Dosage | 351 mg/day or more | 0.55 (0.30 - 1.03) | 0.69 (0.35 - 1.34) |
| Systemic (extraocular) autoimmune disease | Yes | 0.98 (0.60 - 1.62) | 0.76 (0.42 - 1.39) |
RR=Relative risk
CI=Confidence interval
Figure 1.
Kaplan-Meier curve: Discontinuation due to adverse events, by age group
Discussion
These data suggest that the benefits of cyclosporine therapy for absolute ocular inflammation activity control are modest, with 33.4% and 51.9% of patients gaining complete, sustained control of inflammation within 6 and 12 months respectively. A much larger proportion achieved sustained success in bringing inflammation down to a “slightly active” level (60.8% and 76.4% by 6 and 12 months respectively), suggesting that cyclosporine may have had anti-inflammatory benefits that were not sufficient in many cases to achieve the high bar of “complete” control of inflammation. Nevertheless, many experts consider maintaining inflammation at a “slightly active” level unacceptable for long-term management. Relatively few (22.1% and 36.1% by 6 and 12 months respectively) achieved corticosteroid-sparing objectives of tapering of prednisone to 10 mg or less while sustaining complete control of inflammation. Very few patients achieved control of inflammation without the need for systemic corticosteroids (8.2% by one year).
Previous reports have differed in their estimates of what degree of treatment success can be expected with cyclosporine, with several suggesting a higher degree of success than in our experience.6, 10-14, 18, 31, 32 These reports are difficult to compare with ours because of differing methodologies and outcome measures. Our exclusion of patients receiving other non-corticosteroid immunosuppressive drugs along with cyclosporine, and our requirement that complete absence of inflammation be observed over at least two visits spanning at least 28 days before counting as a treatment success, would be expected to produce lower success rates than less stringent approaches, but should provide robust treatment success estimates. Our results are similar to those of a prior randomized controlled trial27 (involving some of the same patients reported herein), and in a report regarding cyclosporine second-line immunosuppressive drug for the treatment of juvenile idiopathic arthritis associated chronic anterior uveitis.33
Among our patients, there were no statistically significant differences between sites of inflammation in response to cyclosporine, although there was a tendency toward improved outcomes among scleritis patients, who comprised only 4.0% (15 patients) of this cohort. Given that there are studies with comparable results in regard to necrotizing and non-necrotizing scleritis,34-35 cyclosporine might be an especially useful drug for the management of scleritis.
Another interesting finding was that the proportion achieving success climbed steadily even after 6 months of therapy, ranging from 7% to 33% higher success rates at 12 months than at 6 months across all success criteria. We have observed this pattern in our studies of methotrexate, azathioprine, and cyclophosphamide as well (data to be published separately), suggesting that immunosuppressive therapy in general—including cyclosporine—takes an extended period of time to be fully effective.
One of the impediments to the wider use cyclosporine as an immunosuppressive agent is the view that cyclosporine has a high rate of side effects.1, 8, 36 Renal toxicity was reported in 1986, manifested as interstitial fibrosis and tubular atrophy.9 The high incidence of nephrotoxicity in these early trials can partly be explained by the high cyclosporine doses used initially (≥10 mg/kg/day). However, nephrotoxicity of cyclosporine has been reported even for patients using lower dosages (4.3±1.6 mg/kg), leading to a recommendation that doses of 3mg/kg/day or less be used.8 In contrast, relatively few of our patients discontinued cyclosporine because of toxicity (10.7% by one year), suggesting that cyclosporine use is feasible. Patients taking cyclosporine 151mg to 250mg/day (approximately 2-3.5 mg/kg/day assuming an average body weight) had an especially low risk of discontinuation because of side effects, and had success on a similar order to that of patients using higher doses. Ozdal et al also reported only small numbers of patients discontinuing cyclosporine therapy for toxicity when lower doses are used.13
In our hands, older patients (over 55 years of age) are several-fold more likely to develop treatment-limiting side effects than patients ages 18-39, suggesting that cyclosporine may not be a good choice for many older patients, and that such patients should be carefully monitored following a decision to use cyclosporine. Renal toxicity and hypertension were the most common reasons for discontinuation observed among the 47 patients for whom cyclosporine was stopped due to side-effects. The incidence of bone marrow suppression was extremely low. Because we only had data available about toxicity leading to discontinuation of therapy, and because the reason for discontinuation in 61 (16%) patients was missing, true toxicity rates are likely somewhat higher. Regarding long-term risks of cyclosporine therapy, our previous comprehensive literature review suggests that cancer risk probably is not substantially elevated with cyclosporine treatment for autoimmune diseases, although data are limited on this subject.37 The overall and cancer mortality data from this cohort will be published separately.
Limitations of the study are those which are typical for a retrospective study at tertiary centers. The participating centers followed a standardized data entry approach, using an extensive array of quality control mechanisms, so as to provide optimal data quality within the constraints of a retrospective design,29 and used treatment and grading approaches very similar to those ultimately adopted by consensus groups.1, 38 Nevertheless, the completeness and standardization of data were not as much as could have been achieved in a prospective study. Also, cases may have been more severe than in non-referral ophthalmology settings; with the result that cyclosporine may appear less effective than had it been studied in alternative settings. Nonetheless, results would be more generalizable to the large proportion of patients requiring immunosuppressive therapy who are managed at tertiary centers. The indications for the choice of cyclosporine as opposed to an alternative immunosuppressive agent are not known, and could have biased the therapeutic results upward or downward, making it difficult to compare the effects of alternative immunosuppressive drugs. The use of survival analysis assumes that patients censored because of starting a second agent would have had similar success to those who did not, whereas a second agent probably was added because of unsatisfactory response to cyclosporine, which could have inflated our calculated success proportions somewhat. On the other hand, patients coming to the clinics from long distances may have been less likely to return for multiple follow-up visits if treatment was successful than if treatment failed, which may have led to some degree of underestimation of treatment benefits.
Strengths of the study are a much larger sample size and amount of person-time observed than in previous reports, providing greater precision in estimating the likelihood of treatment success, safety profiles, side effects and remission rates. We reported and analyzed the clinical outcomes using approaches advocated by a consensus group38 as much as possible in a retrospective study. A more realistic definition of treatment success was used than other prior studies, and the effect of cyclosporine monotherapy was evaluated, thereby eliminating potential confounding effects of additional immunosuppressive agents, problems which may have inflated estimates of the benefit of cyclosporine in previous studies.
In summary, cyclosporine, along with corticosteroids given as indicated according to best medical judgment, was modestly effective in controlling inflammation and accomplishing corticosteroid-sparing goals. Approximately half of patients achieved sustained control of inflammation within a years’ therapy, and an additional one-fourth of patients achieved partial treatment success by one year, with sustained minimal levels of inflammation. Corticosteroid-sparing goals were met in a minority of patients, and fewer than 10% were able to completely discontinue systemic corticosteroids within a year. Our results suggest that cyclosporine is relatively safe in younger patients, but that among patients ages 55 years and older, treatment-limiting toxicity is frequent, a pattern not seen with other immunosuppressive drugs39 40, 41
Financial Support
This study was supported primarily by National Eye Institute Grant EY014943 (Dr. Kempen). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Dr Kempen is an RPB James S. Adams Special Scholar Award recipient. Drs. Jabs and Rosenbaum are Research to Prevent Blindness Senior Scientific Investigator Award recipients. Dr. Thorne is an RPB Harrington Special Scholar Award recipient. Dr. Levy-Clarke was previously supported by and Dr. Nussenblatt continues to be supported by intramural funds of the National Eye Institute. Dr. Suhler was supported in part by the Department of Veterans’ Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Precis:
Within one year of cyclosporine therapy, about half of ocular inflammation patients achieved stable, complete inflammatory control, a minority achieved corticosteroid-sparing goals, and <10% successfully discontinued corticosteroids. Toxicity was frequent after 55 years of age.
References
- 1.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 2.Okada AA. Immunomodulatory therapy for ocular inflammatory disease: a basic manual and review of the literature. Ocul Immunol Inflamm. 2005;13:335–51. doi: 10.1080/09273940590951034. [DOI] [PubMed] [Google Scholar]
- 3.Brunton LL, Parker KL, Murri N, Blumenthal DK, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. 2006. pp. xx–xx. ed-in-chief. chapter author(s). Chapter title. AQ: provide chapter author(s), chapter title, and specific inclusive pagination
- 4.Stepkowski SM. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med. 2000;2:1–23. doi: 10.1017/S1462399400001769. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt RB, Rodrigues MM, Wacker WB, et al. Cyclosporin A: inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest. 1981;67:1228–31. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96:275–82. doi: 10.1016/s0002-9394(14)77814-6. [DOI] [PubMed] [Google Scholar]
- 7.Hesselink DA, Baarsma GS, Kuijpers RW, van Hagen PM. Experience with cyclosporine in endogenous uveitis posterior. Transplant Proc. 2004;36(suppl):372S–7S. doi: 10.1016/j.transproceed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002;13:2962–8. doi: 10.1097/01.asn.0000034945.61533.26. [DOI] [PubMed] [Google Scholar]
- 9.Palestine AG, Austin HA, III, Balow JE, et al. Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N Engl J Med. 1986;314:1293–8. doi: 10.1056/NEJM198605153142005. [DOI] [PubMed] [Google Scholar]
- 10.BenEzra D, Cohen E, Chajek T, et al. Evaluation of conventional therapy versus cyclosporine A in Behcet's syndrome. Transplant Proc. 1988;20(suppl):136–43. [PubMed] [Google Scholar]
- 11.Binder AI, Graham EM, Sanders MD, et al. Cyclosporin A in the treatment of severe Behcet's uveitis. Br J Rheumatol. 1987;26:285–91. doi: 10.1093/rheumatology/26.4.285. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K, Nakajima A, Urayama A, et al. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet. 1989;1:1093–6. doi: 10.1016/s0140-6736(89)92381-7. [DOI] [PubMed] [Google Scholar]
- 13.Ozdal PC, Ortac S, Taskintuna I, Firat E. Long-term therapy with low dose cyclosporin A in ocular Behcet's disease. Doc Ophthalmol. 2002;105:301–12. doi: 10.1023/a:1021227019915. [DOI] [PubMed] [Google Scholar]
- 14.Ozyazgan Y, Yurdakul S, Yazici H, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behcet's syndrome: a single masked trial. Br J Ophthalmol. 1992;76:241–3. doi: 10.1136/bjo.76.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporine therapy for uveitis: long-term followup. J Ocul Pharmacol. 1985;1:369–82. doi: 10.1089/jop.1985.1.369. [DOI] [PubMed] [Google Scholar]
- 16.Wakefield D, McCluskey P. Cyclosporine: a therapy in inflammatory eye disease. J Ocul Pharmacol. 1991;7:221–6. [PubMed] [Google Scholar]
- 17.Nussenblatt RB, Mittal KK, Ryan S, et al. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94:147–58. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 18.Le Hoang P, Girard B, Deray G, et al. Cyclosporine in the treatment of birdshot retinochoroidopathy. Transplant Proc. 1988;20(suppl):128–30. [PubMed] [Google Scholar]
- 19.Kiss S, Ahmed M, Letko E, Foster CS. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005;112:1066–71. doi: 10.1016/j.ophtha.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Silverstein BE, Wong IG. Reduction of cyclosporine dosage with ketoconazole in a patient with birdshot retinochoroidopathy. Am J Ophthalmol. 1998;125:106–8. doi: 10.1016/s0002-9394(99)80246-3. [DOI] [PubMed] [Google Scholar]
- 21.Becker MD, Wertheim MS, Smith JR, Rosenbaum JT. Long-term follow-up of patients with birdshot retinochoroidopathy treated with systemic immunosuppression. Ocul Immunol Inflamm. 2005;13:289–93. doi: 10.1080/09273940490912407. [DOI] [PubMed] [Google Scholar]
- 22.Secchi AG, Tognon MS, Maselli C. Cyclosporine-A in the treatment of serpiginous choroiditis. Int Ophthalmol. 1990;14:395–9. doi: 10.1007/BF00163565. [DOI] [PubMed] [Google Scholar]
- 23.Araujo AA, Wells AP, Dick AD, Forrester JV. Early treatment with cyclosporin in serpiginous choroidopathy maintains remission and good visual outcome. Br J Ophthalmol. 2000;84:979–82. doi: 10.1136/bjo.84.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel SS, Ekong A, Baltatzis S, Foster CS. Multifocal choroiditis and panuveitis: immunomodulatory therapy. Ophthalmology. 2002;109:378–83. doi: 10.1016/s0161-6420(01)00901-0. [DOI] [PubMed] [Google Scholar]
- 25.Murphy CC, Greiner K, Plskova J, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol. 2005;123:634–41. doi: 10.1001/archopht.123.5.634. [DOI] [PubMed] [Google Scholar]
- 26.Walton RC, Nussenblatt RB, Whitcup SM. Cyclosporine therapy for severe sight-threatening uveitis in children and adolescents. Ophthalmology. 1998;105:2028–34. doi: 10.1016/S0161-6420(98)91120-4. [DOI] [PubMed] [Google Scholar]
- 27.Nussenblatt RB, Palestine AG, Chan CC, et al. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. 1991;112:138–46. doi: 10.1016/s0002-9394(14)76692-9. [DOI] [PubMed] [Google Scholar]
- 28.de Vries J, Baarsma GS, Zaal MJ, et al. Cyclosporin in the treatment of severe chronic idiopathic uveitis. Br J Ophthalmol. 1990;74:344–9. doi: 10.1136/bjo.74.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 30.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 31.Towler HM, Whiting PH, Forrester JV. Combination low dose cyclosporin A and steroid therapy in chronic intraocular inflammation. Eye. 1990;4:514–20. doi: 10.1038/eye.1990.68. [DOI] [PubMed] [Google Scholar]
- 32.Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporin A therapy in treating chronic, noninfectious uveitis. Ophthalmology. 1996;103:365–73. doi: 10.1016/s0161-6420(96)30683-0. discussion 373-4. [DOI] [PubMed] [Google Scholar]
- 33.Tappeiner C, Roesel M, Heinz C, et al. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye. 2009;23:1192–8. doi: 10.1038/eye.2008.174. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy JM, Dubord PJ, Chalmers A, et al. Cyclosporine A for the treatment of necrotizing scleritis and corneal melting in patients with rheumatoid arthritis. J Rheumatol. 1992;19:1358–61. [PubMed] [Google Scholar]
- 35.Towler HM, Lightman SL, Forrester JV. Low-dose cyclosporin therapy of ocular inflammation: preliminary report of a long-term follow-up study. J Autoimmun. 1992;5(suppl):259–64. doi: 10.1016/0896-8411(92)90041-n. [DOI] [PubMed] [Google Scholar]
- 36.Kashani S, Mearza AA. Uses and safety profile of ciclosporin in ophthalmology. Expert Opin Drug Saf. 2008;7:79–89. doi: 10.1517/14740338.7.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146:802–12. doi: 10.1016/j.ajo.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangaputra S, Newcomb CW, Liesegang TL, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. doi: 10.1016/j.ophtha.2009.04.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. doi: 10.1016/j.ajo.2009.05.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. doi: 10.1016/j.ophtha.2009.06.060. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]