Abstract
Rationale: No effective treatment exists for idiopathic pulmonary fibrosis, and its pathogenesis remains unclear. Accumulating evidence implicates herpesviruses as cofactors (either initiating or exacerbating agents) of fibrotic lung disease, but a role for latent herpesvirus infection has not been studied.
Objectives: To develop a murine model to determine whether latent herpesvirus infection can augment fibrotic responses and to gain insight into potential mechanisms of enhanced fibrogenesis.
Methods: Mice were infected with murine γherpesvirus 14 to 70 days before a fibrotic challenge with fluorescein isothiocyanate or bleomycin so that the virus was latent at the time of fibrotic challenge. Measurements were made after viral infection alone or after the establishment of fibrosis.
Measurements and Main Results: γHerpesvirus is latent by 14 days post infection, and infection 14 to 70 days before fibrotic challenge augmented fibrosis. Fibrotic augmentation was not dependent on reactivation of the latent virus to a lytic state. Total cell numbers and fibrocyte numbers were increased in the lungs of latently infected mice administered fibrotic challenge compared with mock-infected mice that received fibrotic challenge. Latent infection up-regulates expression of proinflammatory chemokines, transforming growth factor-β1, and cysteinyl leukotrienes in alveolar epithelial cells.
Conclusions: Latent γherpesvirus infection augments subsequent fibrotic responses in mice. Enhanced fibrosis is associated with the induction of profibrotic factors and the recruitment of fibrocytes. Our data complement existing human and animal data supporting the hypothesis that γherpesviruses can serve as initiating cofactors in the pathogenesis of pulmonary fibrosis.
Keywords: chemokine, epithelial cells, fibrocyte, interstitial lung disease
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Herpesviruses have been associated with idiopathic pulmonary fibrosis clinically, and animal models have suggested a role for lytic infection as a fibrotic cofactor.
What This Study Adds to the Field
This study describes the first animal model for latent herpesvirus-induced augmentation of lung fibrosis. Viral augmentation of fibrosis is associated with fibrocyte recruitment and induction of profibrotic mediators by latently infected alveolar epithelial cells.
Idiopathic pulmonary fibrosis (IPF) is characterized by progressive and relentless lung scarring and is the most lethal interstitial lung disease (1). Fibrotic lung disease likely results from a dysregulated healing response to injurious events within the lung, but initiating agents and molecular mechanisms remain unclear.
Herpesviruses have been suggested as cofactors for fibrosis because their life cycle involves lytic replication followed by establishment of lifelong latency with the potential for reactivation. Previous studies have correlated the presence of Epstein-Barr virus (EBV), cytomegalovirus, and human herpesviruses (HHV)-7 and -8 with IPF in human patients (2–5). However, other studies find no association (6, 7). Mouse models have been used to examine whether murine γherpesvirus-68 (γHV-68) contributes to the development of fibrotic lung disease because γHV-68 is genetically and biologically similar to EBV and HHV-8 (8). Although γHV-68 infection alone does not cause fibrosis in wild-type mice, lytic γHV-68 promotes the development of pulmonary fibrosis in the presence of an exogenous injury insufficient to cause fibrosis on its own (9). Furthermore, persistent lytic reactivation of γHV-68 induces fibrosis in Th2-biased mice (10). In addition, we have recently shown that lytic γHV-68 infection can exacerbate established pulmonary fibrosis (11). The mechanisms identified to contribute to lytic virus-induced exacerbation of fibrosis have included Th2 bias, persistent reactivation, alternative activation of macrophages, and fibrocyte recruitment (11–13).
Each of these findings provide clues about roles for lytic γHV-68 infection in fibrosis, but most humans harbor latent herpesviruses for their lifetime, and IPF normally occurs at advanced age (14). A role for latent herpesviruses in fibrosis has not been studied. Thus, we sought to establish an animal model to test the hypothesis that latent γherpesvirus infection can serve as a cofactor or an initiating agent of fibrosis in wild-type mice. In these studies, we first infected mice intranasally with γHV-68 and waited for latency to be established in the lungs. We next challenged the mice with a fibrotic stimulus: either fluorescein isothiocyanate (FITC) or bleomycin. Our results demonstrate the ability of latent γHV-68 to augment a subsequent fibrotic challenge and identify important latent virus-induced alterations of lung epithelial cells that likely promote fibrotic inflammation and extracellular matrix deposition.
METHODS
Mice
Male mice (2–5 mo) were from Jackson Laboratories, Bar Harbor, ME. Animal protocols were approved by the University of Michigan Committee on the Use and Care of Animals.
Viral Infection
Mice were anesthetized and 5 × 104 plaque forming units (PFU) of γHV-68 clone WUMS (American Type Culture Collection, Manassas, VA), ΔORF72 (a v-cyclin mutant virus described previously (15)), or v-cyclin marker rescue virus (“MR”) generated by recombination of wild-type γHV-68 sequence, including the v-cyclin DNA, with a LacZ cassette-containing virus, thus reconstituting an essentially wild-type virus control (15), were suspended in 20 μl saline and delivered intranasally to each mouse. Mock infection was 20 μl of saline.
Virus Plaque Assay
Lytic virus present in two lung lobes of infected mice was measured by plaque assay (11).
Semiquantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction
Semiquantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed and the results quantified using the ΔΔCT method as described in Reference 16. The sequences for all primers and probes are in Table 1. Samples were run in duplicate or triplicate.
TABLE 1.
PRIMERS AND PROBES FOR SEMIQUANTITATIVE REAL-TIME REVERSE TRANSCRIPTASE–POLYMERASE CHAIN REACTION
| Gene | Oligo | Sequences |
|---|---|---|
| M3 | F. primer | AGTGGGCTCACGCTGTACTTGT |
| R. primer | TGTCTCTGCTCACTCCATTTGG | |
| Probe | CATGGGCAAGTGTTCATCTTAGCC | |
| DNApol (ORF9) | F. primer | ACAGCAGCTGGCCATAAAGG |
| R. primer | TCCTGCCCTGGAAAGTGATG | |
| Probe | CCTCTGGAATGTTGCCTTGCCTCCA | |
| gB (ORF8) | F. primer | CGCTCATTACGGCCCAAA |
| R. primer | ACCACGCCCTGGACAACTC | |
| Probe | TTGCCTATGACAAGCTGACCACCA | |
| CCL2 | F. primer | GGCTCAGCCAGATGCAGTTAAC |
| R. primer | CCTACTCATTGGGATCATCTTGCT | |
| Probe | CCCCACTCACCTGCTGCTACTCATTCAC | |
| CCL12 | F. primer | TGGCTGGACCAGATGCG |
| R. primer | GACGTGAATCTTCTGCTTAACAACA | |
| Probe | TGAGCACCCCAGTCACGTGCTGTTA | |
| β-actin | F. primer | CCGTGAAAAGATGACCCAGATC |
| R. primer | CACAGCCTGGATGGCTACGT | |
| Probe | TTTGAGACCTTCAACACCCCCAGCCA |
Definition of abbreviations: F. = forward; R. = reverse.
FITC and Bleomycin Injections
FITC and bleomycin inoculations were performed intratracheally as described previously (17).
Hydroxyproline Assay
Total collagen measurements were made via hydroxyproline assay (17).
Collagenase Digests
Lungs were enzymatically digested using collagenase and DNase (18).
Flow Cytometry
Leukocytes from collagenase digests or bronchoalveolar lavage (BAL) were incubated 15 minutes on ice with Fc block (1:100 dilution, clone 24G2; BD PharMingen, San Diego, CA) before surface staining with CD45-PerCPCy5.5 (1:500 dilution, BD PharMingen) followed by fixation/permeabilization with BD PharMingen Cytofix/cytoperm kit according to manufacturer's instructions. Cells were blocked with goat IgG (1:2,000 dilution; Jackson ImmunoResearch, West Grove, PA) before staining with rabbit anti-mouse collagen 1 (1:400 dilution; Rockland Immunochemicals, Gilbertsville, PA) or rabbit IgG (1:4,500 dilution; Jackson ImmunoResearch, isotype control). Cells were analyzed on a FACScan (BD Biosciences, Mountain View, CA).
Histology
Hematoxylin and eosin and trichrome staining were performed as described previously (18).
ELISA
Chemokines CCL2, CCL12, and transforming growth factor (TGF)-β1 were measured by specific ELISA (DuoSet ELISA; R&D Systems Minneapolis, MN). Cysteinyl leukotriene (CysLT) measurements were made using EIA kits (Cayman Chemical, Ann Arbor, MI). Lower limit of detection was 10 pg/ml. In some airway epithelial cell (AEC) cultures, 5 μM Ca2+ ionophore (A23187) was added for 1 hour in serum-free media as a maximal stimulus for LT synthesis.
AEC Purification
Type II AECs were isolated as previously described (19, 20). Cells cultured on fibronectin for 24 hours were greater than 88% positive for e-cadherin, 3% positive for vimentin, and 0.5% positive for CD45. Western blot analysis confirmed e-cadherin expression and showed no α-smooth muscle actin.
TGF-β1 Bioassay
Mink lung epithelial cells stably transfected with plasminogen activator inhibitor (PAI)1 promoter-driven luciferase gene were mock infected or infected with γHV-68 (multiplicity of infection of 0.005 or 0.05). After 48 to 72 hours of infection, cell lysates were analyzed forTGF-β1 bioactivity (18).
Reagents Used
Complete media is Dulbecco's modified Eagle medium (Lonza; Walkersville, MD) with 10% fetal calf serum, 1% penicillin-streptomycin, 1% l-glutamine, and 0.1% amphotericin B (Lonza). Serum-free media is Dulbecco's modified Eagle medium with 0.1% bovine serum albumin (Sigma), 1% penicillin-streptomycin, 1% l-glutamine, and 0.1% amphotericin B.
Statistical Analyses
Statistical significance was measured by analysis of variance (three or more groups) or Student t test (two groups) using Graphpad Prism 3 software; data represent mean ± SEM; P less than 0.05 was considered significant.
RESULTS
γHV-68 Infection Is Latent in the Lung by 14 Days Post Infection
To test the effect of latent γherpesvirus infection on subsequent pulmonary fibrosis, we first confirmed when lytic virus was cleared from the lungs of normal mice infected with γHV-68. After intranasal inoculation with 5 × 104 PFU, we were not able to detect productive γHV-68 infection in the lung by a standard plaque assay by 14 days post infection (d.p.i.) (Figure 1A). Using real-time RT-PCR, we next measured viral gene expression in the lung over the first 70 d.p.i. and normalized these values to the expression level of each during lytic replication at Day 3. Expression of the lytic gene DNApol was substantially reduced by 14 d.p.i. (Figure 1B). Expression of the M3 gene, which is expressed during both lytic and latent infection, also diminished more slowly over time (Figure 1C). Herpesvirus latency is defined by the expression of latency-associated genes at higher levels than lytic-associated genes when preformed lytic virus is not present. The normalization in Figure 1 makes it difficult to appreciate that this is the case by Day 14. Figure E1 in the online data supplement demonstrates the expression of DNApol and M3 relative to β-actin at 14, 30, 45, and 70 d.p.i. Figure E2 examines the effect of sex on the establishment of latency. Taken together these data confirm that γHV-68 has established a latent infection in the lung by 14 d.p.i.
Figure 1.
γHerpesvirus-68 (γHV-68) infection is latent in the lung by 14 days post infection (d.p.i.). Wild-type C57Bl/6 mice were given an intranasal infection with 5 × 104 plaque-forming units of γHV-68. Subsequently, lytic and latent γHV-68 infection was measured in the lungs. Error bars represent SEM between individual mice. (A) Viral plaque assay demonstrates that there is no active viral replication by 14 d.p.i (nd = not detectable, n = 3). (B) Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) demonstrates that gene expression of the lytic viral gene DNApol decreases to nearly undetectable levels from 14 to 70 d.p.i. (n = 5). Viral gene expression 3 d.p.i. was set at 1, and viral gene expression on subsequent days is expressed in comparison for B and C. (C) Real-time RT-PCR demonstrates that gene expression of the latent viral gene M3 is detectable to 70 d.p.i. (n = 5). It should be noted that M3 is also expressed during lytic infection and remains present during early latent infection.
Latent γHV-68 Augments FITC- or Bleomycin-Induced Pulmonary Fibrosis
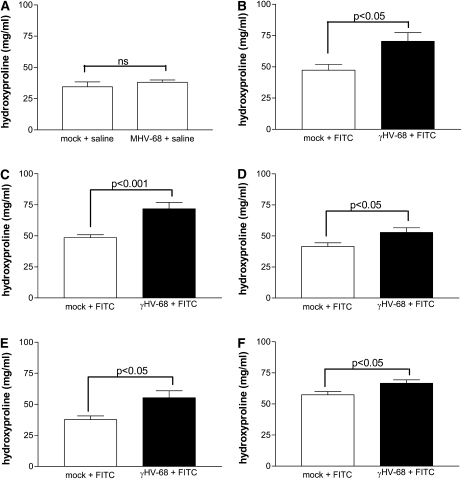
Mice were inoculated intranasally with 5 × 104 PFU γHV-68 or were mock infected with saline on Day 0. Subsequently, at 14, 21, 30, 45, and 70 d.p.i., γHV-68–infected mice and mock-infected mice were administered intratracheal FITC or saline. Lungs were harvested from all mice 21 days after the intratracheal injection, and lung hydroxyproline content was assayed to measure collagen content. γHV-68 infection alone did not increase lung hydroxyproline content above the level of saline-treated mice (Figure 2A). However, established latent γHV-68 infection at all time points resulted in significantly higher hydroxyproline levels in FITC-treated mice than preceding mock infection in FITC-treated mice (Figures 2B–2F). Figure E3 confirms that latent infection augments FITC-induced fibrosis when measured by a different biochemical assay (Sircol) for collagen deposition. Trichrome staining of lung sections confirms the results of the biochemical assays for collagen deposition (Figure E4). To confirm our findings with a different fibrotic insult, we tested whether latent γHV-68 augmented pulmonary fibrosis induced by bleomycin. We observed that intranasal infection with γHV-68 70 days before bleomycin-induced pulmonary fibrosis also significantly augments hydroxyproline levels in the lung (Figure 3). These results demonstrate for the first time that either short-term or long-term γHV-68 latency in the lung significantly augments a subsequent fibrotic stimulus.
Figure 2.
Latent γherpesvirus-68 (γHV-68) augments fluorescein isothiocyanate (FITC)-induced pulmonary fibrosis. (A) Wild-type C57Bl/6 mice were inoculated intranasally with 5 × 104 plaque-forming units of γHV-68 or were mock infected with saline on Day 0. Then, at 14 days post infection (d.p.i.), mice of both groups were administered intratracheal saline. Lungs were harvested 21 days after saline intratracheal administration, which is 35 d.p.i. Hydroxyproline assay of the lungs demonstrates that γHV-68 infection alone does not augment collagen levels in the lung compared with lungs of mock-infected mice, n = 4–5. (B) 14, (C) 21, (D) 30, (E) 45, and (F) 70 d.p.i., γHV-68–infected mice and mock-infected mice were administered intratracheal FITC. In each case, lungs were harvested 21 days after FITC administration. Hydroxyproline assays of the harvested lungs demonstrate that γHV-68 infection before FITC at each time point causes a significant augmentation in collagen levels in fibrotic lungs. For B, C, D, and F data were combined from two to three experiments. The n values for the mock+FITC and γHV-68+FITC groups, respectively, are as follows: B, n = 7, 11; C, n = 14, 12; D, n = 10, 9; F, n = 13, 18. E represents a single experiments with n = 5 mice per group.
Figure 3.
Latent γherpesvirus-68 (γHV-68) augments bleomycin-induced pulmonary fibrosis. Wild-type C57Bl/6 mice were inoculated intranasally with 5 × 104 plaque-forming units of γHV-68 or mock-infected with saline on Day 0. Seventy days post infection, γHV-68–infected mice and mock-infected mice were administered intratracheal bleomycin. Lungs were harvested 21 days after bleomycin administration. Hydroxyproline assays of the harvested lungs demonstrate that γHV-68 infection before bleomycin-induced fibrosis causes a significant augmentation in collagen levels in fibrotic lungs (n = 6 mice per group).
Latent γHV-68 Infection Can Augment a Subthreshold Fibrotic Stimulus
In some experiments, the fibrotic response to FITC alone did not result in a statistically significant increase in lung collagen levels. However, even in these situations, the presence of latent γHV-68 at the time of the FITC challenge resulted in significantly higher hydroxyproline levels after the administration of FITC (Figure 4). This result demonstrates that latent γHV-68 can induce pulmonary fibrosis even in a scenario wherein the subsequent insult is not sufficient to cause measurable levels of fibrosis on its own.
Figure 4.
γHerpesvirus-68 (γHV-68) latency induces fibrosis even with subthreshold fluorescein isothiocyanate (FITC) stimulus. Wild-type C57Bl/6 mice were inoculated intranasally with 5 × 104 plaque-forming units of γHV-68 or mock-infected with saline on Day 0. (A) Fourteen days post infection (d.p.i.) and (B) 21 d.p.i. mice were administered intratracheal saline or intratracheal FITC. Lungs were harvested 21 days after intratracheal injections. A hydroxyproline assay of the lungs demonstrates that in these experiments, FITC alone (hatched bars) does not significantly augment collagen levels in the lungs compared with mice given saline intratracheally (open bars). Despite this, the lungs of FITC-treated mice infected 14 or 21 days previously with γHV-68 (solid bars) displayed a significant augmentation in collagen levels compared with mock-infected mice treated with FITC (n = 3–11 per group).
FITC Induces a Low-Level Reactivation of γHV-68
We first wanted to determine if the fibrotic stimulus was reactivating latent γHV-68 to undergo lytic replication. Thus, we infected mice intranasally with γHV-68 on Day 0, and then mice were administered FITC 30 d.p.i. We harvested lungs immediately before FITC inoculation (on Day 30) or 1 week after FITC (Day 37) to measure the expression of the lytic viral genes, DNApol and gB. Interestingly, we could detect low levels of the capsid protein, gB, when compared with β-actin 30 d.p.i., but no expression of DNApol. After FITC administration, we observed an increase in the expression of both of these lytic genes (Figure 5). However, FITC-induced reactivation of lytic viral RNA transcription was two orders of magnitude less than that of peak viral gene expression on Day 5 after primary infection (Figure 5 and data not shown).
Figure 5.
Fluorescein isothiocyanate (FITC) induces a low-level reactivation of γherpesvirus-68 (γHV-68). Wild-type C57Bl/6 mice were given an intranasal injection with 5 × 104 plaque-forming units of γHV-68 on Day 0. On Day 30, mice were administered intratracheal FITC. The lungs of some mice were harvested on Day 30 before FITC administration, and the expression of γHV-68 genes was measured. The lungs of the remainder of the mice were harvested on Day 37 for analysis of viral gene expression. Real-time reverse transcriptase–polymerase chain reaction demonstrates that levels of gene expression of (A) DNApol and (B) gB are increased 7 days after FITC administration compared with mice that were not administered FITC. Viral gene expression is presented relative to the expression level of a housekeeping gene, β-actin, which was set at 1 for each mouse (n = 4 mice per group). nd = not detectable.
Reactivation of γHV-68 Is Not Necessary to Augment Fibrosis
To determine whether the level of reactivation of lytic viral gene expression by FITC treatment was responsible for the augmentation of fibrosis, we next tested whether a mutant γHV-68 virus lacking v-cyclin expression (ΔORF72) could augment fibrosis. As a result of this mutation, ΔORF72 has a greatly diminished ability to reactivate from latency (approximately 100-fold reduction) (15). Mice were inoculated intranasally with 5 × 104 PFU of ΔORF72, 5 × 104 PFU of a v-cyclin marker-rescue virus (“MR,” essentially a wild-type control), or were mock infected with saline on Day 0. Then, 14 or 21 d.p.i., the mice were administered intratracheal FITC. Lungs were harvested 21 days after the intratracheal injection and lung hydroxyproline content was assayed. There was no significant difference between the augmentation of fibrosis in mice infected with either the ΔORF72 or the “MR” virus 21 or 14 days before the administration of FITC (Figure 6). Thus, we conclude that lytic reactivation is not necessary for latent γHV-68 to augment FITC-induced pulmonary fibrosis.
Figure 6.
Reactivation of γherpesvirus-68 (γHV-68) is not necessary to augment fibrosis. Wild-type C57Bl/6 mice were inoculated intranasally with 5 × 104 plaque-forming units (PFU) of ΔORF72, 5 × 104 PFU of a v-cyclin marker-rescue virus (“MR”), or were mock-infected with saline on Day 0. At 14 or 21 days post infection, all of the mice were administered intratracheal fluorescein isothiocyanate (FITC). Lungs were harvested 21 days after the intratracheal injection and lung hydroxyproline content was assayed. There was no significant difference between fibrosis in mice infected with either the ΔORF72 or the “MR” virus 21 or 14 days before the administration of FITC (n = 5–6 mice per group). i.n. = intranasal.
Latent γHV-68 Augments Inflammation in the Lung during FITC-Induced Pulmonary Fibrosis
To understand why latent virus increases fibrosis, we investigated whether the combined stimulus of latent viral infection plus a fibrotic insult altered cellular recruitment and chemokine production. We first sought to determine whether increased numbers of inflammatory cells correlated with latent virus-induced augmentation of fibrosis. Mice were infected intranasally with 5 × 104 PFU γHV-68 or were mock infected on Day 0. Then 17 days later mice were administered FITC or saline via the intratracheal route. Lungs were harvested on Day 24 or 38 for cellular enumeration. These time points represent 7 days (Figure 7A) or 21 days (Figure 7B) after the intratracheal administration. For simplicity, the treatment groups are referred to as saline (mock infection i.n. followed by saline i.t.), FITC (mock infection i.n. followed by FITC i.t.), γHV-68 (viral infection i.n. followed by saline i.t.), and γHV-68+FITC (viral infection i.n. followed by FITC i.t.). Cellular accumulation was enhanced by treatment with γHV-68 or FITC, but the highest numbers of inflammatory cells were seen in the γHV-68+FITC group. Latent infection in the γHV-68+FITC mice resulted in significantly higher numbers of cells than in mice treated with FITC. Figures 7C–7F show the histologic pattern noted in each group of mice at time of harvest by hematoxylin and eosin staining. Viral infection is associated with accumulation of inflammatory leukocytes, which form focal clusters. This same pattern of focal inflammation was noted in mice infected with the mutant ΔORF72 virus despite the reduced ability of this virus to reactivate (data not shown). These data suggest focal effects as a result of latent viral infection (see below).
Figure 7.
Latent γherpesvirus-68 (γHV-68) augments inflammation in the lung during fluorescein isothiocyanate (FITC)-induced pulmonary fibrosis. (A and B) Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γHV-68 or were mock infected on Day 0. Then 17 days post infection, latently infected mice and mock-infected mice were administered intratracheal saline or FITC. Lungs were harvested and digested (A) 7 days or (B) 21 days after FITC administration, and total cells were enumerated. The lungs of mice treated with γHV-68+FITC (solid bar) display a significant increase in the accumulation of inflammatory cells compared with the lungs of mock-infected mice treated with FITC (hatched bar) (A) 7 days or (B) 21 days after FITC administration (n = 3–5). (C–F) Lung sections of mice treated as above and stained with hematoxylin and eosin. For simplicity, the treatment groups are referred to as saline (mock infection i.n. followed by saline i.t.), FITC (mock infection i.n. followed by FITC i.t.), γHV-68 (viral infection i.n. followed by saline i.t.), and γHV-68+FITC (viral infection i.n. followed by FITC i.t.).
Latent γHV-68 Augments Fibrocyte Accumulation in the Lung during FITC-Induced Pulmonary Fibrosis
Because fibrocytes are important mediators of fibrosis (21, 22), we next investigated whether augmentation of fibrocyte accumulation correlated with latent virus-induced augmentation of fibrosis. Mice were infected intranasally with 5 × 104 PFU γHV-68 or were mock infected on Day 0. Seventeen d.p.i., latently infected mice and mock-infected mice were administered intratracheal FITC. Lungs were harvested 7 or 21 days after FITC administration. Subsequently, we stained the total cells from the digest for CD45 and collagen 1 and used flow cytometry to identify CD45+ collagen 1+ fibrocytes. To illustrate how we identified fibrocytes by flow cytometry, examples of CD45 gating and intracellular collagen staining (Figure 8A) versus isotype control (Figure 8B) are shown. Seven days after FITC, latently infected mice had approximately twice the number of fibrocytes in the lungs (344,900 ± 52,880) compared with mock-infected mice (170,400 ± 22,430) (Figure 8C). Twenty-one days after FITC, latently infected mice had also had significantly more fibrocytes in the lungs (591,000 ± 98,880) compared with mock-infected mice (260,300 ± 55,650) (Figure 8D). Thus, we conclude that the presence of latent γHV-68 augments fibrocyte accumulation in the lungs during FITC-induced pulmonary fibrosis to levels that are significantly higher than those achieved with the fibrotic stimulus alone.
Figure 8.
Latent γherpesvirus-68 (γHV-68) augments fibrocyte accumulation in the lung during fluorescein isothiocyanate (FITC)-induced pulmonary fibrosis. Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γHV-68 or were mock infected on Day 0. At 17 days post infection (d.p.i.), latently infected mice and mock-infected mice were administered intratracheal FITC. Lungs were harvested and digested with collagenase 7 or 21 days after FITC administration, and total cells from the digest were enumerated and stained for CD45 and collagen 1. Flow cytometry was used to determine the percentage of fibrocytes. (A) Flow cytometric analysis of cells from the lung digest of one mouse 30 d.p.i (representative of analysis at each time point post infection). In the left panel, CD45+ cells were gated. In the middle panel, those CD45+ cells were gated to exclude dead cells. In the right panel, living CD45+ collagen 1+ cells were gated. (B) An equal number of cells from each lung digest were stained with a rabbit isotype control antibody instead of the rabbit collagen 1 antibody. To account for nonspecific binding, the percent of cells staining with the isotype control was set to approximately 0.5%. The shape of the gate was chosen to account for autofluorescence. The percentage of fibrocytes was calculated by subtracting the percentage of cells in the irrelevant control (B) from the specific collagen 1 staining percentage (A). Total cells were then calculated by multiplying this percentage by the total number of leukocytes recovered. (C–D) The lungs of mice previously infected with γHV-68 (solid bar) have a significant increase in the accumulation of fibrocytes compared with the lungs of mock-infected mice (open bar) (C) 7 days and (D) 21 days after FITC administration (n = 3–5).
γHV-68 Infection Induces Fibrocyte Accumulation in the Lungs for at Least 45 d.p.i
To understand why the presence of latent virus increased fibrocyte accumulation after FITC administration, we investigated fibrocyte accumulation in the lung during γHV-68 latency alone. Mice were infected intranasally with 5 × 104 PFU γHV-68, and lungs were harvested and digested with collagenase 7, 15, 30, 45, and 70 d.p.i. Lungs of uninfected mice were harvested and digested as controls. Subsequently, total pulmonary fibrocytes were enumerated as described by flow cytometry. We found that the absolute number of fibrocytes in the lungs is significantly increased 15, 30, and 45 d.p.i (Figure 9). This long-term accumulation of fibrocytes due to herpesvirus infection could be one reason that the numbers of fibrocytes are augmented 7 and 21 days after FITC (17 and 38 d.p.i., respectively) in Figure 8. Although fibrocyte accumulation lasted only 45 d.p.i., total numbers of inflammatory cells in response to the virus were elevated through 70 d.p.i. (Figure E5).
Figure 9.
Viral infection induces fibrocyte accumulation in the lungs for at least 45 days post infection (d.p.i.). Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units. The lungs of uninfected mice were harvested on Day 0 as controls, and the lungs of virally infected mice were harvested 7, 15, 30, 45, and 70 d.p.i. All lungs were digested with collagenase, and fibrocytes were enumerated as described for Figure 8. Fibrocytes accumulated to significantly higher levels in the lung 15, 30, and 45 d.p.i. compared with uninfected mice (n = 4 mice per group; *P < 0.05, **P < 0.01 compared with uninfected).
CCL2 and CCL12 Levels Are Increased in the Lungs beyond the Clearance of Lytic γHV-68
CCL2 and CCL12 are potent chemotactic ligands for CCR2-mediated recruitment of inflammatory cells and fibrocytes during pulmonary fibrosis (22, 23). Past studies by our group and others have suggested that γHV-68 induces CCL2 and CCL12 gene expression, and we wished to investigate whether latent infection caused a persistent increase in CCL2 and CCL12 protein production (11, 24, 25). We infected mice intranasally with 5 × 104 PFU γHV-68 on Day 0, and the lungs of the infected mice were harvested 15, 24, and 38 d.p.i. for the measurement of CCL2 and CCL12 in lung homogenates by ELISA. The lungs of uninfected mice were measured as controls. Figure 10 demonstrates that both CCL2 (Figures 10A–10C) and CCL12 (Figures 10E–10G) remain elevated during latent infection of the lung compared with uninfected controls. Figures 10D and 10H show the relative differences in chemokine production between untreated mice and mice in the γHV-68, FITC, or γHV-68+FITC groups.
Figure 10.
Chemokine CCL2 and CCL12 levels are increased in the lungs beyond the clearance of lytic γherpesvirus-68 (γHV-68). Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γHV-68 on Day 0 or were left untreated. The lungs of γHV-68–infected mice were harvested 15, 24, and 38 days post infection (d.p.i.) (solid bars) along with lungs of untreated mice (open bar) and lung homogenates were assayed by ELISA for levels of (A–C) CCL2 and (E–G) CCL12. The lungs of mice latently infected with γHV-68 have significantly higher levels of CCL2 and CCL12 compared with the lungs of uninfected mice (n = 3–10 mice per group; **P < 0.01, ****P < 0.0001 compared with uninfected). In D and H, mice were left uninfected or were infected with γHV-68 on Day 0. Then at 17 d.p.i., mice were given an intratracheal instillation of fluorescein isothiocyanate (FITC) or saline. Lungs were harvested 7 days later (on Day 24) and lung homogenates were analyzed for (D) CCL2 and (H) CCL12; n = 7–10 per group; **P < 0.01, ***P < 0.001 compared with uninfected.
AECs Are Latently Infected at Least 21 Days after Intranasal Infection with γHV-68
AECs have been shown to be a reservoir of latent γHV-68 for at least 54 d.p.i. in vivo (26). We reasoned that latent viral alterations of AECs may play a role in the fibrotic augmentation we observed. Furthermore, the focal nature of the inflammatory cell accumulation suggested focal alterations of AECs (Figures 7E and 7F). First, we tested whether murine AECs are infected by γHV-68 in our in vivo model. We infected mice intranasally with 5 × 104 PFU γHV-68 or mock-infected mice with saline on Day 0, and AECs were purified from the lungs of the infected mice 14 and 21 d.p.i. Using real-time RT-PCR, we measured viral mRNA expression levels in the purified AECs. Viral gene expression was not detectable in the AECs purified from mock-infected mice, but the AECs purified from infected mice expressed the latent viral gene M3 as well as extremely low levels of the lytic viral gene gB (Figure 11). This pattern of viral gene expression is consistent with a latent infection of the lung at 14 and 21 d.p.i.
Figure 11.
Airway alveolar cells (AECs) are latently infected at least 21 days after intranasal infection with γherpesvirus-68 (γHV-68). Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γHV-68 or were mock infected on Day 0. AECs were purified from the lungs of the infected mice 14 and 21 days post infection for analysis of viral gene expression. Real-time reverse transcriptase–polymerase chain reaction demonstrates that viral genes M3 and gB are expressed in the AECs of mice given γHV-68 intranasally Neither M3 nor gB gene expression was detectable in the AECs of mock-infected mice. Viral gene expression is presented relative to the expression level of a housekeeping gene, β-actin, which was set at 1 for each mouse (n = 3–8 mice per group). i.n. = intranasal; nd = not detectable.
Latently Infected AECs Express Higher Levels of CCL2 and CCL12
Next, we investigated if AECs contributed to CCL2 and CCL12 generation that we observed during γHV-68 infection. We infected mice intranasally with 5 × 104 PFU γHV-68 or mock-infected mice with saline on Day 0, and AECs were purified from the lungs of the infected mice 14 and 21 d.p.i. Using real-time RT-PCR, we measured chemokine mRNA expression levels in the purified AECs. CCL2 gene expression increased markedly in latently infected AECs by 21 d.p.i (Figure 12A). On the other hand, CCL12 mRNA levels increased significantly by 14 d.p.i (Figure 12B). Given that our AEC cultures contain fewer than 0.5% CD45+ cells, it is unlikely that contaminating leukocytes contribute to the chemokine levels measured. We conclude that AECs contribute to the increased levels of CCL2 and CCL12 in lungs of latently infected mice. Both chemokines are known to recruit CCR2-expressing fibrocytes.
Figure 12.
Latently infected alveolar epithelial cells (AECs) express higher levels of chemokines CCL2 and CCL12. Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γherpesvirus-68 (γHV-68) or were mock infected on Day 0. AECs were purified from the lungs of the infected mice 14 and 21 days post infection (d.p.i.) for analysis of chemokine gene expression. Real-time reverse transcriptase–polymerase chain reaction demonstrates that (A) CCL2 gene expression is significantly increased in AECs harvested 21 d.p.i., whereas (B) CCL12 gene expression is significantly increased in AECs harvested 14 or 21 d.p.i. (n = 3–8 mice per group; *P < 0.05, ***P < 0.001 compared with mock infected). i.n. = intranasal.
γHV-68 Infection Induces TGF-β1 Production in AECs
We also investigated whether AECs contribute to virus-induced generation of the potent profibrotic factor, TGF-β1. We infected mice intranasally with 5 × 104 PFU of γHV-68 or mock-infected mice with saline, and AECs were purified from the lungs of the γHV-68–infected and mock-infected mice 14 d.p.i. AECs were then cultured for 24 hours, and the culture supernatants were assayed for total TGF-β1 by ELISA. The level of total TGF-β1 was significantly higher in supernatants from AECs isolated from latently infected mice compared with supernatants from AECs isolated from mock-infected mice (Figure 13A). Our ELISA measures total TGF-β1, which would include both latent and active forms of this growth factor. Although it is assumed that an increase in total TGF-β1 would be indicative of an increase in active TGF-β1, we used another method to investigate if γHV-68 infection augments active TGF-β1 production directly. In vitro, we infected mink lung epithelial cells stably transfected with a reporter construct encoding a luciferase gene with a PAI1 promoter. Active TGF-β1 expression corresponds with the amount of luciferase expressed. Epithelial cells were infected at a multiplicity of infection of 0.005 or 0.05. Using a luciferase assay, we found that mink lung epithelial cells infected for 3 and 7 days in vitro produced significantly more active TGF-β1 than uninfected mink lung epithelial cells (Figure 13B). Thus, we conclude that both lytic and latent γHV-68 infection can augment TGF-β1 production in AECs. Furthermore, we also found that TGF-β1 levels are modestly increased in whole lung homogenates of latently infected mice 24 d.p.i. and 38 d.p.i. (data not shown).
Figure 13.
γHerpesvirus-68 (γHV-68) infection induces TGF-β1 production. Alveolar epithelial cells (AECs) from latently infected mice produce more total TGF-β1 than AECs from mock-infected mice. Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units γHV-68 or were mock infected with saline, and AECs were purified from the lungs of both groups 14 days post infection. AECs were then cultured for 24 hours, and the culture supernatants were assayed for total TGF-β1 by ELISA. TGF-β1 from AECs of latently infected mice is represented by the solid bar, and TGF-β1 from AECs of mock-infected mice is represented by the open bar (n = 4–6 per group). (B) Lung epithelial cells infected with γHV-68 in vitro produce more active TGF-β1 than uninfected lung epithelial cells. The bar graph shown reflects the results of a luciferase assay used to measure active TGF-β1 production from epithelial cells in culture for 3 or 7 days. The cells were either uninfected or infected at a multiplicity of infection of 0.005 or 0.05. Active TGF-β1 produced is presented relative to amount of active TGF-β1 produced by uninfected cells after 3 days in culture, which was set at 1 (n = 6–8 mice per group; ***P < 0.001 compared with uninfected cells from same time point). i.n. = intranasal; MOI = multiplicity of infection.
Latent γHV-68 Infection Induces cysLT Production in AECs
Leukotrienes (LTs) are elevated in the lungs of human patients with IPF, and LTs have been shown to be critical for mouse models of pulmonary fibrosis (27, 28). Additionally, we have previously demonstrated that cysLTs have potent proliferative effects on fibrocytes (29). Thus, we investigated whether latent infection of AECs augmented the production of cysLTs. We infected mice intranasally with 5 × 104 PFU of γHV-68 or mock-infected mice with saline, and AECs were purified from the lungs of the infected mice 14 d.p.i. AECs were then cultured for 24 hours in the absence or the presence of the calcium ionophore, A23187, a stimulus for maximal LT production, and the culture supernatants were assayed for cysLTs by ELISA. The level of cysLTs was increased, but not significantly (P = 0.07) in latently infected AECs cultured without ionophore (Figure 14A), but when the AECs were cultured with ionophore, the level of cysLTs was significantly higher in AECs isolated from latently infected mice compared with supernatants from AECs isolated from mock-infected mice (Figure 14B). These data suggest that latent γHV-68 infection alters AECs to produce more cysLTs.
Figure 14.
Latent γherpesvirus-68 (γHV-68) infection induces cysteinyl leukotriene (cysLT) production in alveolar epithelial cells (AECs). Wild-type C57Bl/6 mice were infected intranasally with 5 × 104 plaque-forming units of γHV-68 or mock infected with saline, and AECs were purified from the lungs 14 days post infection. AECs (A) were cultured for 24 hours in serum-free media without A23187 or (B) were cultured for 24 hours with A23187 (5 μM). The culture supernatants were then assayed for cysLTs by ELISA. CysLTs from AECs of latently infected mice are represented by the solid bars, and cysLTs from AECs of mock-infected mice are represented by the open bars (n = 3 per group). i.n. = intranasal.
DISCUSSION
Our results demonstrate for the first time that either short-term or long-term γherpesvirus latency in the lung significantly augments the response to a subsequent fibrotic stimulus. Our observations extend prior reports about a role for γherpesviruses in lung fibrosis in several important ways. First, we demonstrate that latent γHV-68 can augment a subsequent fibrotic stimulus, and this augmentation does not require substantial reactivation to the lytic phase. Second, we demonstrate that latent γHV-68 can augment pulmonary fibrosis in immunocompetent wild-type mice. Third, we demonstrate that latent γHV-68 can serve as a fibrotic cofactor for either FITC or bleomycin as a subsequent stimulus. Fourth, we found that the presence of latent γHV-68 can augment fibrosis even in cases when the fibrotic stimulus is inadequate to cause significant extracellular matrix generation on its own. Finally, we identify latently infected lung epithelial cells as a source of profibrotic chemokines, TGF-β1, and cysLT production, factors which contribute to the recruitment and activation of fibrocytes.
To test our hypothesis about latent γHV-68, we had to first determine when lytic γHV-68 was cleared from the lungs of infected mice. Our finding that lytic virus was cleared from the lung by 14 d.p.i. agrees with previous characterizations of γHV-68 that determined titers of replicating γHV-68 decline to undetectable levels in the lungs between 10 and 15 d.p.i. after intranasal infection (30, 31). Conversely, we detected latent viral RNA through the latest time point we tested, 70 d.p.i. Stewart and colleagues have suggested that latent γHV-68 persists in the lungs of normal mice for at least 12 months post infection (26).
Our results demonstrate that latent γHV-68 infection is able to augment a subsequent fibrotic challenge by either FITC or bleomycin at all time points tested. In a few experiments, the experimental variability of FITC resulted in a total collagen increase that was not statistically greater than the saline controls. Yet, even in this circumstance, latent γHV-68 infection resulted in an accumulation of collagen that was significantly elevated compared with controls. If these findings are able to be extrapolated to humans, it is possible that a latent viral infection could predispose patients to develop fibrotic responses to subsequent injuries that are not themselves sufficient to induce fibrosis. This may explain in part why it has been difficult to elucidate the etiology of IPF.
AEC injury has been suggested to contribute to lung fibrosis. Thus, we originally hypothesized that reactivation of latent γHV-68 in response to the fibrotic challenge may be playing a role in viral-mediated AEC lysis and subsequent fibrosis. Previously, a study using a highly sensitive plaque assay demonstrated that persistent replication does occur at a low level during long-term latency and is detectable in the lungs of immunocompetent mice 90 d.p.i (32). In our model, we found that FITC reactivated transcription of lytic viral RNA in the lungs (although it was to a level two orders of magnitude below peak viral gene expression 5 days after the primary infection [Figure 5 and data not shown]). To further investigate this question, we infected mice with a mutant strain of γHV-68 (ΔORF72) with a 100-fold reduced ability to reactivate (15). There is no evidence that infection with ΔORF72 results in lytic or persistent infection during long-term latency (33). Despite a severely reduced ability to reactivate, latent ΔORF72 infection still caused augmentation of subsequent pulmonary fibrosis to the same degree as “wild-type” γHV-68. Our interpretation of these findings is that latent γHV-68 infection is able to promote a profibrotic lung environment even in the absence of persistent lytic reactivation and potential destruction of AECs.
One potential mechanism whereby γHV-68 latent infection promotes fibrosis is a heightened inflammatory response. Our results demonstrate that prior latent γHV-68 infection significantly augments the total numbers of cells and fibrocytes in the lungs after the instillation of the fibrotic challenge. It is likely that the increased inflammatory cells and fibrocytes contribute in part to the augmentation of subsequent pulmonary fibrosis. Seven and 21 days after FITC administration, there were approximately twice the number of fibrocytes in the lungs of latent γHV-68+FITC mice as there were in the lungs of mice that were administered FITC alone. We speculate that at these two time points the increase could be a result of the fibrocytes present as a result of γHV-68 infection at 24 or 38 d.p.i. adding to the normal number of fibrocytes that accumulate in response to FITC alone. Fibrocyte numbers remain significantly elevated in the lungs of latently infected mice for at least 45 d.p.i. (in the absence of a fibrotic stimulus) but decrease back to basal levels by 70 d.p.i. The increased number of fibrocytes at the time that the fibrotic stimulus is administered may help explain the augmented fibrosis we observe in the lungs of mice infected for 45 or fewer days before challenge because adoptive transfer of fibrocytes has previously been shown to augment FITC-induced fibrotic responses (34). Interestingly, we observed that the presence of latent γHV-68 augmented the fibrotic response to FITC that was instilled 70 d.p.i., although fibrocyte numbers were not increased at this time point in response to virus alone. It is important to note that although total fibrocytes were not elevated at 70 d.p.i., other inflammatory cells were present in increased numbers at this time (Figure E5). Thus, it may be that the augmentation induced in the setting of long-term latency proceeds via different mechanisms than the augmentation induced in the context of short-term latency. Potential mechanisms that may explain enhanced fibrosis during long-term latency include production of profibrotic mediators by inflammatory cells or AECs. Additionally, viral reactivation may be more important during long-term latency than short-term latency.
We were not surprised to find that levels of secreted CCL2 and CCL12, chemokines responsible for fibrocyte recruitment, are increased in the lungs beyond the clearance of lytic γHV-68 infection. Our data agree with Weinberg and colleagues who have previously shown that CCL2 transcript levels are increased for at least 44 days in the lungs after γHV-68 infection (25), and also with our previous findings that CCL12 gene expression is up-regulated during lytic γHV-68 infection (11). CCL2 is also known to up-regulate the production of collagen by fibrocytes (22). CCL2 expression is increased in the lungs of patients with IPF, and CCL12, a murine homolog of human CCL2, is the CCR2 ligand most responsible for fibrocyte recruitment and fibroproliferation in mice (34, 35). These data suggest that the up-regulation of chemokines is one mechanism by which infection with γHV-68 may lead to enhanced accumulation of fibrocytes and increased extracellular matrix deposition by fibrocytes. It is not yet clear whether the enhanced accumulation of fibrocytes in the lungs of latently infected mice results from fibrocyte recruitment or in situ proliferation. We attempted to stain for fibrocytes in the circulation of mice 12 d.p.i. However, we could not detect collagen 1+ cells at levels above the irrelevant control Ab in circulation (data not shown). In contrast, Figure 8 shows that we can readily detect these cells in lung digests. This likely reflects the low levels of collagen 1 that are expressed by fibrocytes in circulation as compared with when they enter the tissue. Additional experiments will be needed to clarify whether the fibrocyte accumulation results from recruitment, proliferation, or both. Although the viral infection alone is able to induce fibrocyte accumulation in the lung, it is likely that maximal differentiation of those fibrocytes to myofibroblasts may require factors present only in the setting of the “second hit” (e.g., the further induction of TGF-β1 or epithelial damage by FITC).
The patchy accumulation of inflammatory cells within the lungs of latently infected mice treated with FITC suggested focal changes in the lung that might be responsible for the increased fibrosis. We speculated that this might be the result of focal areas of infection of lung AECs. Our results confirm that AECs are a reservoir for latent γHV-68 infection in the lung and that latent infection alters the secretion of profibrotic factors by these AECs. We isolated AECs at 14 and 21 d.p.i. The level of M3 gene expression declines in AECs isolated during the third week after infection when compared with AECs isolated 2 weeks post infection. The decline could represent death of infected AECs in vivo or possible differentiation to fibroblasts via epithelial to mesenchymal transition (36). It could also be indicative of a gradual silencing of viral gene expression at later time points during latency. Our data agree with another study that demonstrates AECs to be a reservoir of latent γHV-68 for at least 54 d.p.i. in vivo (26). EBV has previously been localized to AECs of patients with IPF and has been associated with endoplasmic reticulum stress, the unfolded protein response, and a worse prognosis (37, 38). Specifically, the expression of EBV latent membrane protein 1 in AECs is associated with poor prognosis (39).
We hypothesized that latent γHV-68 alters the AEC phenotype to contribute to the augmentation of fibrocyte numbers, inflammation, and TGF-β1 we observed in the whole lungs. Our results demonstrate that latent infection of AECs induces transcription of CCL2 and CCL12 mRNA, production of more TGF-β1, and production of more cysLTs. Data collected by Antoniades and colleagues and Mercer and colleagues corroborate our findings by showing AECs are a prominent source of CCL2 during pulmonary fibrosis (35, 40). We observed a delay in the induction of CCL2 gene expression until 21 d.p.i. in AECs; however, total CCL2 mRNA levels in the lungs of latently infected mice were elevated by 15 d.p.i. Thus, much of the CCL2 gene expression in the lung during this early period after infection may be contributed by inflammatory cells rather than AECs. We also found that AECs harvested from latently infected mice produce significantly more total TGF-β1 than AECs from mock-infected mice. In addition, we were able to use mink lung epithelial cells stably transfected with a reporter construct encoding a luciferase gene with a PAI1 promoter to demonstrate that active TGF-β1 was being produced directly by AECs after γHV-68 infection. We were unable to study latent infection in vitro because the virus eventually destroyed these cells via lytic replication during the second week of culture. Our findings are consistent with the studies of Malizia and colleagues who found that AEC injury with EBV up-regulates TGF-β1 expression in a human cell line (38). TGF-β1 is the most powerful known promoter of extracellular matrix secretion and a number of other fibrogenic processes (41, 42), and we expect that γHV-68–induced TGF-β1 production is one mechanism that contributes to the augmentation of fibrosis in our model.
We also demonstrated that AECs harvested from the lungs of latently infected mice produce significantly more cysLTs than AECs harvested from the lungs of mock-infected mice. This is the first description of the ability of AECs to produce LTs. CysLT levels are elevated in patients with IPF (27), and LT-deficient mice are protected from experimental fibrosis (28, 29). It is possible that the increased cysLT production in AECs induces the secretion of TGF-β1. Leukotriene D4 has been shown to induce TGF-β1 production in human bronchial epithelial cells (43). The production of cysLTs by latently infected AECs is likely to promote fibrosis via effects on mesenchymal cells. We have previously reported that cysLTs can cause fibrocytes to proliferate (29). Additionally, cysLTs are known to induce fibroblast proliferation (44) and collagen secretion (45).
In sum, our model provides evidence that a latent γherpesvirus infection can act as a cofactor in the development of subsequent pulmonary fibrosis, and our results demonstrate for the first time that short-term or long-term latency significantly augments a subsequent fibrotic stimulus in immunocompetent mice even if the stimulus is sub-threshold. Moreover, our results are the first to identify potential mechanisms whereby latent γherpesvirus infection can augment subsequent fibrotic responses. We interpret our findings to suggest that the development of fibrosis in this model requires at least two hits. The first hit may involve the enhanced inflammatory response and alteration of AECs to promote a profibrotic milieu. The enhanced production of chemokines, TGF-β1, and cysLTs by latently infected AECs may then drive fibrotic responses via direct effects on mesenchymal cells. Figure 15 provides a hypothetical model for how latently infected AECs may promote fibrogenesis. These effects would be further amplified and/or may become more persistent in the setting of a second hit with a fibrotic or sub-threshold fibrotic stimulus.
Figure 15.
Hypothesized focal changes induced by latently infected alveolar epithelial cells (AECs) that promote fibrosis. Schematic shows changes in the lung microenvironment around a latently infected AEC that are hypothesized on the basis of our data. Future experiments are necessary to fully confirm this hypothesis. TGF = transforming growth factor.
Clinical data regarding an association of IPF with EBV in particular are controversial. Because up to 95% of the population harbors EBV in circulating B cells, the studies that are the most informative look at lung tissue rather than blood. Several studies have found evidence of EBV infection in the lungs of patients with IPF at levels significantly higher than control patients (2–5). These data suggest that EBV may be preferentially maintained in the lungs of patients who develop IPF. A different study suggested that presence of the EBV latent membrane protein 1 correlated with more rapid progression of IPF (39). However, two other studies found no evidence of EBV in archived IPF tissue sections (6, 7). Thus, it is not clear whether the discrepancies in the human literature represent technical differences, geographical differences, disease heterogeneity, or perhaps smaller sample size in the negative studies. Although γHV-68 infection is not identical to infection with EBV or HHV-8, and FITC inoculation does not recapitulate all aspects of IPF fibrogenesis, this model can be used to better understand particular cell types or profibrotic factors critical to virus-induced augmentation of fibrosis. The studies by Mora and colleagues in Th2-biased mice suggested that antiviral therapies may improve fibrotic outcomes by limiting viral reactivation (13). In contrast, our studies suggest that viral reactivation is not critical to the augmentation of fibrosis in wild-type mice at least during early latency; thus, antiviral drugs may not be as effective as previously hoped. A more fruitful area for therapeutic investigation may be to try to find mechanistic pathways that are similar in both lytic and latent viral exacerbation of fibrosis and exploit these common pathways for therapeutic intervention.
Supplementary Material
Acknowledgments
The authors thank Dr. Thomas A. Moore for help with the gating and analysis of flow cytometry samples.
Supported by NIH grants HL071586 (G.B.T. and B.B.M.), AI065543 (B.B.M.), CA103632 (L.F.v.D.), and a research gift from the Martin Edward Galvin fund for pulmonary fibrosis research. K.M.V. was supported by the Herman and Dorothy Miller Fund.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: K.M.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.R.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.F.v.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.B.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.B.M. has received consultancy fees from Centecor and Pfizer for up to $1,000 and an industry-sponsored grant from Centecor ($100,001 or more).
References
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Lok SS, Stewart JP, Kelly BG, Hasleton PS, Egan JJ. Epstein-Barr virus and wild p53 in idiopathic pulmonary fibrosis. Respir Med 2001;95:787–791. [DOI] [PubMed] [Google Scholar]
- 3.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513. [DOI] [PubMed] [Google Scholar]
- 5.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. [DOI] [PubMed] [Google Scholar]
- 6.Wangoo A, Shaw RJ, Diss TC, Farrell PJ, du Bois RM, Nicholson AG. Cryptogenic fibrosing alveolitis: lack of association with Epstein-Barr virus infection. Thorax 1997;52:888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamo A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, Chilosi M. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:123–128. [PubMed] [Google Scholar]
- 8.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 1990;71:1365–1372. [DOI] [PubMed] [Google Scholar]
- 9.Lok SS, Haider Y, Howell D, Stewart JP, Hasleton PS, Egan JJ. Murine gammaherpes virus as a cofactor in the development of pulmonary fibrosis in bleomycin resistant mice. Eur Respir J 2002;20:1228–1232. [DOI] [PubMed] [Google Scholar]
- 10.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham K, Stecenko AA. Lung Infection with gamma herpesvirus induces progressive pulmonary fibrosis in Th2 biased mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L711–L721. [DOI] [PubMed] [Google Scholar]
- 11.McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 2008;177:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 2006;35:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am J Respir Crit Care Med 2007;175:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol 2006;1:375–404. [DOI] [PubMed] [Google Scholar]
- 15.van Dyk, LF, Virgin HW IV, Speck SH. The murine gammaherpesvirus 68 v–cyclin is a critical regulator of reactivation from latency. J Virol 2000;74:7451–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballinger M, Aronoff D, McMillan T, Cooke K, Olkiewicz K, Toews G, Peters-Golden M, Moore B. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 2006;177:5499–5508. [DOI] [PubMed] [Google Scholar]
- 17.Moore BB, Paine R III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 18.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB. Protection from fluorescein isothiocyanate-induced fibrosis in IL13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 2004;172:4068–4076. [DOI] [PubMed] [Google Scholar]
- 19.Charbeneau RP, Christensen PJ, Chrisman CJ, Paine R III, Toews GB, Peters-Golden M, Moore BB. Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/− mice: implications for fibroproliferation. Am J Physiol Lung Cell Mol Physiol 2003;284:L1103–L1111. [DOI] [PubMed] [Google Scholar]
- 20.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 1996;14:309–315. [DOI] [PubMed] [Google Scholar]
- 21.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C–C chemokines in fibrotic lung disease. J Leukoc Biol 1995;57:782–787. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology 2008;380:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg JB, Lutzke ML, Efstathiou S, Kunkel SL, Rochford R. Elevated chemokine responses are maintained in lungs after clearance of viral infection. J Virol 2002;76:10518–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med 1998;187:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest 1996;97:1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters-Golden M, Bailie M, Marshall T, Wilke C, Phan SH, Toews G, Moore B. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med 2002;165:229–235. [DOI] [PubMed] [Google Scholar]
- 29.Vannella KM, McMillan TR, Charbeneau RP, Wilke CA, Thomas PE, Toews GB, Peters-Golden M, Moore BB. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol 2007;179:7883–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol 1992;73:2347–2356. [DOI] [PubMed] [Google Scholar]
- 31.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med 1996;184:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flano E, Kim IJ, Moore J, Woodland DL, Blackman MA. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J Immunol 2003;170:3828–3834. [DOI] [PubMed] [Google Scholar]
- 33.Lee K, Groshong S, Cool C, Kleinschmidt-DeMasters B, v. Dyk L. Murine gammaherpesvirus 68 infection of IFNγ responsive mice: a small animal model for gammaherpesvirus-associated B cell lymophoproliferative disease. Cancer Res 2009;69:5481–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 1992;89:5371–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malizia AP, Lacey N, Walls D, Egan JJ, Doran PP. CUX1/Wnt signaling regulates epithelial mesenchymal transition in EBV infected epithelial cells. Exp Cell Res 2009;315:1819–1831. [DOI] [PubMed] [Google Scholar]
- 37.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008;294:L1119–L1126. [DOI] [PubMed] [Google Scholar]
- 38.Malizia AP, Keating DT, Smith SM, Walls D, Doran PP, Egan JJ. Alveolar epithelial cell injury with Epstein-Barr virus upregulates TGF-beta1 expression. Am J Physiol Lung Cell Mol Physiol 2008;295:L451–L460. [DOI] [PubMed] [Google Scholar]
- 39.Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax 2000;55:958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Konigshoff M, Howell DC, McAnulty RJ, Das A, Thorley AJ, Tetley TD, et al. The pulmonary epithelium is a prominent source of PAR1- inducible CCL2 in pulmonary fibrosis. Am J Respir Crit Care Med 2008;179:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla A, Meisler N, Cutroneo KR. Perspective article: transforming growth factor-beta: crossroad of glucocorticoid and bleomycin regulation of collagen synthesis in lung fibroblasts. Wound Repair Regen 1999;7:133–140. [DOI] [PubMed] [Google Scholar]
- 42.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308–319. [DOI] [PubMed] [Google Scholar]
- 43.Bosse Y, Thompson C, McMahon S, Dubois CM, Stankova J, Rola-Pleszczynski M. Leukotriene D4-induced, epithelial cell-derived transforming growth factor beta1 in human bronchial smooth muscle cell proliferation. Clin Exp Allergy 2008;38:113–121. [DOI] [PubMed] [Google Scholar]
- 44.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol 1987;138:1190–1195. [PubMed] [Google Scholar]
- 45.Phan SH, McGarry B, Loeffler K, Kunkel S. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry 1988;27:2846–2853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.