Abstract
We previously reported in an ovalbumin-induced model of allergic asthma that Fms-like tyrosine kinase 3 ligand (Flt3-L) reversed airway hyperresponsiveness (AHR) and airway inflammation, and increased the number of regulatory CD11chighCD8αhighCD11blow dendritic cells in the lung. In this study, we investigated the effect of Flt3-L in a clinically relevant aeroallergen-induced asthma on the phenotypic expression of lung T cells. Balb/c mice were sensitized and challenged with cockroach antigen (CRA), and AHR to methacholine was established. These mice received three intraperitoneal injections of anti-CD25 antibody (PC61; 250 μg) and Flt3-L (3 μg) daily for 10 days. Cytokines and Ig levels in the serum were measured and differential bronchoalveolar lavage fluid (BALF) cell counts were examined. Flt3-L reversed AHR to methacholine to the control level. Flt3-L significantly decreased levels of BALF IL-5, IFN-γ, eosinophilia and substantially increased IL-10 and the number of CD4+CD25+ Forkhead winged helix transcription factor box P3 (Foxp3+) IL-10+ T cells in the lung. Administration of PC61 antibody blocked the effect of Flt3-L and substantially increased AHR, eosinophilia, and BALF IL-5 and IFN-γ levels, and decreased BALF IL-10 levels and the number of CD4+CD25+Foxp3+IL-10+ T cells. Flt3-L significantly decreased CD62-L, but increased inducible costimulatory molecule and Foxp3 mRNA expression in the CD4+CD25+ T cells isolated from lungs of Flt3-L–treated, CRA-sensitized mice compared to CRA-sensitized mice without Flt3-L treatment and PBS control group. Flt3-L significantly inhibited the effect of CRA sensitization and challenge to increase GATA3 expression in lung CD4+CD25+ T cells. Collectively, these data suggest that the therapeutic effect of Flt3-L is mediated by increased density of naturally occurring CD4+CD25+Foxp3+IL-10+ICOS+ T-regulatory cells in the lung. Flt3-L could be a therapeutic strategy for the management and prevention of allergic asthma.
Keywords: airway hyperresponsiveness, anti-CD25 antibody, Fms-like tyrosine kinase 3 ligand, Forkhead winged helix transcription factor box P3, naturally occurring CD4+CD25+ T-regulatory cells
CLINICAL RELEVANCE.
Fms-like tyrosine kinase 3 ligand could prove to be a novel mediator in controlling clinically-relevant allergen-induced immune response by increasing the density of CD4+CD25+Foxp3+ICOS+IL-10+ T-regulatory cells in asthmatic lung.
Asthma is a disease of the lungs characterized by reversible airway obstruction, airway hyperresponsiveness (AHR), tissue and blood eosinophilia, mucus hypersecretion, and chronic airway inflammation (1). Autopsies of subjects who died of severe acute asthma attacks have indicated the presence of lymphocyte infiltration in the lungs, suggesting that lymphocytes play an important role in the pathogenesis of asthma (2). Increased numbers of activated CD4+ T lymphocytes have been found in asthmatic airways (3). T helper (Th) 2 cells deliver a potent cytokine milieu that initiates the onset of the disease. However, Th1 cells are the counterweights to the Th2 cells for immune balance, and they have been shown to exacerbate allergic asthma (4–6). Nonetheless, the Th2 cell is the dominant player in the progression and pathological changes of asthma (4, 7, 8). There are a plethora of environmental antigens that trigger the development of Th2 cells. The cockroach antigen (CRA), which is a composition of the insects' feces, exoskeleton, and saliva, is categorized as a hazardous environmental antigen that can elicit a Th2-polarized response. The current treatments being used for this inflammatory disorder are antihistamines, leukotriene blockers, and glucocorticoids.
Fms-like tyrosine kinase 3 (Flt3) is identical to fetal liver kinase 2 (Flk2) and is a member of the class III tyrosine kinase receptor family. The murine Flt3 or Flk2 was independently cloned by two groups of investigators. Flt3 was cloned from murine placenta based upon its similar sequence homology to c-fms (9). The c-fms (cellular) oncogene is a homolog of v-fms (viral) oncogene, which was originally encoded by the Susan McDonough strain of feline sarcoma virus (10). The human homolog of murine Flt3 gene was also cloned (11–13), and found to be expressed in CD34+ progenitor cells and in some leukemic cells (12). The murine and human ligands (Flt3-L) for the Flt3/Flk2 receptor were cloned, and demonstrated to share structural similarities with c-kit-L and M-CSF-L (14, 15). In vivo treatment of mice with Flt3-L results in a significant increase of dendritic cells (DCs) in all primary and secondary lymphoid tissues (16), and, in humans, it induces both CD11c+ and CD11c− subsets (17). The development of distinct populations of DCs by Flt3-L suggests that there is a regulation of the Th1/Th2 cell profile in allergic asthma, and this action maybe by the induction of CD4+ CD25+ T-regulatory cells (Tregs).
Naturally occurring CD4+CD25+ Tregs (NTregs) play an active role in establishing and maintaining immunological unresponsiveness to self-constituents and negative control of various immune responses to non–self-antigens (18). The concept of Tregs for immunologists is certainly not a new idea, but could be a plausible therapeutic target for allergy and asthma. Immune surveillance by Tregs is critical to modulate T cell response to maintain immune homeostasis, and in orchestrating immunologic tolerance (19, 20). NTregs constitutively express CD25, the α-chain of the IL-2 receptor complex (21, 22), and it is postulated that they suppress effector T cells by a cell-to-cell–dependent mechanism. Understanding the underlying mechanisms of Treg modulation remains elusive, and further studies are warranted to provide insight as to how Tregs develop and suppress effector T cells.
Recently, we reported that treatment with Flt3-L could prevent the progression and reverse asthma in an ovalbumin (OVA)-induced mouse model of allergic asthma, resulting in the complete abolition of AHR to methacholine (23). In addition, we found that OVA-sensitized mice treated with Flt3-L increased the number of regulatory CD11chighCD8αhighCD11blow DCs in the lung (23). In this study, we investigated the effect of Flt3-L in cockroach-sensitized and -challenged mice, and the phenotypic expression of T cells. Involvement of CD4+CD25+ Tregs in the therapeutic effect of Flt3-L was examined by using anti-CD25 antibody (PC61). Flt3-L reversed existing AHR and airway inflammation, and caused a substantial increase of CD4+CD25+ Forkhead winged helix transcription factor box P3 (Foxp3+) inducible costimulatory molecule (ICOS+) IL-10+ Tregs in the lung. The beneficial effect of Flt3-L was blocked by PC61.
MATERIALS AND METHODS
Animals
Female Balb/c mice (4–5 wk old) were purchased from Harlan Laboratories (Indianapolis, IN), and were housed in separate cages. Food and water were provided ad libitum. In accordance with the National Institutes of Health guidelines, the research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University (Omaha, NE).
Sensitization, Treatment, and Pulmonary Function
Allergic airway inflammation was induced by intraperitoneal injection of 10 μg cockroach mix allergenic extract (CRA; Hollister-Stier, Spokane, WA) emulsified in 2.25 mg imject alum (Pierce, Rockford, IL) in a total volume of 100 μl on Days 0 and 14, followed by aerosol sensitization with 1% CRA for 30 minutes using an Aeroneb Pro nebulizer (Aerogen, Somerset, PA) on Days 28, 29, and 30. Mice were again challenged with 5% CRA for 30 minutes on Day 32, and pulmonary functions were measured 24 hours later to establish AHR to methacholine (data not shown). Starting on Day 33, CRA-sensitized mice were randomized into five groups: the first experimental group received 3 μg intraperitoneal Flt3-L (Peprotech, Inc., Rocky Hill, NJ) treatment for 10 days; the second group received 3 μg intraperitoneal Flt3-L in sterile PBS for 10 days with 250 μg of PC61 three times on Days 34, 38, and 42; the third group received 3 μg intraperitoneal Flt3-L in sterile PBS for 10 days without PC61; the fourth group received 250 μg intraperitoneal PC61 three times on Days 34, 38, and 42; and the fifth group of nonsensitized control mice were sham treated with the vehicle (sterile PBS). On Day 43, all groups of mice were challenged with 5% aerosolized CRA, and nonsensitized control mice were treated only with the vehicle (sterile PBS). On Day 44, AHR to methacholine was measured using whole-body plethysmography (Buxco Electronics, Troy, NY), and the data reported in enhanced pause (Penh) values (23). On Day 45, AHR to methacholine was measured with the invasive tracheotomy method to measure specific airway resistance, followed by collection of bronchoalveolar lavage fluid (BALF), blood, lungs, and spleen.
Tissue Preparation and Isolation of Tregs
To isolate CD4+ CD25+ Tregs, after the 45-day protocol, mice were killed and lungs were harvested. These tissues were cut into fragments and digested with collagenase-D (1 mg/ml; Roche Applied Science, Indianapolis, IN) and 5 ml of RPMI 1,640 (Cambrex, East Rutherford, NJ). The samples were incubated at 37°C in a CO2 incubator for 60 minutes. Tissues were disrupted using a 1-ml syringe, and red blood cells were removed from the suspension using Tris-buffered ammonium chloride solution and neutralized with PBS4 solution. Tregs were isolated with CD4+ microbead kit (Miltenyi Biotec, Auburn, CA) and sorted by AutoMACS (Miltenyi Biotec). The sorted cells were labeled with conjugated allophycocyanin (APC) CD25 antibody and analyzed by flow cytometry.
Flow Cytometry and Antibodies
A FACScan (Becton Dickinson, San Jose, CA) was used for analytical Flow cytometry and data were processed with CellQuest Pro (Becton Dickinson) (5) using a standard protocol for cell preparation. Cells were stained with: peridinin chlorophyll protein (PerCP) CD4 (L3T4), phycoerythrin (PE) CD69 (H12F3), FITC CD62L (MEL-14), and APC ICOS (7e.17G9), purchased from BD Pharmingen (San Diego, CA), and PE CD25 (BC96), purchased from eBioscience (San Diego, CA). In addition, cells were also stained with FITC anti-mouse/rat Foxp3 using a staining kit (eBioscience).
Serum IgE Analysis
On Day 44, serum was collected, then centrifuged, separated, and stored at −80°C for later analysis. ELISA for total IgE was conducted as previously described (24) and according to the manufacturer's recommendations using rat anti-mouse IgE (BD Pharmingen), IgE standard (BD Pharmingen), and rat anti-mouse IgE–horseradish peroxidase (HRP; Southern Biotechnology Associates, Birmingham, AL) for the total IgE assay. IgE assays were developed with 3,3′,5,5′-tetramethylbenzidine substrate and read at 450 nm using a Bio-Rad microplate reader and software (Bio-Rad, Hercules, CA). Sensitivity for total IgE was 1 ng/ml.
Serum and Anti-CRA IgE and IgGs Analysis
Serum levels of anti-CRA IgE and IgGs were determined as previously described (25). Briefly, microtiter plates were coated with 100 μg/ml CRA. The coated plates were washed several times with PBS containing 0.05% Tween (PBS-T) and blocked with 10% FBS for 2 hours at 37°C. Diluted serum was incubated in duplicates overnight, washed with PBS-T, incubated with anti-mouse avidin conjugates (IgE, IgG1, or IgG2a; BD Pharmingen) for 2 hours, and again washed several times with PBS-T. Assays were developed with tetramethylbenzidine (TMB) substrate reagent (BD Pharmingen), and reactions were stopped with 2 N H2SO4 and read at 450 nm using a Bio-Rad microplate reader and software.
BALF
BALF was collected from each animal via cannulation of the exposed trachea and gently flushing the lungs with 1 ml of 37°C sterile PBS. Total cell counts were determined by counting the cells in a hemocytometer. BALF was centrifuged, and the supernatant was collected and immediately frozen for later cytokine measurement. Cells were suspended in sterile PBS and cytospin slides were stained (Hema 3 stain set; Biochemical Sciences, Inc., Swedesboro, NJ) for differential counts.
Cytokine Assays
BALF cytokines (IL-4, IL-5, IFN-γ, and IL-10) were measured by ELISA. Antibody pairs for individual cytokines, as well as standards, were used according to the manufacturer's recommendations (BD Pharmingen) with streptavidin–HRP and 3,3′, 5,5′-tetramethyl benzidine (Sigma-Aldrich, St. Louis, MO). The sensitivity of the assays was 3–5 pg/ml.
Secretion of IL-10 by CD4+CD25+ T Cells from Flt3-L–Treated Mice
IL-10 secreted by CD4+CD25+ T cells was examined by ELISPOT (eBioscience, San Diego, CA). Isolated and purified Tregs from the lungs of each experimental group were sorted by BDFACS Aria (Becton Dickinson) with a purity of 99.0%. These cells were seeded in equal densities of 1.5 × 104 per well in sterile polyvinylidene fluoride microtitered plates, precoated with anti–IL-10 antibody (Millipore, Temecula, CA), and cultured for 48 hours at 37°C in a 5% CO2 incubator. Biotinylated detection antibody was added and incubated for 2 hours, then thoroughly washed, followed by the addition of the avidin–HRP reagent and incubated for an additional 45 minutes. Plates were air dried for counting with a dissecting microscope.
RT-PCR
To analyze the expression of Foxp3, the mRNA was prepared from isolated Tregs using Trizol (Sigma-Aldrich) reagent protocol. Gene Amp PCR System 2,400 (Perkin Elmer, Waltham, MA) was used at 35 cycles for Foxp3, 31 cycles for GATA3, and 29 cycles for hypoxanthine phosphoribosyltransferase (HPRT). Primers were as follows: Foxp3, forward, 5′-TACACCCAGGAAAGACAGCAACCT-3′, reverse, 5′-TCT GAAGTAGGCGAACATGCGAGT-3′ (melting point [Tm], 55°C); GATA-3, forward, 5′-AGGCAAGAT GAGAAAGAGTGCCTC-3′, reverse, 5′-CTCGACTTACATCCGAACCCGGTA-3′ (Tm, 55.0°C); and HPRT, forward, 5′-GATACAGGCCAGACTTTGTTG-3′, reverse, 5′-GGTAGGCTGGCCTATAGGCT-3′ (Tm, 50°C).
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA) statistical analysis and graphing software. Unpaired Student's t test was used to determine differences between two groups. Multiple group comparison was made using ANOVA. A P value of less than 0.05 was considered significant.
RESULTS
Assessment of AHR to Methacholine in Cockroach-Sensitized and -Challenged Mice after Flt3-L Treatment
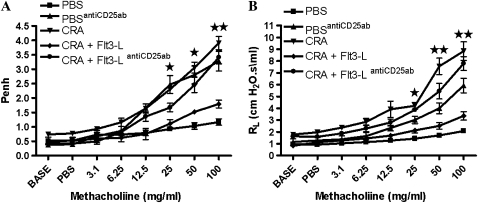
Before the treatment with Flt3-L or PBS, CRA-sensitized and -challenged mice had established AHR to methacholine (data not shown). Treatment with Flt3-L induced a significant reduction in AHR to methacholine to levels comparable to PBS-treated mice (Figure 1A). This was confirmed by measuring specific airway resistance in tracheostomized mice (Figure 1B). The therapeutic effect of Flt3-L was lost after the administration of PC61 in CRA-sensitized and -challenged mice (Figure 1). Administration of PC61 in PBS control mice increased AHR to methacholine (Figure 1).
Figure 1.
Airway hyperresponsiveness (AHR) to methacholine. (A) On Day 33, AHR to methacholine (Mch) was established, followed by 10-day treatment with Fms-like tyrosine kinase 3 ligand (Flt3-L; 3 μg/d, intraperitoneal). On Day 44 AHR to Mch was measured, and enhanced pause (Penh) values were recorded. (B) On Day 45, airway resistance (RL) to Mch was recorded. Data are shown as mean (±SEM) for six mice in each group (*P < 0.01; **P < 0.001).
Effect of Flt3-L treatment on total and differential cells in the BALF.
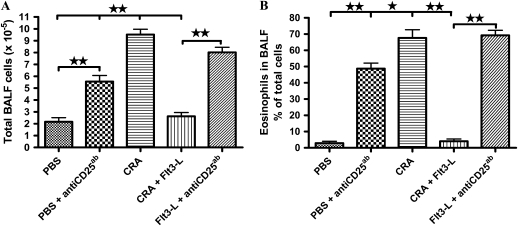
Sensitization and challenge of mice with CRA significantly increased the total number of cells in the BALF with a predominant increase in eosinophils. Treatment with Flt3-L significantly decreased the total number of cells and eosinophils in the BALF (Figure 2). PBS control and Flt3-L–treated mice that were administered PC61 exhibited substantial increase in the number of total cells and eosinophils in the BALF (Figure 2).
Figure 2.
Bronchoalveolar lavage fluid (BALF) and cellularity. Recovered total cells in the BALF were counted, and differential analysis was performed using standard morphological criteria. A total of 300 cells were examined per cytospin slide, and absolute cell numbers were calculated per milliliter of the BALF based on the percentage of individual cells in a slide. Data are shown as mean (±SEM) for six animals in each group (**P < 0.001).
Effect of Flt3-L treatment on serum Ig in cockroach-presensitized and -challenged mice.
Sensitization and challenge of mice with CRA significantly increased the levels of serum total IgE, anti-CRA IgE, anti-CRA IgG2a, and anti-CRA IgG1 compared with PBS control. Interestingly, Flt3-L did not decrease the levels of total serum or anti-CRA IgE. In addition, there was no significant effect of Flt3-L on serum IgG1 or IgG2 (Table 1).
TABLE 1.
SERUM Ig LEVELS
| PBS | CRA | CRA + Flt3-L | |
|---|---|---|---|
| Anti-CRA IgG2a | 0.001 ± 0.005 | 0.086 ± 0.015* | 0.084 ± 0.023* |
| Anti-CRA IgG1 | 0.006 ± 0.004 | 0.404 ± 0.039* | 0.500 ± 0.040* |
| Total IgE | 15.224 ± 4.176 | 69.62 ± 7.011† | 52.187 ± 4.535 |
| Anti-CRA IgE | 0.036 ± 0.014 | 0.276 ± 0.062* | 0.352 ± 0.086* |
Definition of abbreviations: CRA, cockroach antigen; Flt, Fms-like tyrosine kinase.
P < 0.01.
P < 0.001.
Effect of Flt3-L treatment on BALF cytokines in cockroach-sensitized and -challenged mice.
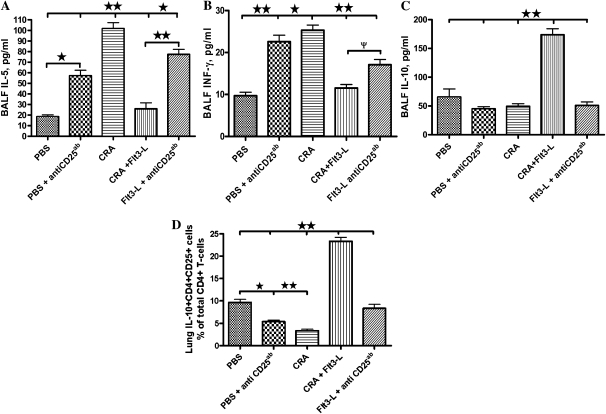
Sensitization and challenge of mice with CRA significantly increased the levels of BALF IL-5 and decreased IL-10. In contrast, treatment with Flt3-L significantly lowered the BALF IL-5 and increased IL-10 levels. In addition, BALF INF-γ levels were elevated in CRA-sensitized and -challenged mice. However, Flt3-L treatment lowered BALF INF-γ (Figures 3A–3C). Administration of PC61 in Flt3-L–treated or control PBS-treated mice blocked the effect of Flt3-L, increased IL-5 and IFN-γ, and decreased IL-10 levels in the BALF. IL-4 and IL-13 were not detectable in the BALF (data not shown).
Figure 3.
BALF cytokine level and IL-10–secreting CD4+CD25+ T-regulatory cells. BALF samples were immediately centrifuged and level of cytokines, IL-5 (A), IFNγ (B), and IL-10 (C), in the supernatants was measured. Data are shown as means (±SEM) for six animals in each group (*P < 0.01 and **P < 0.001). (D) CD4+CD25+ T cells were isolated, cultured, and plated on ELISPOT plates to evaluate expression of IL-10. Data are shown as means (±SEM) for five animals in each group (**P < 0.001).
Effect of Flt3-L and PC61 on CD4+CD25+ T cells secreting IL-10 in cockroach-sensitized and -challenged mice.
Adaptive or inducible CD4+CD25− Tregs suppress effector T cells in an IL-10–dependent manner, whereas NTregs mediate suppression in a cell-to-cell manner, and the use of low-to-moderate levels of IL-10 to reverse immune response. We determined the effect of Flt3-L and PC61 administration on CD4+CD25+ T cell isolated from the lung of cockroach-sensitized and -challenged mice and their ability to produce IL-10. We evaluated the number of sorted CD4+CD25+ T cells producing IL-10 from each experimental group using the ELISPOT. Flt3-L significantly increased the number of IL-10–secreting CD4+CD25+ T cells in the lungs (P < 0.001) compared with cockroach-sensitized mice without Flt3-L and the PBS control group (Figure 3D). Although, there was a significant increase in IL-10–secreting CD4+CD25+ T cells from the lungs of Flt3-L–treated mice, only about 23% of the CD4+CD25+ T cells were IL-10 positive (Figure 3D). The therapeutic effect of FLt3-L was blocked after administration of PC61. (Figure 3D).
Effect of Flt3-L on CD4+CD25+ T Cells Isolated from Lungs of Flt3-L–Treated Mice
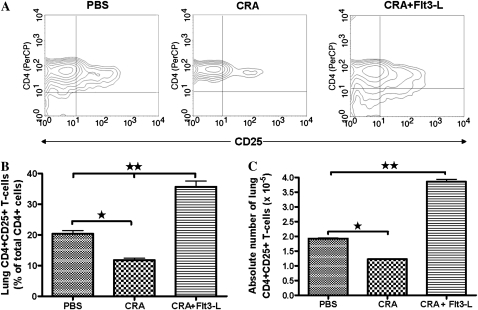
CD4+CD25+ Tregs have suppressive properties and modulate immune response. The expression of CD4+CD25+ Tregs is shown in representative contour plots isolated from the lungs of PBS, CRA, and CRA plus Flt3-L mice (Figure 4A). The absolute number of CD4+CD25+ T cells isolated from the lungs of PBS control mice was normal (Figures 4B and 4C). However, CD4+CD25+ T cells were significantly decreased in the lungs of CRA-sensitized mice compared with PBS (Figures 4B and 4C). There was a substantial increase in CD4+CD25+ T cells isolated from the lungs of Flt3-L–treated mice compared with CRA-sensitized mice and PBS control.
Figure 4.
Expression of CD4+CD25+ T cells. Contour plots show a comparative analysis of CD4+CD25+ expression of T cells isolated from lungs of PBS, cockroach antigen (CRA), and CRA/Flt3-L mice (A). Statistical analysis of the percentage of lung CD4+CD25+ T cells in each experimental group and Flt3-L–treated mice exhibits a significant increase of CD4+CD25+ T cell expression compared with CRA and PBS mice (B). Statistical analysis of the absolute number of lung CD4+CD25+ T cells in each experimental group is shown, and these data paralleled the percentage of CD4+CD25+ T cell (C) (*P < 0.01 and **P < 0.001).
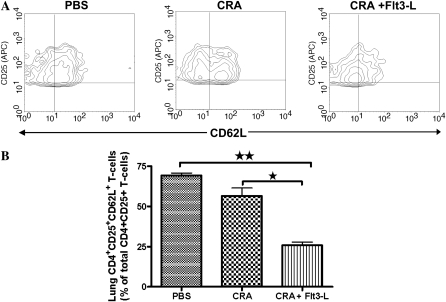
Effect of Flt3-L on the Expression of CD62L in CD4+CD25+ T Cells Isolated from the Lungs of Flt3-L–Treated Mice
The expression of CD62-L on lung CD4+CD25+ Tregs is shown in representative contour plots (Figure 5A). CD4+CD25+ Tregs isolated from the lungs of PBS control mice expressed significantly high levels of L-selectin (CD62L) (Figures 5A and 5B). CRA sensitization and challenge did not significantly affect the expression of CD62L on lung CD4+CD25+ Tregs. However, CD4+CD25+ Tregs isolated from lungs of CRA-sensitized and -challenged mice treated with Flt3-L exhibited a substantial decrease in CD62L expression compared with the CRA group and the PBS control group (Figure 5B).
Figure 5.
Expression of CD62L on CD4+CD25+ T cells. Contour plots showed a comparative analysis of CD62L expression with CD4+CD25+ T cells isolated from lungs of PBS, CRA, and CRA/Flt3-L mice (A). Statistical analysis of CD62L expression of each experimental group and Flt3-L–treated mice exhibits a significant decrease of CD62L expression compared with CRA and PBS mice (B) (*P < 0.01 and **P < 0.001).
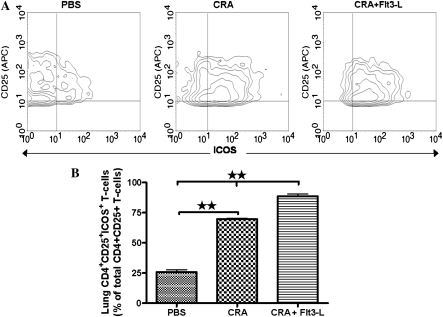
Effect of Flt3-L on the Expression of ICOS in CD4+CD25+ T Cells Isolated from the Lungs of Flt3-L–Treated Mice
The expression of ICOS on lung CD4+CD25+ Tregs is shown in representative contour plots (Figure 6A). CD4+CD25+ Tregs isolated from the lungs of PBS control mice expressed very low levels of ICOS expression (Figures 6A and 6B). CRA sensitization and challenge significantly increased the expression of ICOS on lung CD4+CD25+ Tregs, which was further increased by Flt3-L treatment, expressing substantially higher levels of ICOS than CRA and PBS control mice (Figure 6B).
Figure 6.
Expression of inducible costimulatory molecule (ICOS) on CD4+CD25+ T cells. Contour plots showed a comparative analysis of ICOS expression on CD4+CD25+ T cells isolated from lungs of PBS, CRA, and CRA/Flt3-L (A). Statistical analysis of ICOS expression of each experimental group and Flt3-L–treated mice exhibits a significant increase of ICOS expression compared with CRA and PBS mice (B) (**P < 0.001).
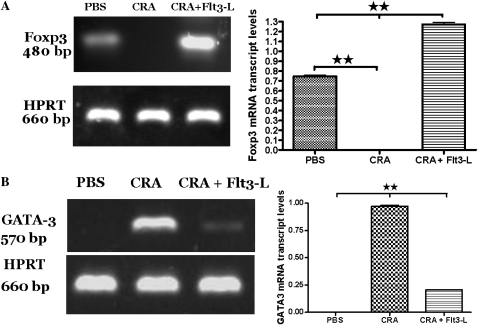
Effect of Flt3-L Treatment on mRNA Expression of Foxp3 and GATA3 in Lung CD4+CD25+ T Cells
Lung CD4+CD25+ T cells isolated from PBS control mice exhibited moderate expression of Foxp3, and no detectable levels of GATA3, which is indicative of a normal distribution of Tregs and the absence of Th2 cells. However, sensitization and challenge with CRA abolished Foxp3 expression, but increased GATA3 expression in the lung CD4+CD25+ T cells. Administration of Flt3-L in cockroach-sensitized and -challenged mice restored Foxp3 expression and significantly suppressed GATA3 expression in the lung CD4+CD25+ T cells, and the expression of Foxp3 was substantially stronger in the lung cells of Flt3-L–treated than PBS control mice (Figures 7A and 7B). This suggests that high expression of Foxp3 in the lung CD4+CD25+ T cells could be required to reduce GATA3 expression and reverse pre-existing AHR and airway inflammation.
Figure 7.
(A and B) The effect of Flt3-L on lung CD4+CD25+ T cells expressing Forkhead winged helix transcription factor box P3 (Foxp3) and GATA3. CD4+CD25+ T cell isolated from the lungs of PBS control mice showed normal expression of Foxp3 mRNA (480 bp) and no detectable level of GATA3 (570 bp). However, cockroach sensitization induced a significant decrease in Foxp3 expression, but substantially increased GATA3 expression in CD4+CD25+ T cell isolated from the lung tissue. Flt3-L caused a significant increase in Foxp3 and decreased expression of GATA3 compared with CRA mice and PBS control group. HPRT (660 bp) was used as the housekeeping gene. Densitometric analyses confirmed the PCR data by showing the ratio of mRNA intensity of Foxp3 and GATA3 by dividing by the intensity of the housekeeping gene, HPRT (**P < 0.001).
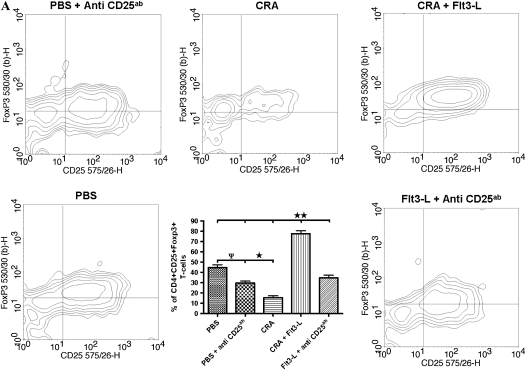
Flow Cytometric Analysis of Foxp3 Expression and Density of CD4+CD25+ T Cells after Flt3-L and PC61 Treatment
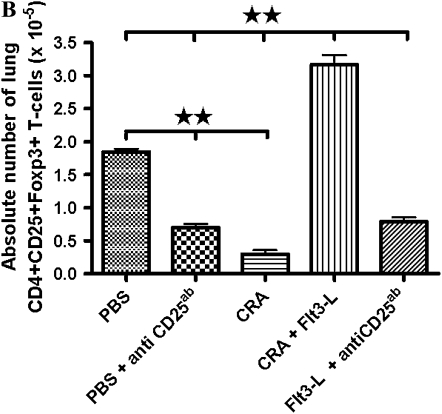
Representative contour plots (Figure 8A) show intracellular Foxp3 expression in the lung CD4+CD25+ T cells of all experimental groups. Foxp3 was normally expressed in the lung CD4+CD25+ cells of PBS control mice, and this was significantly reduced by treatment with PC61 (Figure 8A). CRA sensitization and challenge substantially reduced Foxp3+ cells, which was reversed by treatment with Flt3-L (Figure 8A). The CD25 antibody, PC61, blocked the effect of Flt3-L to increase Foxp3+ cells in the lung of CRA-sensitized and -challenged mice, and there was no significant difference in the number of CD4+CD25+Foxp3+ cells after PC61 administration than in cockroach-sensitized and -challenged mice without Flt3-L (Figure 8B).
Figure 8.
Intracellular protein expression of Foxp3 and the number of lung CD4+CD25+ after Flt3-L and PC61 treatment. (A) Contour plots of CD4+CD25+ T cells that are representative of all five groups. Foxp3 expression was significantly expressed in the PBS control compared with a substantial decrease in the CRA and PBS mice with PC61. Flt3-L–treated mice with PC61 showed significant decrease in Foxp3 on lung CD4+CD25+ T cells; however, Foxp3 was restored after Flt3-L treatment only (*P < 0.01; ΨP < 0.05; **P < 0.001). (B) In cockroach-sensitized and -challenged mice treated with Flt3-L, there was a significantly increased number of lung CD4+CD25+Foxp3+ T cells compared with sensitized mice without Flt3-L. This effect was completely inhibited by PC61, with a significant decrease in the number of lung CD4+CD25+Foxp3+ T cells (** P < 0.001).
DISCUSSION
In this article, we present a cockroach-sensitized and -challenged mouse model of allergic asthma characterized by pulmonary eosinophilia and AHR to methacholine. Previously, we reported that treatment with Flt3-L prevented and reversed the development of allergic airway inflammation and histologic features of asthma, and abolished AHR to methacholine in an OVA-induced mouse model of allergic asthma (26). Here, we present evidence that Flt3-L is capable of modulating immune response to a clinically relevant cockroach-induced murine model of allergic asthma with existing AHR. Furthermore, the therapeutic effect of Flt3-L on AHR was associated with a significant increase in CD4+CD25+ Tregs isolated from the lungs of Flt3-L–treated mice. These CD4+CD25+ Tregs showed a substantial decrease in CD62L expression, and a significant increase in ICOS expression compared with mice that were CRA-sensitized without Flt3-L treatment. The BALF obtained from these Flt3-L–treated mice showed a significant decrease of eosinophilia and IL-5. Interestingly, there was a significant increase of IL-10 in the Flt3-L–treated mice compared with CRA-sensitized mice without Flt3-L therapy and PBS control animals.
A novel finding observed in this study was the substantial decrease in CD62-L expression from CD4+CD25+ T cells isolated from the lungs of Flt3-L–treated mice. Suppression of inflammation and autoimmunity can occur due to Tregs with high expression of CD62L. Ermann and colleagues (27) showed that CD62Lhigh Tregs prevented graft-versus-host disease and slowed the onset of diabetes in the severe combined immunodeficiency (SCID) mouse model. Other groups demonstrated that naive Tregs are able to maintain the expression of CD62L and CCR7 in vitro (28). Our data suggest that Flt3-L induces a decrease in CD62L expression on Tregs to facilitate their migration from lymph nodes to the inflammatory sites in the lung.
CD62L (Mel-14) is a member of the L-selectin family, and is highly expressed on naive CD4+ T cells. However, it is down-regulated upon T cell activation, resulting in the migration of T cells to the inflammatory site (29). Through rolling and adhesion within the circulatory system, naive T cells are decelerated by CD62L binding with peripheral lymph node vascular addressins (GlyCAM-1 and MAdCAM-1) expressed on the face of the lumen of high endothelial venules. Although not examined in this study, stabilization of the CD62L binding is mediated by binding of CCR7, which leads to CD4 T cell transmigration into the lymph node (30). After antigen exposure in the lymph node, there will be a down-regulation of CD62L in T cells and up-regulation of adhesion molecules, CD49d and lymphocyte function-associated antigen (LFA)-1. The change in the expression of adhesion molecules allows the movement to inflammatory tissue (31). The decreased expression of CD62L on CD4+CD25+ Tregs from the lungs of Flt3-L–treated mice suggests that these cells were activated in the lymph nodes and then migrated to inflamed lung tissue. The migration of Tregs with decreased expression of CD62L has been confirmed by other investigators in retinoic acid–treated Tregs (32). CD4+CD25+ Tregs modulate inflammation by cell-to-cell contact and/or the use of the immunosuppressive cytokine, IL-10.
Furthermore, we evaluated, for the first time (to our knowledge), the effect on Flt3-L on Tregs using an anti-CD25 antibody (PC61) in a murine model of cockroach-induced allergic asthma. Many investigators in various disease models have shown that PC61 depletes Tregs in vivo (33–36). In this study, Flt3-L–treated mice given PC61 showed high AHR and airway inflammation; however, administration of Flt3-L alone restored AHR and airway inflammation nearly to the PBS control level. Interestingly, normal healthy mice given PC61 alone challenged with PBS showed elevated AHR and airway inflammation, as indicated by increased BALF cellularity and eosinophils. The elevated AHR and inflammation suggest the loss of the proper immune response, which appears to be due to the lack of proper Treg development during the initial T cell priming events, and, in their absence, probably alters and more uncontrolled T cell population to develop and expand (37). In addition, the administration of PC61 to Flt3-L–treated mice caused a significant reduction of CD4+CD25+Foxp3+ T cells; however, treatment with Flt3-L restores and increases the number of CD4+CD25+Foxp3+ T cells significantly higher than the PBS control. This suggests that more Tregs are needed to reverse the associated changes of established AHR and airway inflammation. Interestingly, the ELISPOT assay of IL-10–secreting Tregs showed nearly 25% of the CD4+CD25+Foxp3+ T cells secreting IL-10. It is tempting to speculate that the 20–25% of Tregs secreting IL-10 could be IL-10–producing CD4+ CD25-type 1 (TR1) inducible Tregs, and the remaining may be NTregs. However, this will warrant further investigation to confirm the two subtypes. Nevertheless, these IL-10–secreting Tregs correlate with the IL-10 found in the BALF of mice treated with Flt3-L.
In this study, BALF IL-10 was elevated in mice treated with Flt3-L. The coexistence of Tregs with aggressive CD4+ T cells is maintained by the secretion of IL-10, which may be regulated by the expression of ICOS. Hutloff and colleagues (38) showed that IL-10 secretion by T cells is modulated by the expression of ICOS. Costimulation by ICOS induces a three- to fivefold increase in IL-10 secretion from ICOS+ CD4+ T cells. Binding of ICOS-L with ICOS strongly promotes IL-10 secretion, whereas the CD28/B7 pathway acts as a potent attenuator of IL-10 release (39). Immunomodulators can reverse allergic airway inflammation, with a concomitant increase in BALF IL-10 and decrease in serum IgE levels; these results strongly correlate with the presence of tolerogenic CD4+ CD25+ T cells in the lung of mouse models (40). In our study, cockroach sensitization and challenge caused a significant elevation in both total and anti–CRA-specific serum Ig (IgE, IgG2a, and IgG1). Interestingly, we found no effect of Flt3-L treatment on serum Ig, and this is consistent with several other immunomodulators, including Mycobacterium bovis–bacillus Calmette-Guerin, which has been reported to exert little-to-no effect on serum IgE (41). Alternatively, it is possible that a 10-day regimen of Flt3-L may not be sufficient to remove previously existing antibodies. In addition, IgE might not be required for the development of eosinophilic airway inflammation and AHR in mice, as reported by several investigators (42).
We found that approximately 23% of the CD4+CD25+ Tregs were positive for IL-10, with the secretion of moderate levels of IL-10 in the BALF of mice treated with Flt3-L. In addition, there was a robust increase in CD4+CD25+ Tregs, and these cells showed substantial increases in mRNA expression for Foxp3; this was correlated well with the expression of Foxp3 in CD4+CD25+ T cells in the lungs of Flt3-L–treated mice. Because inducible CD4+CD25− Tregs release a copious amount of IL-10 in the lung, our data suggest that Flt3-L preferentially increases the density of NTregs, as opposed to the inducible Tregs. In our in vivo model, suppression of AHR and airway inflammation appears to be independent of high levels of IL-10 and inducible Tregs, but dependent on increased number of naturally occurring Tregs and moderate levels of IL-10. Suppression of inflammation in vivo in some models, but not all, requires IL-10 produced by naturally occurring Tregs (43). Sarangi and colleagues (44) found increases in skin lesions in IL-10 knockout mice compared with wild-type mice in a herpes simplex virus model, and skin lesions were worsened in IL-10 knockout mice depleted of naturally occurring Tregs (39). This further supports the notion that the modulation of immune response is dependent on NTregs and moderate levels of IL-10. Interestingly, Foxp3 mRNA expression was absent in CD4+CD25+ T cells from the lungs of CRA-sensitized and -challenged mice without Flt3-L. However, there was significant increase in Foxp3 expression in CD4+CD25+ T cells from the lungs of Flt3-L–treated mice.
The lack of Foxp3 expression and the low number of CD4+CD25+ T cells in the lung of cockroach-sensitized mice without Flt3-L treatment suggest that these cells could be deficient in their density and/or function. In patients with multiple sclerosis, CD4+CD25+ Tregs had significantly reduced levels of Foxp3 expression, which was increased after treatment with a copolymer-1 in these patients (45). Nonetheless, we have found that Flt3-L has the potential to elicit immunomodulatory effects by increasing naturally occurring CD4+CD25+Foxp3+ Tregs. Interestingly, we observed that the CD4+CD25+ T cells found in the mice of CRA-sensitized mice without Flt3-L treatment expressed high levels of GATA3 compared with low levels in Flt3-L–treated mice, and undetectable levels in PBS control CD4+CD25+ T cells. It is likely that these CD4+CD25+GATA3+ T cells are expressing CD25 in transient stages or at low levels (46), and the induction of CD25 is needed for developing Th2 cells (47); however, they lack regulatory capacities, due to the deficiency of Foxp3 expression (48). The high expression of GATA3 and the high level of IL-5 found in the BALF of CRA mice demonstrate that these CD4+CD25+ T cells are Th2 cells. The low expression of GATA3 in the Flt3-L–treated mice suggests that the treatment with Flt3-L may need to go beyond 10 days to completely resolve and clear the number of activated Th2 cells in the airways of subjects with allergic asthma.
Currently, the precise molecular mechanism by which Flt3-L treatment increases naturally occurring CD4+CD25+Foxp3+ Tregs and mediates its anti-inflammatory and antiasthmatic effects remains to be elucidated. Activation of Flt3, the receptor for Flt3-L, induces downstream signaling of Ras/ERK pathway to cause gene expression, possibly via STAT molecules (41). Flt3-L increases proliferation of natural killer cell (NK)-progenitors, which was augmented by IL-2 with a robust increase in NK cells (42). Recently, Burchill and colleagues (49) showed that signaling through IL-2Rβ was dependent on STAT5 activation and translocation to the Foxp3 promoter for development of functional CD4+CD25+Foxp3+ Tregs. This could be a plausible mechanism mediated by Flt3-L signaling, causing the activation of IL-2/IL-2R axis pathway to cause Foxp3 activation in Tregs. Obviously, additional studies are warranted to confirm or refute this theory. Nevertheless, CD4+CD25+ Tregs play an essential role in the maintenance of peripheral self-tolerance by preventing the activation and proliferation of autoreactive T lymphocytes that have escaped thymic deletion (50–52). Flt3-L appears to have a dual effect in modulating immune response by mediating the development and migration of Tregs and regulatory DCs. These specialized immunosuppressive cells appear to have a symbiotic relationship to modulate effector T lymphocyte function both in vitro and in vivo by the use of cell surface membrane proteins and the immunosuppressive cytokine, IL-10. The acceleration and deceleration of T cell and DC interaction is tightly regulated by costimulatory molecules. In this study, increase in the expression of ICOS, a molecule for T cell growth and a possible modulator of IL-10, may help to provide insight to understand the function of Tregs. Costimulatory molecules, such as programmed death (PD)-1 and cytotoxic T-lymphocyte antigen (CTLA)-4, have been shown to be negative regulators of T cell activation. Recently, the molecule, signaling lymphocyte activation molecule, was shown to be another modulator of T cell activation and the maintenance and function of Tregs (47). Clearly, the role of costimulatory molecules is at the forefront of understanding the function of Tregs and how they modulate disease.
In summary, we report a novel murine model of allergic asthma induced by CRA, and the therapeutic effect of Flt3-L in this model. Flt3-L modulated the progression of asthma by abolishing AHR to methacholine, and by causing an increase in naturally occurring CD4+CD25+Foxp3+IL-10+ICOS+ Tregs. In addition, we delineated that Flt3-L with PC61 blocks the development of CD4+CD25+Foxp3+IL-10+ICOS+ Tregs and, in absence of PC61, Flt3-L is able to cause a significant increase in CD4+CD25+Foxp3+IL-10+ICOS+ Tregs to suppress effector T cells, possibly by secretion of IL-10 and cell-to-cell interaction. Understanding the role of Flt3-L and CD4+CD25+Foxp3+ICOS+IL-10+ Tregs warrants further investigation, and it will be interesting to see what effect Flt3-L has on other costimulatory molecules, such as PD-1, CTLA-4, and signaling lymphocyte activation molecule. In conclusion, these results support the hypothesis that Flt3-L may be an effective immunotherapeutic agent in the treatment of AHR and associated changes in airway inflammation in allergic asthma.
This work was supported was supported by National Institutes of Health grant R01HL070885.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0397OC on May 15, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ohtomo T, Miyatake S, Kajiyama Y, Umezu-Goto M, Kobayashi N, Kaminuma O, Mori A. Airway eosinophilic inflammation is attenuated in conserved noncoding sequence-1–deficient mice. Int Arch Allergy Immunol 2008;146:2–6. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan SM. Asthma death, CD8+ T cells, and viruses. Proc Am Thorac Soc 2005;2:162–165. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest 2005;115:1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang-Hoover J, Finn P, Stein-Streilein J. Modulation of ovalbumin-induced airway inflammation and hyperreactivity by tolerogenic APC. J Immunol 2005;175:7117–7124. [DOI] [PubMed] [Google Scholar]
- 5.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol 2003;15:627–633. [DOI] [PubMed] [Google Scholar]
- 6.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS–ICOS–ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 2002;8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 7.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med 2003;9:582–588. [DOI] [PubMed] [Google Scholar]
- 8.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 2001;167:4668–4675. [DOI] [PubMed] [Google Scholar]
- 9.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene 1991;6:1641–1650. [PubMed] [Google Scholar]
- 10.Scheijen B, Griffin JD. Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene 2002;21:3314–3333. [DOI] [PubMed] [Google Scholar]
- 11.Rosnet O, Schiff C, Pebusque MJ, Marchetto S, Tonnelle C, Toiron Y, Birg F, Birnbaum D. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood 1993;82:1110–1119. [PubMed] [Google Scholar]
- 12.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol 2003;171:1216–1223. [DOI] [PubMed] [Google Scholar]
- 13.Namikawa R, Muench MO, Roncarolo MG. Regulatory roles of the ligand for Flk2/Flt3 tyrosine kinase receptor on human hematopoiesis. Stem Cells 1996;14:388–395. [DOI] [PubMed] [Google Scholar]
- 14.Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood 1994;83:2795–2801. [PubMed] [Google Scholar]
- 15.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 1994;368:643–648. [DOI] [PubMed] [Google Scholar]
- 16.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996;184:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 2000;96:878–884. [PubMed] [Google Scholar]
- 18.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531–562. [DOI] [PubMed] [Google Scholar]
- 19.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol 2002;169:4850–4860. [DOI] [PubMed] [Google Scholar]
- 20.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood 2001;98:2736–2744. [DOI] [PubMed] [Google Scholar]
- 21.Levings MK, Sangregorio R, Sartirana C, Moschin AJ, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 2002;196:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164. [PubMed] [Google Scholar]
- 23.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol 2004;172:5016–5023. [DOI] [PubMed] [Google Scholar]
- 24.Dohi M, Tsukamoto S, Nagahori T, Shinagawa K, Saitoh K, Tanaka Y, Kobayashi S, Tanaka R, To Y, Yamamoto K. Noninvasive system for evaluating the allergen-specific airway response in a murine model of asthma. Lab Invest 1999;79:1559–1571. [PubMed] [Google Scholar]
- 25.Saloga J, Renz H, Lack G, Bradley KL, Greenstein JL, Larsen G, Gelfand EW. Development and transfer of immediate cutaneous hypersensitivity in mice exposed to aerosolized antigen. J Clin Invest 1993;91:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal DK, Hopfenspirger MT, Chavez J, Talmadge JE. Flt3 ligand: a novel cytokine prevents allergic asthma in a mouse model. Int Immunopharmacol 2001;1:2081–2089. [DOI] [PubMed] [Google Scholar]
- 27.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood 2005;105:2220–2226. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 2006;108:4260–4267. [DOI] [PubMed] [Google Scholar]
- 29.Waters WR, Rahner TE, Palmer MV, Cheng D, Nonnecke BJ, Whipple DL. Expression of L-selectin (CD62L), CD44, and CD25 on activated bovine T cells. Infect Immun 2003;71:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G Jr, Yang J, Lempicki RA, Sereti I, Lane HC. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol 2003;170:28–32. [DOI] [PubMed] [Google Scholar]
- 31.Gardner LM, Spyroglou L, O'Hehir RE, Rolland JM. Increased allergen concentration enhances IFN-gamma production by allergic donor T cells expressing a peripheral tissue trafficking phenotype. Allergy 2004;59:1308–1317. [DOI] [PubMed] [Google Scholar]
- 32.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 2007;204:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte–associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 2001;194:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaparakis M, Laurie KL, Wijburg O, Pedersen J, Pearse M, van Driel IR, Gleeson PA, Strugnell RA. CD4+ CD25+ regulatory T cells modulate the T-cell and antibody responses in helicobacter-infected BALB/c mice. Infect Immun 2006;74:3519–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeill A, Spittle E, Backstrom BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol 2007;65:63–69. [DOI] [PubMed] [Google Scholar]
- 36.Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Brauer R. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther 2005;7:R291–R301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci USA 2005;102:17418–17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999;397:263–266. [DOI] [PubMed] [Google Scholar]
- 39.Witsch EJ, Peiser M, Hutloff A, Buchner K, Dorner BG, Jonuleit H, Mages HW, Kroczek RA. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol 2002;32:2680–2686. [DOI] [PubMed] [Google Scholar]
- 40.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest 2004;114:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopfenspirger MT, Parr SK, Hopp RJ, Townley RG, Agrawal DK. Mycobacterial antigens attenuate late phase response, airway hyperresponsiveness, and bronchoalveolar lavage eosinophilia in a mouse model of bronchial asthma. Int Immunopharmacol 2001;1:1743–1751. [DOI] [PubMed] [Google Scholar]
- 42.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis–bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med 1998;187:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev 2006;212:60–73. [DOI] [PubMed] [Google Scholar]
- 44.Sarangi PP, Sehrawat S, Suvas S, Rouse BT. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus–induced ocular immunopathology. J Immunol 2008;180:6297–6306. [DOI] [PubMed] [Google Scholar]
- 45.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA 2005;102:6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol 2004;172:3842–3849. [DOI] [PubMed] [Google Scholar]
- 47.Hwang ES, White IA, Ho IC. An IL-4–independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proc Natl Acad Sci USA 2002;99:13026–13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stassen M, Jonuleit H, Muller C, Klein M, Richter C, Bopp T, Schmitt S, Schmitt E. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol 2004;173:267–274. [DOI] [PubMed] [Google Scholar]
- 49.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta–dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007;178:280–290. [DOI] [PubMed] [Google Scholar]
- 50.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol 1998;160:1212–1218. [PubMed] [Google Scholar]
- 51.Kataoka H, Takahashi S, Takase K, Yamasaki S, Yokosuka T, Koike T, Saito T. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol 2005;17:421–427. [DOI] [PubMed] [Google Scholar]
- 52.Mason D. T-cell–mediated control of autoimmunity. Arthritis Res 2001;3:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]