Abstract
Formoterol, a long-acting β2-receptor agonist, is used to relieve bronchial constriction. However, formoterol is often a racemic formulation, and contains both (R,R)- and (S,S)-enantiomers. Because the activity of each isomer is poorly defined, the mechanisms by which formoterol relaxes smooth muscle cells (SMCs) of intrapulmonary airways are not well understood. Consequently, we compared the effects of (S,S)-, (R,R)-, and racemic formoterol, as well as (R)-albuterol, on the contraction and Ca2+ signaling of airway SMCs in mouse lung slices with phase-contrast and confocal microscopy. Small airways were contracted with methacholine and the associated SMCs displayed sustained Ca2+ oscillations and an increase in Ca2+ sensitivity. These contracted airways displayed a substantial, concentration-dependent relaxation in response to (R,R)-formoterol. Racemic formoterol had a similar potency as (R,R)-formoterol for relaxing airways. By contrast, (S,S)-formoterol only induced a small relaxation. In conjunction with relaxation, (R,R)- and racemic formoterol stopped and decreased the methacholine-induced Ca2+ oscillations and Ca2+ sensitivity of the SMCs, respectively, whereas (S,S)-formoterol only decreased the Ca2+ sensitivity. In these studies, (R,R)- and racemic formoterol had a similar, but much greater, potency than (R)-albuterol for relaxing mice airways. This action was quickly initiated at high concentrations by decreasing the frequency of Ca2+ oscillations, but was more usually mediated at lower concentrations by decreasing the Ca2+ sensitivity of the SMCs.
Keywords: Ca2+ sensitivity, Ca2+ oscillations, confocal microscopy, lung slices, methacholine
CLINICAL RELEVANCE.
The efficacy and mechanisms of short- or long-acting β2-adrenergic agonists are important for evaluating treatments designed to induce bronchodilation to facilitate the management of asthma and chronic obstructive pulmonary disease.
β2-Receptor agonists have been in use for many years to relieve bronchial constriction associated with asthma or chronic obstructive pulmonary disease. Formoterol is a long-acting β2-receptor agonist (>12-h duration of action), often used, in combination with glucocorticosteroids, in the maintenance therapy of asthma, whereas short-acting β2-receptor agonists (SABAs) (3- to 6-h duration of action) are essentially used as rescue medications to relieve symptoms and treat exacerbations (1–3). However, formoterol has a rapid onset of action, whether given alone or in combination with glucocorticosteroids, and appears to be as effective as SABAs in relieving acute asthma exacerbations (1, 4–6). Formoterol occurs in two formulations: racemic formoterol (50% of (R,R)- and (S,S)-enantiomers) and purified (R,R)-formoterol. Clinical and animal data suggest that (R,R)- and racemic formoterol have a similar potency to promote airway relaxation (7–9). However, others studies suggest that (S,S)-formoterol may not be biologically inactive as previously thought, but may reduce the relaxant efficacy of the racemic mixture (10). In addition, (S,S)-formoterol may promote inflammation (11).
It has been well established that smooth muscle cell (SMC) contraction usually requires an increase in intracellular calcium concentration ([Ca2+]i), and this has been observed to occur as a series of Ca2+ oscillations in airway SMCs of many species (12–17). The frequency of the Ca2+ oscillations within SMCs is a key parameter that correlates with the contractility of SMCs. Agents that elevate cAMP, such as SABAs (i.e., isoproterenol [ISO] and albuterol) or forskolin (FSK), have been shown to slow down the frequency of the Ca2+ oscillations, and this has been correlated with airway relaxation (18, 19).
A second mechanism that determines the extent of airway contraction is the Ca2+ sensitivity of the SMC contractile apparatus (20, 21). As a result, β2-receptor agonists may also induce airway relaxation by reducing the Ca2+ sensitivity of airway SMCs. We have previously shown that (R)-albuterol was more effective than racemic albuterol in decreasing the Ca2+ sensitivity of airway SMCs, and this was correlated with a stronger efficacy for airway relaxation (19).
Because it is unknown if formoterol induces airway relaxation by altering the Ca2+ oscillations and Ca2+ sensitivity of airway SMCs, the aim of this study was to characterize and compare the effects of (R,R)-, (S,S)-, and racemic formoterol on the contraction and Ca2+ signaling of airway SMCs. Although previous studies have used tissues or cells isolated from the trachea to investigate the effects of β2-receptor agonists, a simultaneous correlation of the contractile responses with cellular changes in Ca2+ was not possible in these preparations. In addition, it is unclear if the responses of tracheal SMCs reflect those of the small airway. Consequently, we have compared the effects of isomers of formoterol and (R)-albuterol on small intrapulmonary airways in mouse lung slices where contraction and Ca2+ signaling are more easily studied.
MATERIALS AND METHODS
Animals and Reagents
Male BALB/C mice, 7 to 10 weeks old, were purchased from Charles River Breeding Labs (Needham, MA). Mice were killed by intraperitoneal injection of pentobarbital sodium (nembutal), as approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Hanks' balanced salt solution (HBSS) without phenol red was supplemented with 25 mM Hepes (sHBSS [pH 7.4]). (R)-albuterol, (R,R)-, (S,S)-, and racemic formoterol were donated by Sepracor Inc. (Marlborough, MA). Cell-permeant, caged-IP3 was obtained from Alexis Biochemicals (San Diego, CA). Other reagents were obtained from Invitrogen Corp. (Carlsbad, CA), Calbiochem (La Jolla, CA), or Sigma-Aldrich Corp. (St. Louis, MO).
Preparation of Mouse Lung Slices
Lung slices were prepared by the methods previously described (14). In brief, the lungs were inflated with warm 2% agarose in sHBSS. Subsequently, a small bolus of air was injected to flush the agarose-sHBSS out of the airways and into the distal alveolar space. The inflated lungs were cooled with cold sHBSS to gel the agarose. A lung lobe was sectioned into roughly 130-μm-thick lung slices with a vibratome in cold sHBSS. Lung slices were maintained in Dulbecco's modified Eagle's Medium supplemented with antibiotics and antimycotics at 37°C in 10% CO2. Lung slices were used within 3 days. Similarly sized airways (150–210 μm in diameter) were used in all experiments.
Measurement of the Contractile Response of Airways
Lung slices were mounted in a custom-made chamber and perfused by a gravity-fed perfusion system (14). Lung slices were observed with phase-contrast microscopy on an inverted microscope with a 10× objective, and images were recorded using a charge-coupled device camera and image acquisition software (Video Savant; IO Industries, London, ON, Canada). Digital images were recorded in time lapse (0.5 Hz), stored on a hard drive, and analyzed using custom-written scripts with Video Savant. The area of the airway lumen was calculated with respect to time by pixel summing. Area values were normalized to the initial lumen area.
Measurement of Intracellular Ca2+
Lung slices (∼12–15 slices) were incubated with Oregon Green 488 BAPTA-1-AM (20 μM, 2 ml), sulfobromophthalein (100 μM), and 0.05% pluronic F-127 for 40 minutes at 30°C. For de-esterification, the slices were washed for 40 minutes in sHBSS containing 100 μM sulfobromophthalein at 30°C. Imaging of loaded slices was performed using a video-rate confocal microscope, as previously described (14, 22). Fluorescence images were saved to a hard disk using Video Savant software at 15 frames per second. Changes in fluorescence (F) were analyzed by selecting a region of interest (5 × 5 pixels) in a single SMC and were normalized to the initial level of fluorescence (F0). Final fluorescence values were expressed as a ratio (F/F0). Additionally, a line-scan analysis of the images was performed by extracting a row of pixels from each image and placing them sequentially, as a time sequence, in a single image.
Flash Photolysis of Caged-IP3
Flash photolysis of cell-permeant caged-IP3 was used to increase intracellular IP3 concentration. Slices were initially loaded with Oregon Green 488 BAPTA-1-AM, as described previously here. Subsequently, lung slices were incubated at room temperature with 2 μM caged-IP3 for 1 hour in sHBSS containing 0.1% pluronic F-127 and 100 μM sulfobromophthalein, followed by de-esterification for 30 minutes in the sHBSS containing 100 μM sulfobromophthalein. The details of the flash photolysis setup have been previously described (18, 23). Briefly, a flash of ultraviolet (UV) light was produced from a mercury arc lamp with an electronic shutter and a band-pass filter (330 nm). The intensity of the flash was regulated by neutral density filters. The size of the area illuminated was adjusted to be a small spot with an iris diaphragm.
Preparation of Ca2+-Permeabilized Lung Slices
The SMCs of lung slices were made permeable to Ca2+ by exposure to 20 mM caffeine and 50 μM ryanodine in sHBSS for at least 4 minutes. In this procedure, caffeine activates or opens the ryanodine receptors of the sarcoplasmic reticulum, and this allows ryanodine to lock the ryanodine receptor in an open state. This results in the depletion of the intracellular Ca2+ store (the sarcoplasmic reticulum), which, in turn, increases the Ca2+ permeability of the plasma membrane by the activation of store-operated Ca2+ channels (21). Under these conditions, the [Ca2+]i is determined by the extracellular Ca2+ concentration, which was set at 1.3 mM (sHBSS). Importantly, this Ca2+ permeabilization was irreversible when caffeine and ryanodine were removed.
Statistical Analysis
Statistical analysis of the data showed no difference between the individual mice. Data are expressed as means (±SD) of three or more experiments per mouse for n mice. Statistical analysis was assessed by ANOVA. Comparison of paired data was done using a paired samples t test. A P value of less than 0.05 was considered statistically different.
RESULTS
Response of Airways to Formoterol Enantiomers
The response of contracted airways to formoterol enantiomers was investigated by exposing airways from mice to 400 nM methacholine (MCh) to induce contraction, followed by a range of formoterol concentrations (Figure 1). A MCh concentration of 400 nM was used, because this induced a midrange contraction. The concentrations of the formoterol enantiomers tested ranged from 0.5 to 4,000 nM, but all data points are not always shown in figures.
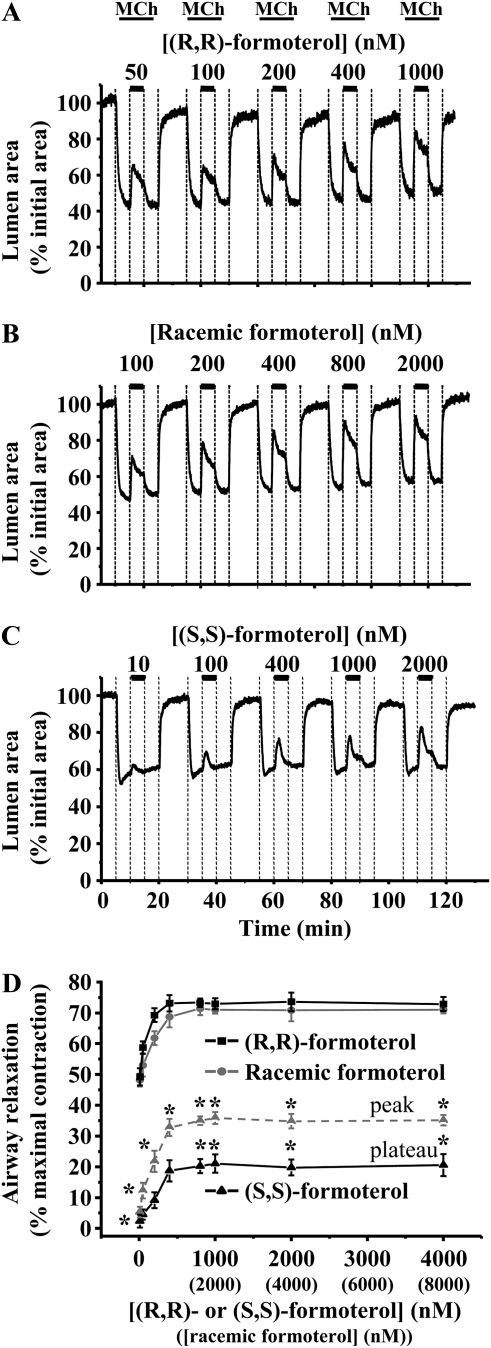
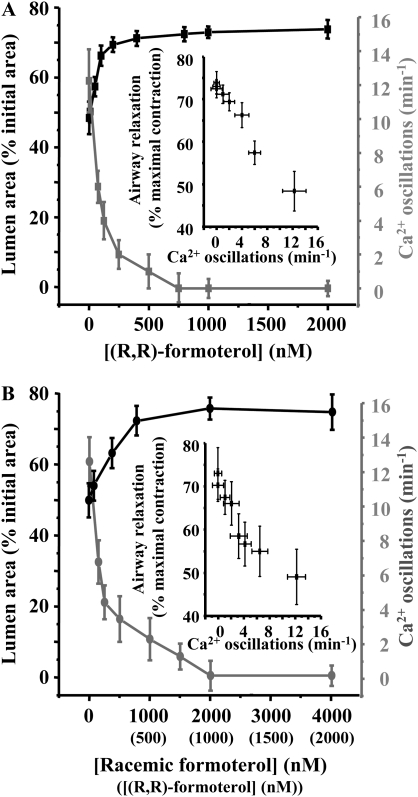
Figure 1.
The relaxant effect of (R,R)-, racemic, and (S,S)-formoterol on contracted airways. Airways were contracted with 400 nM methacholine (MCh) and exposed to a series of formoterol concentrations: (A) (R,R)-formoterol (50–1,000 nM), (B) racemic formoterol (100–2,000 nM), and (C) (S,S)-formoterol (10–2,000 nM). (D) The mean concentration-dependent relaxation response of airways contracted by MCh (400 nM) to (R,R)-, racemic, and (S,S)-formoterol. The maximal relaxation induced by each formoterol isomer was quantified during the plateau phase (solid lines). The transient response induced by (S,S)-formoterol was also quantified at peak relaxation (dashed line). The relaxant effect of (R,R)-formoterol and racemic formoterol (containing an equal concentration of (R,R)-formoterol) was not significantly different. However, both (R,R)- and racemic formoterol induced a significantly larger relaxation than (S,S)-formoterol. Mean (±SD) values are presented; a minimum of four experiments was performed for each concentration for each mouse (*P < 0.05; n = 7 mice for (R,R)-formoterol, n = 6 for racemic formoterol and (S,S)-formoterol).
At concentrations above 1 nM, (R,R)- and racemic formoterol induced a sustained relaxation of MCh-contracted airways in a concentration-dependent manner (Figures 1–3). However, the initial relaxation was often followed by a small recontraction of the airway, which subsequently stabilized, and the airway remained at a constant relaxed state for the following 30 minutes (Figure 2). Therefore, we quantified the relaxation response at the beginning of this plateau phase (4 min after addition of formoterol). The relaxant effect of (R,R)- or racemic formoterol (containing an equal concentration of (R,R)-formoterol) was reversible, and the airways recontracted to their initial levels after formoterol removal (Figures 1A, 1B, and 2). (R,R)- and racemic formoterol induced a similar airway relaxation at all concentrations tested (Figure 1D). The effective concentration range of relaxation was relatively narrow. A substantial relaxation of approximately 50% was induced by a concentration of 50 nM (R,R)-formoterol or 100 nM racemic formoterol, whereas a maximal relaxation was induced by 800 nM (R,R)-formoterol or 1,600 nM racemic formoterol. Maximal relaxation was 73.29 (±1.375)% (% maximal contraction; n = 7 mice) and 71.36 (±2.13)% (n = 6 mice) for (R,R)- and racemic formoterol, respectively.
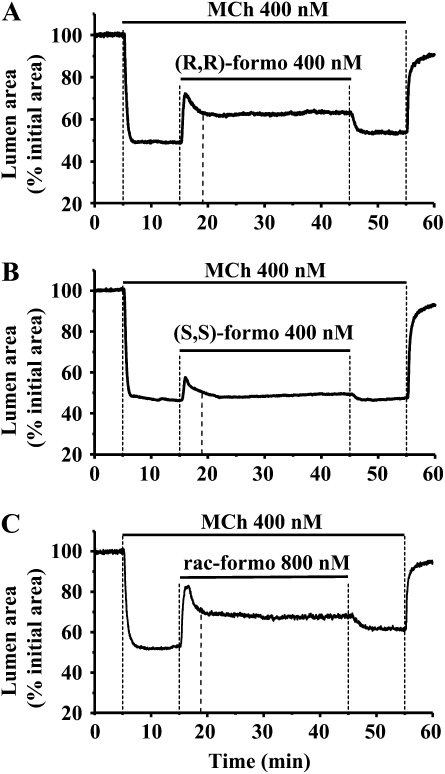
Figure 2.
Duration of the relaxant effect of (R,R)-, racemic, and (S,S)-formoterol on contracted airways. Airways were contracted with 400 nM MCh and exposed for 30 minutes to 400 nM of (A) (R,R)-formoterol, (B) (S,S)-formoterol, and (C) 800 nM racemic formoterol. Each formulation of formoterol induced an initial strong and fast relaxation, followed by a small recontraction of the airway that subsequently stabilized after approximately 4 minutes (dashed line) of exposure to formoterol, and remained constant for the next 30 minutes.
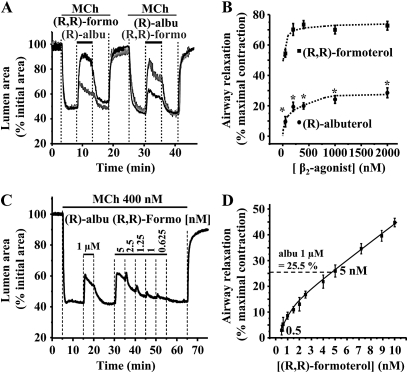
Figure 3.
Comparison of airway relaxation induced by (R)-albuterol and (R,R)-formoterol. (A) Representative experiments showing the effect of (R,R)-formoterol (1 μM) and (R)-albuterol (1 μM) on the same MCh-contracted airway. (R,R)-formoterol induced a larger relaxation than the subsequent exposure to (R)-albuterol (black line). Similar results were obtained if the application order of (R)-albuterol and (R,R)-formoterol was reversed (gray line). (B) The mean concentration-dependent relaxation induced by (R)-albuterol and (R,R)-formoterol (50–2,000 nM) in airways contracted with MCh (400 nM). Relaxation induced by (R,R)-formoterol was significantly greater than that induced by (R)-albuterol (*P < 0.05; n = 4 mice for (R,R)-formoterol and (R)-albuterol). Mean (±SD) values are presented; a minimum of three experiments was performed for each concentration for each mouse. (C) Representative experiment showing the relaxant effect of (R)-albuterol (1 μM) followed by 5, 2.5, 1.25, 1, and 0.625 nM of (R,R)-formoterol on the same MCh-contracted airway. (D) The mean concentration-dependent relaxation to low concentrations of (R,R)-formoterol (0.5–10 nM). The maximal relaxation induced by 5 nM (R,R)-formoterol was 26.65 (±2.35)% (% maximal contraction; n = 5 mice; a minimum of three experiments was performed for each concentration for each mouse), which was equivalent to the relaxation induced by 1 μM (R)-albuterol.
In a similar series of experiments, (S,S)-formoterol was found to induce a small relaxation of MCh-contracted airways (Figure 1C). In addition, (S,S)-formoterol had mainly a transient relaxation effect at low concentrations (<400 nM) (Figures 1C and 2B). The relaxation induced by (S,S)-formoterol (quantified at either the peak relaxation or during the plateau phase) was significantly smaller than that induced by (R,R)- or racemic formoterol at all concentrations tested (P < 0.05) (Figure 1D). (S,S)-formoterol induced a maximal relaxation of 21.03 (±2.97)% (plateau; n = 6 mice) at 1,000 nM.
Comparison of (R)-Albuterol and (R,R)-Formoterol on Airway Contraction
Because long-acting β2-receptor agonists have been considered as replacements for SABAs, we compared the effectiveness of (R,R)-formoterol and (R)-albuterol on the same MCh-contracted airways (Figure 3A). We found that, although both agonists (at equal concentrations) relaxed the airways with a similar timing, (R,R)-formoterol induced a significantly larger relaxation than (R)-albuterol at all concentrations tested (Figures 3A and 3B). At 1,000 nM, (R,R)-formoterol induced a relaxation of 70.05 (±2.77)% (% maximal contraction; n = 4 mice), which was more than double the relaxation induced by the same concentration of (R)-albuterol (25.5 ± 2.56%). This action was not influenced by the sequence of drug application (Figure 3A). In an additional series of experiments, the exposure of an airway several times (6 times) to (R)-albuterol (1 μM) did not change the subsequent airway relaxation induced by 500 nM or 1 μM (R,R)-formoterol. Similar results were also obtained if the airway was first exposed to formoterol (6 times) followed by albuterol. These results emphasize that the sequence of drug application was irrelevant, and that, more importantly, the desensitization of the tissue to either agonist does not readily occur.
Our comparison of formoterol with albuterol at equal concentrations suggests that formoterol has greater efficacy. However, an alternative approach to examine the mechanisms by which these two β2-receptor agonists induce relaxation is to compare their action during an equivalent relaxation. Consequently, we determined the concentration of (R,R)-formoterol required to match the relaxation induced by 1 μM albuterol (Figures 3C and 3D). With the same MCh-contracted airways, we found that 5 nM (R,R)-formoterol induced a relaxation approximately equivalent to the relaxation induced by 1 μM (R)-albuterol (Figures 3C and 3D). We will subsequently use, in this study, these concentrations of formoterol and albuterol to address the underlying mechanism of formoterol action as compared with albuterol.
Effects of Formoterol Enantiomers on Ca2+ Oscillations
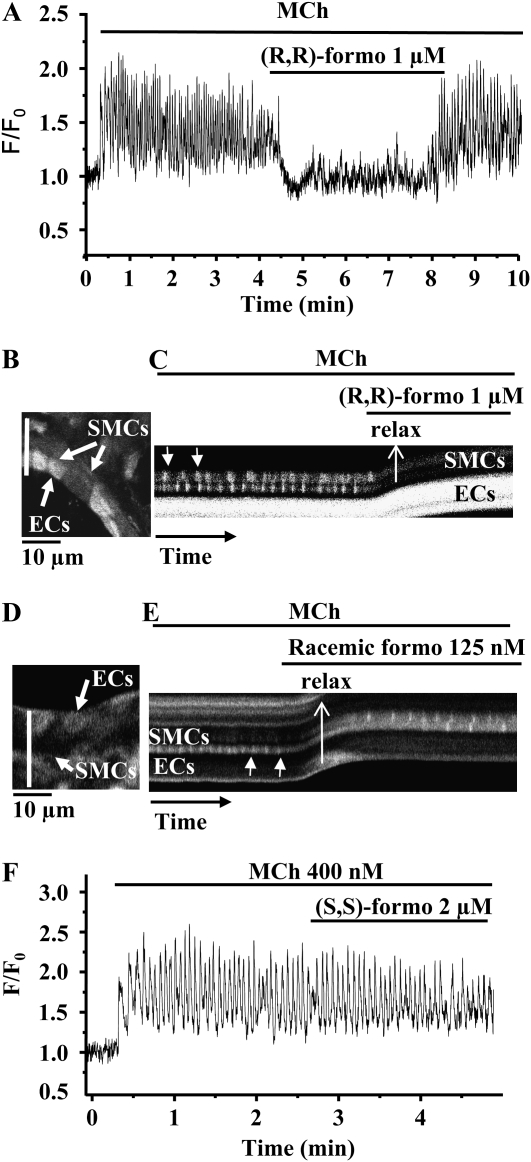
In response to 400 nM MCh, mouse airway SMCs displayed an increase of [Ca2+]i that rapidly developed (within 1 min) into sustained rhythmic Ca2+ oscillations (14.08 ± 1.86 oscillations per min; six mice; Figures 4 and Figures 5). The subsequent addition of either (R,R)- or racemic formoterol, at high concentrations of approximately 1 μM quickly stopped (Figures 4A–4C), or, at lower concentrations, slowed (Figure 4E), the Ca2+ oscillations. This inhibition of the Ca2+ oscillations was accompanied by airway relaxation (Figures 4C and 4E). Upon the removal of formoterol, the Ca2+ oscillations resumed with a frequency equal to the original rate (Figure 4A), and the airway recontracted (Figures 1 and 2). By contrast, (S,S)-formoterol (2 μM) had no effect on the frequency of the Ca2+ oscillations (Figure 4F).
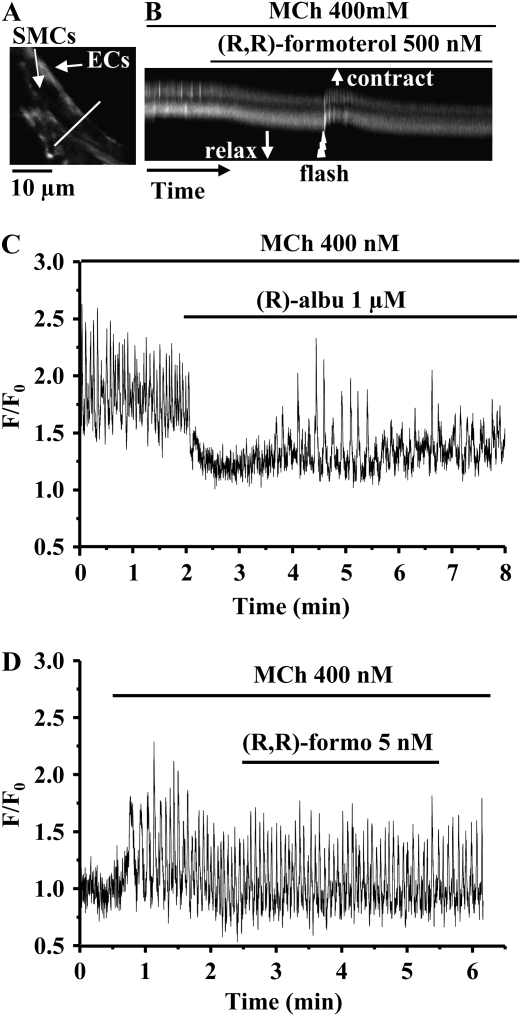
Figure 4.
The effect of formoterol isomers on the frequency of MCh-induced Ca2+ oscillations. (A) Representative experiment showing that the Ca2+ oscillations induced in an airway smooth muscle cell (SMC) by 400 nM MCh were quickly abolished by exposure to (R,R)-formoterol (1 μM). (B) Fluorescence image indicating the location of SMCs and epithelial cells (ECs) from an observed airway. (C) Line scan analysis (for 90 s from white line indicated in [B]) showing rhythmic Ca2+ oscillations induced by MCh 400 nM (arrows) in two SMCs. These Ca2+ oscillations were inhibited by the addition of 1 μM (R,R)-formoterol. The upward shift of the fluorescence trace (C) away from the lumen (in the direction of arrow) indicates the onset of the relaxation of the airway. (D) Fluorescence image showing SMCs and ECs of a second airway. (E) Line scan plot (for 150 s, indicated by the white line in [D]) showing rhythmic Ca2+ oscillations (arrows) induced by 400 nM MCh in two SMCs. The frequency of the Ca2+ oscillations was decreased by a low concentration of racemic formoterol (125 nM) and the airway relaxed. (F) Representative experiment showing that Ca2+ oscillations induced by 400 nM MCh were not altered by (S,S)-formoterol (2 μM).
Figure 5.
The mean concentration-dependent relaxation (black line and left scale) and the change in the frequency of the Ca2+ oscillations (gray line and right scale) induced by (A) (R,R)-formoterol (0–2,000 nM) and (B) racemic formoterol (0–4,000 nM) in airways contracted with MCh (400 nM). Numbers in parentheses indicate the equivalent (R,R)-formoterol concentration in racemic formoterol. Insets: the relationship between the reduction in Ca2+ oscillation frequency in SMCs and airway relaxation. As the concentration of (R,R)-formoterol or racemic formoterol increased, the relaxation increased and the Ca2+ oscillation frequency decreased (n = 4 mice for (R,R)-formoterol and racemic formoterol). Mean (±SD) values are presented; a minimum of three experiments was performed for each concentration for each mouse.
To characterize the relationship between the frequency of the Ca2+ oscillations of the SMCs and the relaxation of the airway induced by formoterol, MCh-contracted airways (400 nM) were exposed to a range of concentrations (up to 2 μM) of (R,R)- or racemic formoterol (Figure 5), and the frequency of the Ca2+ oscillations was measured. At concentrations up to 750 nM, (R,R)-formoterol decreased the frequency of the Ca2+ oscillations induced by MCh, whereas, at concentrations greater than 750 nM, (R,R)-formoterol inhibited the Ca2+ oscillations (Figure 5A). Racemic formoterol also decreased the frequency of Ca2+ oscillations in a concentration-dependent manner (Figures 4E and 5B), and stopped the Ca2+ oscillations at concentrations of greater than 2,000 nM (equal to 1,000 nM (R,R)-formoterol). No significant differences in the decrease of the frequency of the Ca2+ oscillations induced by (R,R)- and racemic formoterol (at equal concentrations of (R,R)-formoterol) were observed. (S,S)-formoterol had no effect on the Ca2+ oscillations at all concentrations tested (10–4,000 nM).
The formoterol concentration–dependent Ca2+ oscillation frequency data were plotted against the formoterol concentration–dependent airway size data to obtain the relationship between the Ca2+ oscillation frequency and airway relaxation (Figure 5, insets). (R,R)-formoterol induced a relaxation of the airway area that was a near linear function of the Ca2+ oscillation frequency. The relaxation relationship for racemic formoterol was similar. It is interesting to note that, although the Ca2+ oscillations are fully inhibited (i.e., no Ca2+ signaling), the airways are not fully relaxed.
Effect of Formoterol on the Release of Intracellular Ca2+
To investigate the mechanism by which formoterol inhibited the Ca2+ oscillations, we examined the effect of formoterol on IP3-induced Ca2+ release from internal Ca2+ stores. In control studies, airway SMCs were loaded with caged-IP3 and briefly illuminated with UV light (a 500-ms flash). This single UV flash initiated several Ca2+ oscillations that propagated as Ca2+ waves from the illuminated area to the rest of the SMC (data not shown). A flash exposure had no effect on Ca2+ release of SMCs not loaded with caged-IP3.
Similarly, airway SMCs loaded with caged-IP3 were subsequently contracted with MCh to induce Ca2+ oscillations. The exposure of these SMCs to (R,R)-formoterol stopped the Ca2+ oscillations. However, in the continued presence of MCh and (R,R)-formoterol, a UV flash exposure applied to the SMCs to release IP3 also initiated a short period of Ca2+ oscillations (Figures 6A and 6B). A transient recontraction of the airway was associated with this brief resumption of the Ca2+ oscillations. Equivalent results were found with racemic formoterol. These results suggest that formoterol may either limit the production of IP3 and/or decrease the sensitivity of the IP3 receptor (IP3R) to concentrations of IP3 within the cell.
Figure 6.
The effect of the photolytic release of IP3 in SMCs exposed to (R,R)-formoterol. (A) Fluorescence image indicating the location of SMCs and ECs from the airway observed. (B) Line scan analysis (for 180 s from the white line indicated in [A]) showing that the rhythmic Ca2+ oscillations in two SMCs induced by 400 nM MCh were inhibited by 500 nM (R,R)-formoterol, and that this allowed the airway to relax. The subsequent flash photolysis (500 ms, jagged arrow) of caged–iso-Ins(1,4,5)P3/PMIP3 (caged-IP3) in presence of MCh and (R,R)-formoterol induced a short period of Ca2+ oscillations. The upward shift of the fluorescence trace indicates the transient contraction of the airway induced by IP3 release from caged-IP3. As the Ca2+ oscillations slowed again, the airway relaxed. (C) Representative experiment showing that Ca2+ oscillations induced in an airway SMC by 400 nM MCh were initially abolished by (R)-albuterol (1 μM), but, after 2 minutes, the Ca2+ oscillations resumed at a lower frequency. (D) Representative experiment showing that the Ca2+ oscillations induced by 400 nM MCh were not affected by 5 nM (R,R)-formoterol.
Comparison of (R)-Albuterol and (R,R)-Formoterol on Ca2+ Oscillations
As previously reported (19), we found that 1 μM (R)-albuterol stopped Ca2+ oscillations induced by MCh 400 nM during the initial 1–2 minutes of exposure. However, in the continued presence of albuterol, the Ca2+ oscillations resumed, but at a lower frequency than with MCh alone (Figure 6C). By contrast, we found that “equivalent effective concentrations” of (R,R)-formoterol (<10 nM) had no effect on MCh-induced Ca2+ oscillations (Figures 5A and 6D). Therefore, although 5 nM formoterol induced a similar relaxation as compared with 1 μM (R)-albuterol, this was achieved without a change in the Ca2+ oscillation frequency.
Effects of Formoterol Enantiomers on the Ca2+ Sensitivity of SMCs
The sensitivity of the contractile apparatus to Ca2+ provides a second potential mechanism of action for formoterol to relax airways. To investigate this possibility, it is necessary to clamp the SMC [Ca2+]i at a high level. This was achieved by creating Ca2+-permeabilized lung slices by treatment with ryanodine and caffeine. The details of this procedure have been previously published (21).
In Ca2+-permeabilized lung slices, the addition of MCh induced airway contraction (Figure 7) without altering the elevated [Ca2+]i (data not shown [21]). The subsequent addition of either, racemic, (R,R)-, or (S,S)-formoterol relaxed the contracted airway (Figures 7A and 7B) in a concentration-dependent manner (Figure 7C), but, again, without altering the elevated [Ca2+]i. The relaxation induced by formoterol in Ca2+-permeabilized lung slices was ranked in the following order: racemic formoterol > (R,R)-formoterol ≫ (S,S)-formoterol (Figure 7C). These results demonstrate that all three formoterol preparations decreased SMC Ca2+ sensitivity.
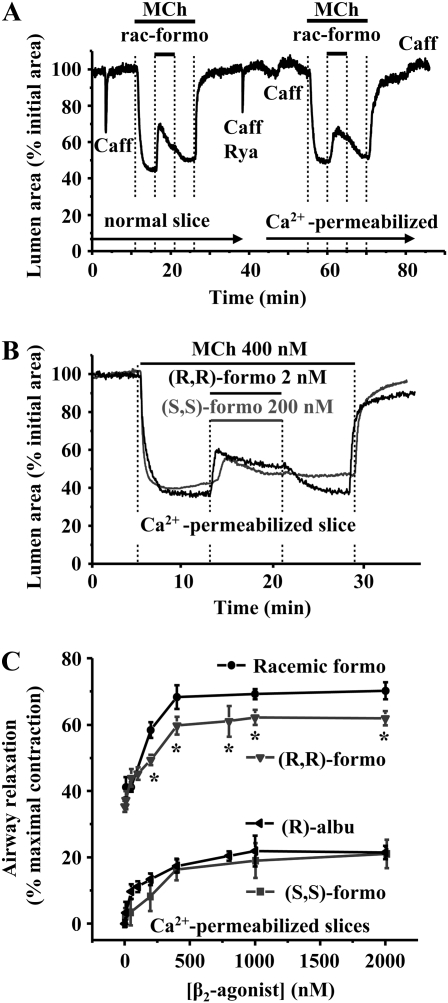
Figure 7.
Effect of formoterol isomers and (R)-albuterol on Ca2+ sensitivity in Ca2+-permeabilized lung slices. (A) A normal airway was contracted with 400nM MCh. The addition of racemic formoterol (400 nM) induced relaxation of the airway. To convert this lung slice into the Ca2+-permeabilized state, the slice was perfused simultaneously with 20 mM caffeine and 50 μM ryanodine, which induced a transient contraction. A second caffeine application did not evoke a contraction, confirming the Ca2+-permeabilized status of the lung slice. Exposure to 400 nM MCh induced contraction of the same airway in a similar manner to that induced in the normal airway, indicating that MCh increased Ca2+ sensitivity. Exposure to racemic formoterol relaxed the contracted airway, indicating a decrease in Ca2+ sensitivity. (B) Representative experiments showing airway contraction induced by 400 nM MCh, followed by relaxation induced by (R,R)-formoterol (2 nM) (black line) and (S,S)-formoterol (200 nM) (gray line) in a Ca2+-permeabilized lung slice. Under these conditions (SMCs with a high clamped intracellular calcium concentration [[Ca2+]i]), contraction indicates an increase in Ca2+ sensitivity; relaxation indicates a decrease in Ca2+ sensitivity. (C) The mean concentration-dependent relaxation of airways contracted by MCh (400 nM) to (R,R)-, racemic, (S,S)-formoterol, and (R)-albuterol in Ca2+-permeabilized slices (n = 6 mice for (R,R)-formoterol and racemic formoterol; n = 5 mice for (S,S)-formoterol and (R)-albuterol). Mean (±SD) values are presented; a minimum of three experiments was performed for each concentration for each mouse.
To compare the mechanism of action of formoterol with that of albuterol, we also examined the response of (R)-albuterol on Ca2+ sensitivity. In keeping with our previous results, (R)-albuterol also induced relaxation in MCh-contracted airways of Ca2+-permeabilized lung slices. However, the extent of this response was significantly less than that induced by equal concentrations of (R,R)-formoterol, but similar to that induced by (S,S)-formoterol (Figure 7C).
Relative Contribution of Ca2+ Signaling and Sensitivity to Airway Relaxation
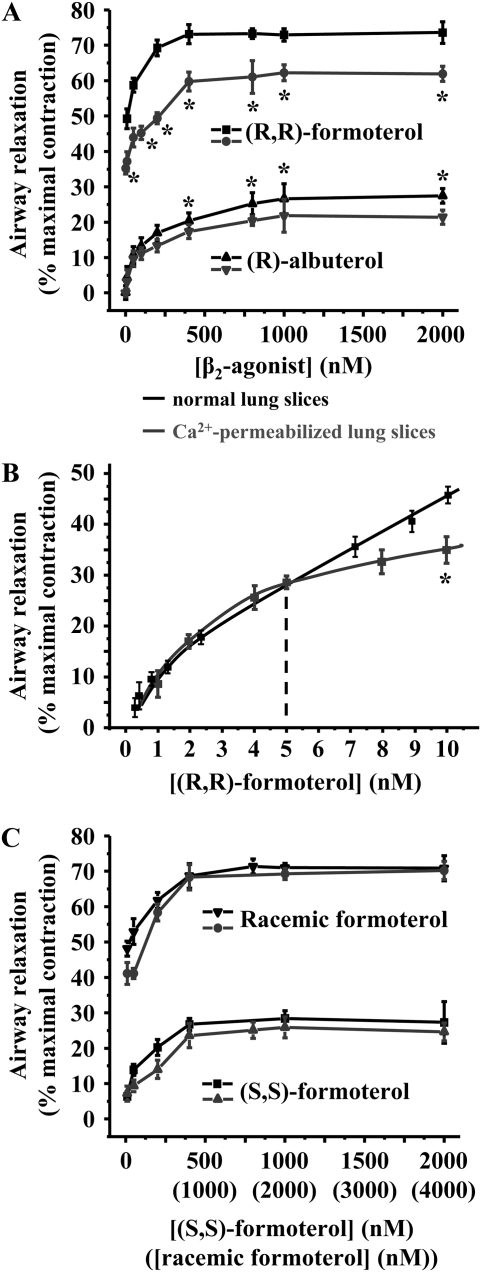
To determine the relative contribution of the decrease in the frequency of the Ca2+ oscillations and Ca2+ sensitivity induced by formoterol or albuterol in airway relaxation, we compared (in Figures 8A–8C) the concentration–relaxation data of normal (from Figures 1D, 3B, and 3D) and Ca2+-permeabilized lung slices (from Figure 7C). At concentrations equal to or higher than 10 nM, (R,R)-formoterol induced a greater relaxation in normal as compared with Ca2+-permeabilized slices (Figure 8A, top). This indicates that the relaxation induced by a decrease in Ca2+ signaling and sensitivity were additive. Similarly, the relaxation induced by (R)-albuterol in normal airways was greater (small difference, but significant) than in Ca2+-permeabilized airways (Figure 8A, bottom). From this analysis, it is also evident that (R,R)-formoterol is significantly more effective at relaxing airways than (R)-albuterol in either normal or Ca2+-permeabilized lung slices. One implication of these results is that the decrease in Ca2+ sensitivity alone induced by formoterol is substantially more effective than the combined effect of (R)-albuterol on the Ca2+ signaling and Ca2+ sensitivity. This was confirmed by the fact that low concentrations of (R,R)-formoterol (<10 nM) induced a similar relaxation in both normal and Ca2+-permeabilized airways (Figure 8B). Whereas (R)-albuterol at 1 μM induced relaxation by a decrease in Ca2+ signaling and sensitivity (Figure 8A, bottom), 5 nM formoterol induced a similar relaxation by only decreasing the Ca2+ sensitivity (Figure 8B).
Figure 8.
Relative contribution of Ca2+ signaling and Ca2+ sensitivity to airway relaxation induced by formoterol isomers and (R)-albuterol. (A) The mean concentration-dependent relaxation of airways (contracted by MCh, 400 nM) induced by (R,R)-formoterol and (R)-albuterol (1–2,000 nM) in normal (black line, data from Figures 1D and 3B) and Ca2+-permeabilized lung slices (gray line, data from Figure 7C). Relaxation induced by (R,R)-formoterol and (R)-albuterol in normal and Ca2+-permeabilized lung slices was significantly different (*P < 0.05). (B) The mean concentration-dependent relaxation of airways (contracted by MCh, 400 nM) induced by low concentrations of (R,R)-formoterol (0.5–10 nM) in normal (black line, data from Figure 3D) and Ca2+-permeabilized lung slices (gray line; *P < 0.05; n = 5 mice). Mean (±SD) values are presented; a minimum of three experiments was performed for each concentration for each mouse. (C) The mean concentration-dependent relaxation of airways (contracted by MCh, 400 nM) induced by racemic- and (S,S)-formoterol in normal (black line, data from Figure 1D) and Ca2+-permeabilized slices (gray line, data from Figure 7C).
In contrast to (R,R)-formoterol or (R)-albuterol, the relaxation induced by racemic formoterol or (S,S)-formoterol in normal slices, as compared with its effect in Ca2+-permeabilized lung slices (Figure 8C), was similar at all concentrations tested. The implication of these results is that the decrease in Ca2+ sensitivity predominately mediates the relaxant effect. Interestingly, the relaxation induced by (R,R)-formoterol or racemic formoterol in normal slices was similar to the relaxation induced in Ca2+-permeabilized lung slices by racemic formoterol (compare Figures 8A and 8C). The implication of this result is that the decrease in the Ca2+ sensitivity induced by (R,R)- and (S,S)-formoterol is additive to give the maximal response. Importantly, because (S,S)-formoterol does not effect Ca2+ oscillations even at high concentrations, its mechanism of action appears to be different from that of (R,R)-formoterol.
β2-Adrenoceptors and Airway Relaxation Induced by Formoterol and (R)-Albuterol
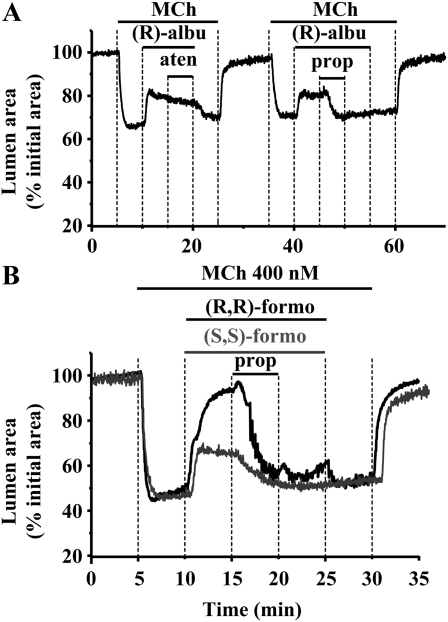
Albuterol, (R,R)-formoterol, and (S,S)-formoterol are believed to act on β2-adrenoceptors. However, (R,R)-formoterol, but not (S,S)-formoterol, decreased the frequency of Ca2+ oscillations. In addition, (R,R)-formoterol is more effective at relaxing airways than similar concentration of (S,S)-formoterol or (R)-albuterol. Consequently, the effects of (R,R)-formoterol, (S,S)-formoterol, or (R)-albuterol may be mediated by mechanisms other than via β2-adrenoceptors. To explore this possibility, we examined the effect of β-adrenoceptor antagonists on the relaxation responses. We found that atenolol (1 and 10 μM), a specific β1-antagonist, failed to modify the relaxation response of airways to formoterol or (R)-albuterol. However, propranolol (1 μM), a β1/β2–antagonist, inhibited the effects of both formoterol and albuterol (Figures 9A and 9B). This is consistent with previous data indicating that the β2 receptor primarily mediates the responses to albuterol and formoterol.
Figure 9.
Contribution of the β2-adrenoceptors to airway relaxation induced by (R)-albuterol or formoterol isomers. (A) An airway was contracted with 400 nM MCh and exposed to 1 μM (R)-albuterol to induce relaxation. The subsequent exposure to atenolol (aten; 1 μM), a specific β1-antagonist, failed to modify the response of airways to (R)-albuterol. After washing for 10 minutes with Hanks' balanced salt solutions supplemented with 25 mM Hepes, the same airway was contracted with 400 nM MCh, exposed to (R)-albuterol (1 μM) to induce relaxation, and then treated with the β1/β2–antagonist, propranolol (prop; 1 μM). Propranolol inhibited the relaxation induced by (R)-albuterol. (B) Representative experiments showing that 1 μM propranolol inhibited the relaxation induced by 1 μM (R,R)-formoterol (black line) or 2 μM (S,S)-formoterol (gray line).
DISCUSSION
In this study, we characterized the acute effects of (R,R)-, (S,S)-, and racemic formoterol on mouse small airways contracted with MCh. (R,R)- and racemic formoterol were found to have a similar relaxant efficacy; they both induced a fast, large, and sustained relaxation. We also found that (S,S)-formoterol had a relaxant effect, although significantly smaller than (R,R)- or racemic formoterol. Although caution is always required when extrapolating data from mouse airways to human airways, these results are consistent with clinical data that showed no difference between (R,R)-formoterol and racemic formoterol on bronchodilation up to 24 hours for patients with asthma (9). It is not clear if longer exposure would have a similar outcome. However, a recent study of patients with chronic obstructive pulmonary disease showed a similar bronchodilator efficacy for (R,R)- and racemic formoterol after 14 days of treatment (24). Previous studies with guinea pig tracheal strips support the idea that (R,R)- and racemic formoterol have a similar relaxant efficacy, but, in those studies, (S,S)-formoterol had no significant effect on airway contractility (7, 8). It is possible that this difference results from the differences between SMCs from trachea and intrapulmonary airways, between species, or the contractile agonists used. In addition, the method for studying airway relaxation is different. On the other hand, the efficacy of formoterol is high, which makes the detection of small differences difficult.
We also examined if (R,R)- and (S,S)-formoterol act through β2 receptor. The β1-specific antagonist, atenolol, and a nonselective β receptor blocker, (β1/β2) propranolol, were used with our mouse lung slices. Atenolol had no effect on the relaxation induced by (R,R)- or (S,S)-formoterol. However, propranolol inhibited the relaxation induced by (R,R)- or (S,S)-formoterol and (R)-albuterol. This implies the β2 receptor was primarily involved in relaxation induced by all the isomers examined.
Changes in Ca2+ are believed to be the principle mechanism by which airway SMC contraction is mediated, and data from our previous studies indicate that the frequency of Ca2+ oscillations within SMCs is a key parameter determining their contractility (14, 16). The frequency of these Ca2+ oscillations is modified during the relaxation induced by agents that elevate cAMP, such as FSK, ISO (18), and (R)- and racemic-albuterol (19). FSK and ISO slowed the frequency of Ca2+ oscillations, whereas (R)-albuterol (at a high concentration) briefly arrested the Ca2+ oscillations induced by MCh during the first 1–2 minutes of exposure. However, the Ca2+ oscillations usually resumed, but at a slower rate than with MCh alone. By contrast, (R,R)- and racemic formoterol (at concentrations >1 μM) completely stopped the Ca2+ oscillations, and this was correlated with substantial airway relaxation. The Ca2+ oscillations did not recover even after several minutes of exposure to (R,R)- or racemic formoterol. The strong effect of (R,R)-formoterol on the Ca2+ oscillations explains, in part, why (R,R)-formoterol has a greater relaxant effect than similar concentrations of albuterol, ISO, or FSK. Because the relaxant effect of FSK was found to be mediated by inhibiting the IP3-induced Ca2+ release from internal Ca2+ stores, we tested the possibility that formoterol could affect the Ca2+ oscillations by a similar mechanism. We found that the Ca2+ oscillations could be restored by the release of additional IP3 from caged-IP3. This result suggests that the inherent source of IP3 (phospholipase C–β activity) has been depleted or inhibited by (R,R)- or racemic formoterol (at concentrations around 1 μM). Alternatively, the sensitivity of the IP3R to the endogenous IP3 may be greatly reduced. Although it is likely, in view of our previous results with ISO, that the IP3R sensitivity to IP3 is decreased, the continued occurrence of Ca2+ oscillations in the presence of ISO or (R)-albuterol suggests that this decrease in IP3R sensitivity was insufficient to render the receptor totally unresponsive to IP3. Thus, a lack of IP3 is a more likely explanation for the sustained lack of Ca2+ oscillations.
Interestingly, (S,S)-formoterol or low concentration of (R,R)-formoterol that induced airway relaxation had no effect on Ca2+ oscillations; this result suggests that formoterol primarily induces relaxation by a different mechanism. It is well recognized that the contractility of airway SMCs is also regulated by the sensitivity of the contractile apparatus to Ca2+ or “Ca2+ sensitivity” (20, 21). However, the details of this mechanism are poorly understood in airway SMCs. Using Ca2+-permeabilized SMCs, in which the [Ca2+]i was clamped at a high constant level, we found that (S,S)-formoterol induced a relaxation similar to that induced in normal airways. Therefore, we concluded that (S,S)-formoterol relaxed airways by altering the Ca2+ sensitivity. This could also explain why the effect of the S-isomer is relatively slow acting in comparison with (R,R)- and racemic formoterol.
Importantly, (R,R)-formoterol was found to significantly decrease the Ca2+ sensitivity, although the relaxation induced by (R,R)-formoterol in Ca2+-permeabilized airway was smaller than in normal airways. However, the difference in (R,R)-formoterol–induced relaxation between normal and Ca2+-permeabilized airways is relatively small, and this suggests that most of the relaxant effect of (R,R)-formoterol can be mediated by a decrease of the Ca2+ sensitivity, even though the [Ca2+]i remains elevated. The combination of a low [Ca2+]i brought about by slowing or stopping the Ca2+ oscillations, together with a decrease in Ca2+ sensitivity, gave approximately 10–15% more relaxation. On the other hand, racemic formoterol was found to induce a similar relaxation in both normal and Ca2+-permeabilized slices. The difference between racemic and (R,R)-formoterol on Ca2+-permeablized lung slices could be explained by the additional effect of the S-isomer on Ca2+ sensitivity, and supports the idea that a decrease in Ca2+ sensitivity is a major mechanism determining the relaxation induced by formoterol.
We also compared relaxation induced by (R,R)-formoterol and the SABA, (R)-albuterol. As expected, (R,R)-formoterol is much more potent than similar concentration of (R)-albuterol at relaxing mouse airways, and this is correlated with a stronger effect of (R,R)-formoterol on both Ca2+ oscillations and Ca2+ sensitivity. β2-Agonists have different affinity and efficacy (25, 26), and this could explain why similar concentration of (R,R)-formoterol, (S,S)-formoterol, and (R)-albuterol induced a different amount of relaxation. Formoterol is believed to have a higher affinity for the β2-adrenoceptor than albuterol (26). Therefore, we compared (R,R)-formoterol and (R)-albuterol at an equivalent relaxation. We found that, at 5 nM, (R,R)-formoterol induced a similar relaxation of mouse airways compared with 1,000 nM albuterol. Importantly, at these concentrations, formoterol exerted its relaxant effect by only decreasing the Ca2+ sensitivity; relaxation induced by (R)-albuterol was mediated by both a decrease in the Ca2+ sensitivity and by slowing the Ca2+ oscillations.
It is also interesting to note that 1 μM (S,S)-formoterol induced a maximal relaxation of 21%, which is equivalent to the relaxation induced by approximately 3.5 nM (R,R)-formoterol (compare Figures 1D and 3D). In both cases, the relaxation of mouse airways was mediated by a decrease in Ca2+ sensitivity without a change in Ca2+ oscillations. However, unlike (R,R)-formoterol, (S,S)-formoterol had no ability to stop or slow the Ca2+ oscillations even at higher concentrations (up to 4 μM). (S,S)-formoterol did not appear to compete with its R-isomer, because racemic formoterol (at all the concentrations tested) induced a similar relaxation to that of (R,R)-formoterol (for equivalent concentrations of (R,R)-formoterol). In addition, the decrease in the Ca2+ sensitivity induced by (R,R)- and (S,S)-formoterol observed in Ca2+-permeabilized lung slices was found to be additive. Whereas (S,S)-formoterol acts though β2-adrenoceptors, but with a lower affinity than (R,R)-formoterol, our results suggest that (S,S)-formoterol exerts its action by a different process than that used by (R,R)-formoterol. The difference in molecular size and structure of the β2-agonists, their different hydrophilic properties, and their residency time at the active site of the β2-adrenoceptors are mainly responsible for their respective affinity and efficacy. However, our results also suggest that the β2-adrenoceptors could activate different molecular pathways according to the β2-agonists and isomers used. The regulation of the Ca2+ sensitivity would most likely reflect the activity of myosin light-chain phosphatase, and this appears to mediate most of the relaxant effect induced by albuterol and formoterol in mouse lung slices.
In summary, (R,R)- and racemic formoterol quickly induced sustained airway relaxation by a combination of two processes: a decrease in Ca2+ sensitivity, and a decrease in the frequency of Ca2+ oscillations. The strong efficacy of both (R,R)- and racemic formoterol appears to be associated primarily with its ability, especially at low concentrations, to substantially reduce Ca2+ sensitivity and bring about relaxation, even in the presence of ongoing Ca2+ oscillations. In this respect, (S,S)-formoterol had a similar, but weaker, behavior, and induced relaxation without the ability to influence Ca2+ oscillations. This capability of (S,S)-formoterol to decrease Ca2+ sensitivity also appears to enhance the effect of (R,R)-formoterol. The rapid onset of relaxation induced by higher concentrations of the (R,R)- and racemic formoterol appears to be mediated by the additional simultaneous reduction or stoppage of the frequency of the Ca2+ oscillations. These studies with short-term exposures to β2-agonists in mouse lung slices also indicate that formoterol is a more effective bronchodilator than albuterol, and that its mechanism of action is not identical. A final important point is that variable degrees of relaxation were induced by the various isomers of formoterol; this implies that the isomer formulation of bronchodilators must be taken into consideration when evaluating their therapeutic potential.
This work was supported by a research grant from Sepracor Inc., and by National Institutes of Health grants HL-71930 and HL087401 (M.J.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0403OC on June 5, 2009
Conflict of Interest Statement: M.J.S. has received research grants ($78,735 in 2007 and $84,904 in 2008) for non–principal investigator salary and supplies, $5,000 to serve on a scientific research advisory committee, $1,500 to present a research review lecture (from Sepracor Inc.), and $5,000 in 2007 and $4,000 in 2008 for speaking at conferences sponsored by Sepracor Inc. P.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet]. [updated 2008. Jun 24; accessed 2009 March 28]. Available from: http://www.ginasthma.org
- 2.Prenner BM. Role of long-acting beta2-adrenergic agonists in asthma management based on updated asthma guidelines. Curr Opin Pulm Med 2008;14:57–63. [DOI] [PubMed] [Google Scholar]
- 3.Donohue JF. Safety and efficacy of beta agonists. Respir Care 2008;53:618–622; discussion 623–624. [PubMed] [Google Scholar]
- 4.Bateman ED, Fairall L, Lombardi DM, English R. Budesonide/formoterol and formoterol provide similar rapid relief in patients with acute asthma showing refractoriness to salbutamol. Respir Res 2006;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, Petermann W, Aubier M, Schwabe G, Bengtsson T. Formoterol as relief medication in asthma: a worldwide safety and effectiveness trial. Eur Respir J 2003;22:787–794. [DOI] [PubMed] [Google Scholar]
- 6.Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, Selroos O. Safety of formoterol by turbuhaler as reliever medication compared with terbutaline in moderate asthma. Eur Respir J 2002;20:859–866. [DOI] [PubMed] [Google Scholar]
- 7.Kallstrom BL, Sjoberg J, Waldeck B. Steric aspects of formoterol and terbutaline: is there an adverse effect of the distomer on airway smooth muscle function? Chirality 1996;8:567–573. [DOI] [PubMed] [Google Scholar]
- 8.Trofast J, Osterberg K, Kallstrom BL, Waldeck B. Steric aspects of agonism and antagonism at beta-adrenoceptors: synthesis of and pharmacological experiments with the enantiomers of formoterol and their diastereomers. Chirality 1991;3:443–450. [DOI] [PubMed] [Google Scholar]
- 9.Lotvall J, Palmqvist M, Ankerst J, Persson G, Rosenborg J, Bengtsson T, Rott Z, Poczi M, Devai A, Waldeck B. The effect of formoterol over 24 h in patients with asthma: the role of enantiomers. Pulm Pharmacol Ther 2005;18:109–113. [DOI] [PubMed] [Google Scholar]
- 10.Handley DA, Senanayake CH, Dutczak W, Benovic JL, Walle T, Penn RB, Wilkinson HS, Tanoury GJ, Andersson RG, Johansson F, et al. Biological actions of formoterol isomers. Pulm Pharmacol Ther 2002;15:135–145. [DOI] [PubMed] [Google Scholar]
- 11.Abraha D, Cho SH, Agrawal DK, Park JM, Oh CK. (S,S)-formoterol increases the production of IL-4 in mast cells and the airways of a murine asthma model. Int Arch Allergy Immunol 2004;133:380–388. [DOI] [PubMed] [Google Scholar]
- 12.Dai JM, Kuo KH, Leo JM, Pare PD, van Breemen C, Lee CH. Acetylcholine-induced asynchronous calcium waves in intact human bronchial muscle bundle. Am J Respir Cell Mol Biol 2007;36:600–608. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Kuo KH, Dai J, van Breemen C. Asynchronous calcium waves in smooth muscle cells. Can J Physiol Pharmacol 2005;83:733–741. [DOI] [PubMed] [Google Scholar]
- 14.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACh, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 2005;125:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol 2001;91:488–496. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 2008;5:23–31. [DOI] [PubMed] [Google Scholar]
- 17.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol 1997;272:C966–C975. [DOI] [PubMed] [Google Scholar]
- 18.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a camp-mediated inhibition of the IP3 receptor. Respir Res 2006;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delmotte P, Sanderson MJ. Effects of albuterol isomers on the contraction and Ca2+ signaling of small airways in mouse lung slices. Am J Respir Cell Mol Biol 2008;38:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325–1358. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 2006;291:L208–L221. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson MJ, Parker I. Video-rate confocal microscopy. Methods Enzymol 2003;360:447–481. [DOI] [PubMed] [Google Scholar]
- 23.Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds: a sensitive method to evaluate gap junctional coupling. Methods Mol Biol 2001;154:407–430. [DOI] [PubMed] [Google Scholar]
- 24.Kharidia J, Fogarty CM, Laforce CF, Maier G, Hsu R, Dunnington KM, Curry L, Baumgartner RA, Hanrahan JP. A pharmacokinetic/pharmacodynamic study comparing arformoterol tartrate inhalation solution and racemic formoterol dry powder inhaler in subjects with chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2008;21:657–662. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol 2006;117:18–24, quiz 25. [DOI] [PubMed] [Google Scholar]
- 26.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med 1998;158:S146–S153. [DOI] [PubMed] [Google Scholar]