Abstract
Flavonoids are known to be effective scavengers of free radicals. In particular, proanthocyanidins are flavonoids that possess cardiovascular protection, antioxidative activities, and immunomodulatory activities. Here, we evaluated proanthocyanidin contents in the total polyphenolic compounds of pine needle extracts prepared by hot water, ethanol, hexane, hot water-hexane (HWH), and hot water-ethanol (HWE). Analysis of each extract indicated that the ethanol extract contained the highest proanthocyanidin concentration. The HWH and hexane extracts also contained relatively high concentrations of proanthocyanidin. On the other hand, proanthocyanidin content analyses out of the total polyphenolic compounds indicated that the HWH extract contained the highest content. These results suggest that HWH extraction is a suitable method to obtain an extract with a high level of pure proanthocyanidins and a relatively high yield. The HWH extract possessed superior activity in diverse antioxidative analyses such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferrous ion chelating (FIC), and ferric-ion reducing power (FRAP) assays. In addition, upon assessing the effects of the pine needle extracts on macrophages (Raw 264.7 cell), the HWH extract exhibited the highest activity. In this study, we discerned an efficient extraction method to achieve relatively pure proanthocyanidins from pine needles and evaluated the biological functions of the resulting extract, which could potentially be used for its efficacious components in functional food products.
Keywords: Antioxidative activity, immunological activity, pine needle, proanthocyanidin
Introduction
Proanthocyanidins (PAs) are widely present in fruits, vegetables, nuts, seeds, flowers, and bark [1] and regarded as an important class of secondary metabolites in plants [2]. PAs are a group of naturally occurring polyphenolic bioflavonoids, specifically taking the form of oligomers or polymers of polyhydroxy flavan-3-ol units, such as (+)-catechin and (-)-epicatechin [3]. PAs have been reported to have a wide range of biological and pharmacological activities including antioxidative, cardioprotective, antitumor, antibacterial, antiviral, anti-inflammatory, and immunomodulatory effects [1,4]. Among these diverse pharmacological activities, the antitumor and antioxidative effects of PAs are most notable. Hence, PAs have received attentions in areas of health, medicine, and nutrition. PAs are shown to be some of the most potent free radical scavengers and antioxidants both in vitro and in vivo [5], as well as potential chemopreventive agents to ameliorate the normal cell toxicity associated with chemotherapeutic agents used in cancer treatment.
Pine trees (Pinus densiflora) belong to the family Pinaceae and are widely distributed around the world. In East-Asian countries such as Korea and China, various parts of pine trees, including the needles, cones, cortices, and pollen, are widely consumed as foods or dietary supplements to promote health [6], and have shown a wide spectrum of biological and pharmacological actions such as anti-inflammatory, antibacterial, antiviral, antidepressant, and triglyceride decreasing effects [7]. Pine bark acts as a good protective agent of collagen from collagenase [2] and pine needles have shown anti-hypertension effects [8], and protection against oxidative DNA damage and apoptosis induced by hydroxyl radicals [7]. The effective components of pine needles are chlorophyll, carotenes, dietary fiber, terpenoids, phenolic compounds, tannins, and alkaloids. Extracts from pine bark are reported to be effective scavengers of reactive oxygen, can lower serum lipids, and may help delay aging [9].
Extraction is essential for using PAs from pine trees and many methods have been applied for this purpose. Jeong et al. [7] used cold water, freeze drying, and ethanol for PA extraction from pine needles. On the other hand, hot water and hexane [2] and ethanol [9] were used for PA extraction from pine bark. However, there are scant systematic comparisons of pine needle extraction methods. Therefore, this study compared diverse extraction methods for PA extraction from pine needles.
To establish an effective method of PA extraction, we evaluated proanthocyanidin contents in the total polyphenolic compounds of pine needle extracts prepared by various extraction methods and compared their antioxidative and immunomodulatory activities.
Materials and Methods
Chemicals
Sodium carbonate, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, and iron (III) chloride 6-hydrate were purchased from Fisher Scientific (Louhgborough, UK). L-(+)-ascorbic acid, butylated hydroxytoluene (BHT), Folin-Ciocalteau's reagent, iron (II) sulfate 7-hydrate, β-carotene (Type 1: synthetic), 2,2-diphenyl-1-picrylhydrazyl, and lipopolysaccharide (LPS) were obtained from Sigma (St. Louis, MO, USA). Gallic acid, Tween 40, and potassium ferricyanide were obtained from MP biomedicals LLC (Solon, OH, USA). Trichloroacetic acid was obtained from Junsei Kagaku (Tokyo, Japan). Linoleic acid was obtained from Fluka (Buchs, Switzerland). RPMI-1640 medium, fetal bovine serum (FBS), and penicillin-streptomycin were obtained from Bio Whittaker (Walkersville, MD, USA).
Preparation of pine needle extracts
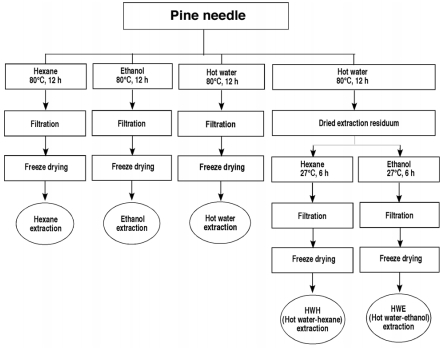
The pine needle extracts were prepared by hot water, ethanol, hexane, hot water-hexane (HWH), and hot water-ethanol (HWE) extraction (Fig. 1). The needles were crushed mechanically into a powder form using a mortar and a pestle. The pine needle powder (3 kg) was then extracted with 4 L each of ddH2O, hexane, and ethanol at 80℃ for 12 hr to obtain the hot water, hexane, and ethanol extracts, respectively. To obtain the hot water-hexane (HWH) and hot water-ethanol (HWE) extracts, the pine needle powder (3 kg) was extracted with 4 L of ddH2O. After removing the supernatant of the hot water extract, the residuum was extracted with 4 L each of hexane (HWH) or ethanol (HWE) at 80℃ for 12 hr. Each extract was filtered with Whatman filter paper, evaporated under vacuum at 40℃, and then lyophilized. The extracts were stored at -20℃ until used.
Fig. 1.
Schematic diagram of the extraction processes of the pine needles
Analysis of total polyphenolic compounds (TPC) in extracts
The amounts of TPC in the extracts were determined using the Folin-Ciocalteau assay [10]. Each extract was dissolved and diluted in 99% methanol. Three-hundred microliters of sample were added to test tubes followed by 1.5 ml of Folin-Ciocalteau's reagent (10 x dilutions) and 1.2 ml of sodium carbonate (7.5% w/v). The mixture was allowed to stand for 30 min before absorbance was measured at 765 nm. TPC was expressed as gallic acid equivalents (GAE) in mg per 100 g of material.
Analysis of proanthocyanidin in extracts
The amounts of proanthocyanidins in the extracts were determined by the method of Brand-Williams et al. [11]. Each extract was dissolved and diluted in 99% methanol. One milliliter of extract was added to 6 ml of 5% (v/v) HCl (in butanol) solution and 0.2 ml of FeNH4(SO4)2·H2O (in 2M HCl) solution. The sample mixture was incubated at 95℃ for 40 min and then centrifuged at 14,000×g for 5 min. The supernatant was transferred to a new vial and 5% (v/v) HCl (in butanol) solution was added. The absorbance of each tube was measured at 550 nm. The amount of proanthocyanidin in each sample was calculated using 96% grape seed proanthocyanidin (Naturex, Avignon, France) as a standard.
Analysis of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity in extracts
The free radical scavenging activities of the extracts were measured by the DPPH method proposed by Brand-Williams et al. [11]. Each extract was dissolved and diluted in 99% methanol. One milliliter of extract was added to 2 ml of DPPH (5.9 mg in 100 ml methanol) solution. The mixture was incubated for 30 min at room temperature and then the absorbance was measured at 517 nm. The DPPH radical scavenging activity was calculated according to the following equation:
DPPH radical scavenging activity (%) = [(A0-A1)/A0] × 100
where A0 was the absorbance of the control, and A1 was the absorbance with the test compound.
Ferric-reducing antioxidant power (FRAP) assay of extracts
The FRAP assay was performed according to the Benzie and Strain method [12] with some modifications. Each extract was dissolved and diluted in 99% methanol. One milliliter of extract was added to 2.5 ml of 0.2 M potassium phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide. The mixture was incubated for 20 min at 50℃, after which 2.5 ml of 10% trichloroacetic acid was added. The mixture was then separated into aliquots of 2.5 ml and mixed with 2.5 ml of ddH2O. Then, 0.5 ml of 0.1% (w/v) FeCl3 was added to each tube and allowed to stand for 30 min. The absorbance of each tube was measured at 700 nm.
Ferrous ion chelating (FIC) assay of extracts
Metal chelating activity was determined according to the method of Dinis et al. [13]. FeSO4 (2 mM) and ferrozine (5 mM) were prepared and diluted 20 times. The extracts (10, 50, 100, 250, and 500 µl, diluted to 1 ml) were mixed with 1 ml of diluted FeSO4, followed by 1 ml of diluted ferrozine. The tubes were mixed well and allowed to stand for 10 min at room temperature. The absorbance of each tube was measured at 562 nm. The ability of the sample to chelate ferrous ions was calculated and expressed as follows:
Chelating effect (%) = [1-(A1/A0)] × 100
where A0 was the absorbance of the control, and A1 was the absorbance with the test compound.
Cell culture and analysis of nitric oxide (NO) production
RAW 264.7 cells, a murine macrophage cell line, were obtained from the Korean Cell Line Bank (Korea). The cells were cultured in RPMI-1640 medium and supplemented with 10% FBS and 100 U/ml penicillin-streptomycin at 37℃ in a humidified 5% CO2 atmosphere. The cells (2×105 cells per well) were seeded in a 96-well plate and incubated in RPMI-1640 medium and supplemented with 10% dextran-coated charcoal stripped medium and 100 U/ml penicillin-streptomycin. After 18 h of cultivation, LPS (1 µg/ml) and various concentrations of the pine needle extracts (0-500 µg/ml) were added to the cells. After an additional 24 h of incubation, the NO released from the macrophage cells was assessed by measuring the nitrite concentration using the culture supernatant. One-hundred fifty microliters of the culture media were incubated with 50 µl of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine in 2.5% phosphoric acid solution) at room temperature for 10 min in a 96-well microplate [14]. Nitrite concentration was determined with an ELISA microplate reader at an absorbance of 540 nm in comparison with NaNO2 as a standard. Background levels of nitrite were determined in cell-free RPMI-1640, with or without the additives, and were subtracted from the total amount of nitrite formed.
Results
Extraction yield and proanthocyanidin contents
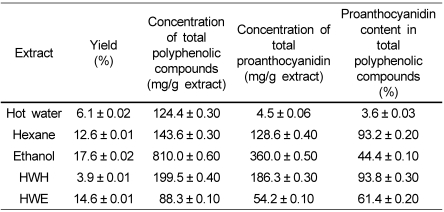
Phenolic compounds are commonly found in both edible and inedible plants and have been reported to have multiple biological effects, including antioxidant activity [15]. Table 1 shows the effects of various solvents on the yield, total phenolics, and proanthocyanidin contents of the pine needles. Out of the TPC, PA content was highest in the HWH (93.8%) and hexane (93.2%) extracts, while the ethanol extract showed only 44.4%. This indicated that a greater amount of non-PA substances existed in the ethanol extract. On the other hand, the hot water extract showed the lowest PA content (3.6%) out of the TPC.
Table 1.
Concentrations of total polyphenolic compounds and proanthocyanidin in pine needle extracts
Means ± SEM for three samples are shown.
This experiment was repeated at least twice yielding reproducible results.
The ethanol extract harbored the highest mass of TPC (810 mg/g) and PA (360 mg/g). Yu et al. [9] reported a 114.15 mg amount of TPC for one gram dry weight of pine bark by ethanol extraction, indicating pine bark as a rich source of TPC. Our study showed TPC amounts from pine needles in ranges from 88.24 to 810.00 mg/g dry weight (Table 1), and the highest amount of TPC was found in the ethanol extract. Our ethanol extraction conditions (80% ethanol, 80℃, 12 hours) were different from Yu et al. [9] (60% ethanol, 50℃, 2 hours), suggesting that the pine needles or our extraction conditions may be superior to those of Yu et al. [9].
Absolute amounts of PA were much higher in the HWH and HWE extracts than in the hot water extract (Table 1). These results suggest that hydrophilic compounds were mainly removed by hot water extraction, and the extraction rate of hydrophobic PAs was enhanced. In addition, this combined extraction method could be applicable to many other residua or wastes for materials of interest.
DPPH radical scavenging activity
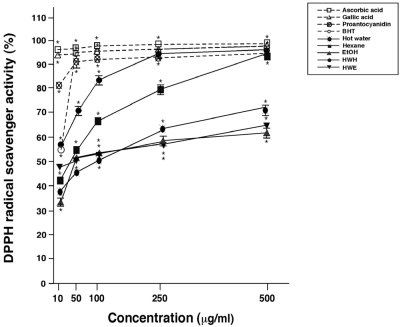
The DPPH assay was used to determine the extracts' abilities to scavenge free radicals, and the results are shown in Fig. 2. The stable radical DPPH has been widely used to determine the primary antioxidant activity of pure antioxidant compounds, plant and fruit extracts, and food materials [16]. The HWH extract showed the most powerful scavenging activity against DPPH radicals, in a concentration dependent manner ranging from 10 to 250 µg/ml. On the other hand, HWE showed the lowest DPPH radical activity. The hexane extract of pine needles is recognized for its primary antioxidants, which terminate the chain reactions by major initiators in the autoxidation of fatty acids [17,18]. The abundance of TPC within plant extracts is correlated with their enhanced antioxidant activity [19]. This is mainly due to their redox properties, which can play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides [20].
Fig. 2.
DPPH radical scavenging activities of pine needle extracts. Means ± SEM for three samples are shown as percentages compared with no treatment (0%). Factorial ANOVA with Fisher's PLSD post-hoc test* P < 0.001 was compared with no treatment. This experiment was repeated at least twice yielding reproducible results. BHT: butylated hydroxytoluene, Proanthocyanidin: 96% grape seed proanthocyanidin.
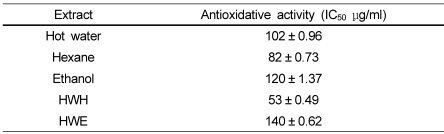
Table 2 shows IC50 values for the antioxidative activity of the extracts. The HWH and hexane extracts represented 53 and 82 µg/ml, respectively. The hot water, ethanol, and HWE extracts showed IC50 values over 100 µg/ml. These results confirmed the superior antioxidative activity of the HWH extract compared to the other extracts.
Table 2.
Antioxidative activities of pine needle extracts estimated from DPPH radical scavenger activity
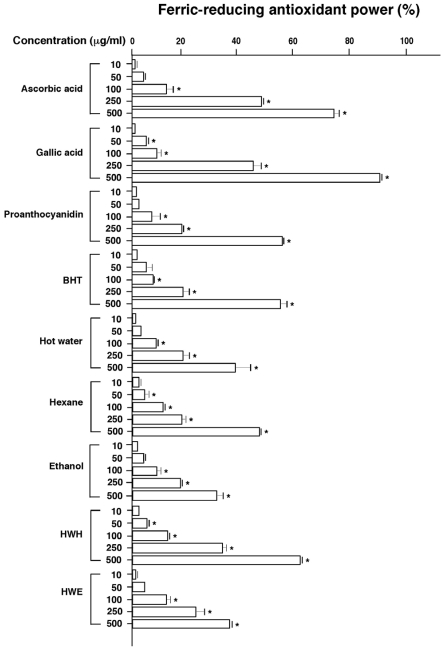
Antioxidative activity by FRAP assay
The FRAP assay measures the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) in the presence of antioxidants, which are reductants with half-reaction reduction potentials above that of Fe3+/Fe2+. Fig. 3 shows the FRAP assay results of the pine needle extracts compared with ascorbic acid, gallic acid, PA, and BHT. All extracts showed dose-dependent FRAPs and the HWH extract showed the highest reducing power among the extracts. The HWH extract showed higher activity than both purchased PA and BHT. The ethanol, HWE, and hot water extracts showed the lowest FRAPs. Table 1 shows that total PA content was about 2-fold higher in the ethanol extract compared to the HWH extract. Hence, total PA mass was about 4-fold in 500 µg/ml of the ethanol extract than in 250 µg/ml of the HWH extract; however, they showed similar FRAPs. This may suggest the presence of inhibiting compounds or differences in extracted PA classes with respect to FRAP activity in the ethanol extract, as well as the importance of extraction methods for biologically functional compounds.
Fig. 3.
Ferric-reducing antioxidant powers of pine needle extracts. Means ± SEM for three samples are shown as percentages compared with no treatment (0%). Factorial ANOVA with Fisher's PLSD post-hoc test* P < 0.001 was compared with no treatment. This experiment was repeated at least twice yielding reproducible results. BHT: butylated hydroxytoluene, Proanthocyanidin: 96% grape seed proanthocyanidin.
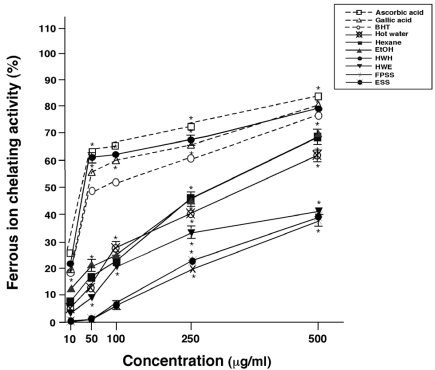
Antioxidative activity by FIC assay
Gardner et al. [21] reported that chelating agents are effective as secondary antioxidants, as they reduce reduction potential and stabilize the oxidized form of metal ions. Fe2+ ions can encourage lipid peroxidation by generating hydroxyl radicals through the Fenton reaction and increase lipid peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals, thereby initiating the chain reaction of lipid peroxidation [22]. The Fe2+ ion chelating abilities of the various extracts were determined by measuring their capacities to compete with ferrozine for the Fe2+ ion, as ferrozine can quantitatively form complexes with Fe2+ ions. Fig. 4 represents the FIC activity of the pine needle extracts. All extracts showed dose-dependent FIC activities and the HWH extract showed outstanding chelating activity compared to the other extracts, especially at 50 and 100 µg/ml. HWH extract was superior to BHT and similar to gallic acid or ascorbic acid in FIC activity. The HWE extract presented the lowest FIC activity overall.
Fig. 4.
Ferrous ion chelating activities of pine needle extracts. Means ± SEM for three samples are shown as percentages compared with no treatment (0%). Factorial ANOVA with Fisher's PLSD post-hoc test* P < 0.001 was compared with no treatment. This experiment was repeated at least twice yielding reproducible results.
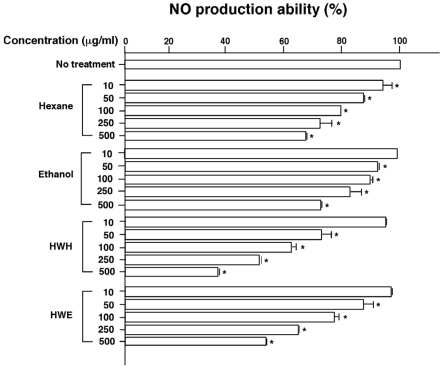
Immunomodulatory activities of pine needle extracts
The stimulation of macrophages to produce NO demands a signal and LPS. Therefore, RAW 264.7 cells were treated with LPS along with the pine needle extracts. To determine appropriate concentrations of the pine needle extracts, the RAW264.7 macrophages were treated with 0-500 µg/ml of the extracts and nitrite in culture broths that were then analyzed. As shown in Fig. 5, all extracts decreased NO production in a concentration dependent manner. In particular, the HWH extract showed the highest inhibition of LPS-induced NO production (52 and 38% inhibition of NO production abilities at 250 and 500 µg/ml, respectively). NO production showed a steep decline according to increasing concentrations of the HWH extract, while the ethanol and hexane extracts represented gentle declines. Table 1 shows similar PA contents out of the TPC for the HWE and hexane extracts. The hexane extract contained about 72 and 69% of TPC and PA, respectively, compared with the HWH extract. However, NO production was reduced more effectively with 100 and 250 µg/ml of the HWH extract than with 250 and 500 µg/ml of the hexane extract, respectively.
Fig. 5.
Inhibitory effects of LPS-induced NO production by pine needle extracts. Means ± SEM for three samples are shown as percentages compared with no treatment (100%). Factorial ANOVA with Fisher's PLSD post-hoc test* P < 0.001 was compared with no treatment. This experiment was repeated at least twice yielding reproducible results.
Discussion
Proanthocyanidin is a kind of phenolic product (oligonols are catechin-type monomers, dimers and trimers, as well as oligomeric proanthocyanidins) present in plant, and has received attention recently [23,24]. Experimental and clinical studies have shown that proanthocyanidin has an antioxidant effect, antinociceptive and cardioprotective properties, without inducing significant toxicological effects [25-27]. Pine trees (Pinus densiflora) have shown a wide range of biologically functional activities and many methods have been applied for PA extraction from pine trees [2,7-9]. We compared diverse extraction methods for PA extraction from pine needles as there are less systematic comparisons of those extraction methods.
Table 1 showed TPC amounts from pine needles in ranges from 88.24 to 810.00 mg/g dry weight. In other studies, TPC contents were less than 100 mg/g dry weight from the seed of Caesalpinia bonducella using ethanol extraction [28], and less than 30 mg from cantaloupe methanol extracts [29]. Baydar et al. [30] extracted 667.87 and 627.98 mg/g from the seeds of grapes with acetone:water:acetic acid and ethyl acetate:methanol: water, respectively. Ku et al. [2] extracted PA from pine bark by sequencing the use of hot water, acetone, and hexane. In Korea, however, methanol and acetone are prohibited for use as extraction solvents due to oral usage by humans and could not be employed in the present study. We used HWH, HWE, hexane, ethanol, and hot water extraction methods for PA preparation from pine needles (Fig. 1). Hence, a mere comparison is not appropriate between our method and that of Baydar et al. [30].
In our study, the ethanol extract showed the highest quantity of TPC, however, its PA content was 44.4%, while the HWH and hexane extracts showed PA contents of 93.8 and 93.2%, respectively (Table 1). For the HWH and HWE extracts, PA was extracted from the residuum of following hot water extraction. Hexane extraction with the residuum of the hot water (HWH) extract would have two advantages. First, biologically functional components can be extracted by their hydrophilic and hydrophobic characteristics. Second, impurities in the hydrophilic portion would be diminished. Souquet et al. [31] has reported that six polymeric PAs (tannin) could be purified from grape skins by using size exclusion chromatography followed by normal phase HPLC, however, this procedure is too complicate and costly to be applied for the commercial use of PA mass production.
Several methods have been developed to estimate the total antioxidative activities of fruits, plants, and animal tissues. Among them, assays for oxygen radical absorption capacity [32], total radical absorption potentials [33], Trolox equivalent antioxidant [34], and ferric reducing ability (FRAP) [13] are generally used in various examinations. The TPC and PA of the extracts were compared by diverse antioxidative activities. HWH and hexane extracts showed first and second position, respectively, at DPPH radical scavenging activity (Fig. 2 and Table 2). HWH demonstrated the best activity among diverse extracts and superior activity to both purchased PA and BHT at FRAP assay (Fig. 3). In addition, HWH showed the outstanding activity at ferrous ion chelating activity (Fig. 4). Hence, we could conclude that HWH extract showed the outstanding antioxidative activity. Indeed, antioxidative activity of HWH extract from pine needle in this study revealed higher than that of hot water extract from pine needle reported by Jeong et al. [7]. Oxidative stress is caused by a disturbance of the balance between the antioxidant defense mechanisms of the human organism and the level of reactive oxygen species (ROS), and has been associated with many pathological disorders such as atherosclerosis, diabetes and cancer [35]. Supplementation with exogenous antioxidants, including phenolic compounds from plant sources, may help to restore this balance.
NO is involved in neurotransmission, cytotoxicity against microbes and tumors, and vasoregulation [4,36]. However, a high level of NO production may induce host cell death and inflammatory tissue damage [37]. Therefore, the overproduction of NO can be harmful and result in various inflammatory and autoimmune diseases [37-39], and pharmacological interference of the NO production cascade presents a promising strategy for therapeutic interventions against inflammatory disorders. In our study, immunomodulating activities were much higher in the HWH extract compared to the other tested extracts (Fig. 5). NO production represented a sharp decline in proportion to increasing concentrations of the HWH extract, while the ethanol and hexane extracts represented gentle declines. Hence, the method of HWH extraction would be appropriate for PA preparation from pine needles.
The results from this study demonstrate that we discerned an efficient extraction method to achieve relatively pure proanthocyanidin from pine needles and evaluated the biological functions of it, which could potentially be used for an efficacious component in functional food products.
Footnotes
This research was financially supported by the Ministry of Education and Science Technology (MEST) and the Korea Industrial Technology Foundation (KOTEF) thorough the Human Resource Training Project for Regional Innovation.
References
- 1.Liu YZ, Cao YG, Ye JQ, Wang WG, Song KJ, Wang XL, Wang CH, Li RT, Deng XM. Immunomodulatory effects of proanthocyanidin A-1 derived in vitro from Rhododendron spiciferum. Fitoterapia. 2009;81:108–114. doi: 10.1016/j.fitote.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007;67:1618–1627. doi: 10.1016/j.chemosphere.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Porter LJ. The Flavonoids. In: Harborne JB, editor. Advances in Research since 1986. London: Chapman & Hall; 1986. pp. 23–55. [Google Scholar]
- 4.Bagchi M, Mimes M, Williams C, Balmoori J, Ye X, Stohs S, Bagchi D. Acute and chronic stress-induced oxidative gastrointestinal injury in rats and the protective ability of a novel grape seed proanthocyanidin extract. Nutr Res. 1999;19:1189–1199. [Google Scholar]
- 5.Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, Stohs SJ. Protective effects grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998;30:771–776. doi: 10.1016/s0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Chen J, Xu Z, Qiao X, Huang L. Disappearance of polycyclic aromatic hydrocarbons sorbed on surfaces of pine [Pinua thunbergii] needles under irradiation of sunlight: Volatilization and photolysis. Atmos Environ. 2005;39:4583–4591. [Google Scholar]
- 7.Jeong JB, Seo EW, Jeong HJ. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem Toxicol. 2009;47:2135–2141. doi: 10.1016/j.fct.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Kim KY, Chung HJ. Flavor compounds of pine sprout tea and pine needle tea. J Agric Food Chem. 2000;48:1269–1272. doi: 10.1021/jf9900229. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Zhao M, Wang JS, Cui C, Yang B, Jiang Y, Zhao Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Inno Food Sic & Emer Tech. 2009;9:122–128. [Google Scholar]
- 10.Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 11.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. [Google Scholar]
- 12.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 13.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavenges. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 14.Duan XW, Jiang YM, Su XG, Zhang ZQ, Shi J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2006;101:1365–1371. [Google Scholar]
- 15.Lee JC, Lim KT, Jang YS. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim Biophys Acta. 2002;1570:181–191. doi: 10.1016/s0304-4165(02)00196-4. [DOI] [PubMed] [Google Scholar]
- 16.Shih PW, Lai PL, Jen HWK. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]
- 17.Frankel EN. Recent advances in lipid oxidation. J Agric Food Chem. 1991;54:495–511. [Google Scholar]
- 18.Gordon MH. The mechanism of the antioxidant action in vitro. In: Hudson BJF, editor. Food Antioxidants. London: Elsevier; 1990. pp. 1–18. [Google Scholar]
- 19.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii extracts. Food Chem. 2003;82:593–597. [Google Scholar]
- 21.Gardner PT, White TC, McPhail DB, Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. [Google Scholar]
- 22.Miller DD. Mineral. In: Fennema OR, editor. Food Chemistry. New York: Marcel Dekker; 1996. pp. 618–649. [Google Scholar]
- 23.Dalbo S, Jurgensen S, Horst H, Soethe DN, Santos ARS, Pizzolatti MG, Valle RMR. Analysis of the antinociceptive effect of the proanthocyanidin-rich fraction obtained from Croton Celtidifolius barks: evidence for a role of the dopaminergic system. Pharmacol Biochem Behav. 2006;85:317–323. doi: 10.1016/j.pbb.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- 25.Preuss HG, Wallerstedt D, Talpur N, Tutuncuoglu SO, Echard B, Myers A, Bui M, Bagchi D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: a pilot study. J Med. 2000;31:227–246. [PubMed] [Google Scholar]
- 26.Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–737. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 27.Uchida S, Hirai K, Hatanaka J, Hanato J, Umegaki K, Yamada S. Antinociceptive effects of St. John's wort, Harpagophytum procumbens extract and grape seed proanthocyanidins extract in mice. Biol Pharm Bull. 2008;31:240–245. doi: 10.1248/bpb.31.240. [DOI] [PubMed] [Google Scholar]
- 28.Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol. 2009;47:1848–1857. doi: 10.1016/j.fct.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Ismail HI, Chan KW, Mariod AA, Ismail M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010;119:643–647. [Google Scholar]
- 30.Baydar NG, Özkan G, Sağdiç O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control. 2004;15:335–339. [Google Scholar]
- 31.Souque JM, Cheynier VR, Brossaud F, Moutounet M. Polymeric proanthocyanidins from grape skins. Phytochemisty. 1996;43:509–512. [Google Scholar]
- 32.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 33.Evelsona P, Travacioa M, Repettoa M. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys. 2001;388:261–266. doi: 10.1006/abbi.2001.2292. [DOI] [PubMed] [Google Scholar]
- 34.Van den Berg R, Haenenb GRMM, Van den Berga H. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. [Google Scholar]
- 35.Halliwell B, Gutteridge JMC. Antioxidant defences. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. pp. 200–216. [Google Scholar]
- 36.Kunkel SL. Th1- and Th2-type cytokines regulate chemokine expression. Biol Signals. 1996;5:197–202. doi: 10.1159/000109190. [DOI] [PubMed] [Google Scholar]
- 37.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 38.Manthey CL, Perera PY, Salkowski CA, Vogel SN. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994;152:825–831. [PubMed] [Google Scholar]
- 39.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]