Abstract
The actions of alcohols and anesthetics on ion channels are poorly understood. Controversy continues about whether bilayer restructuring is relevant to the modulatory effects of these surface active agents (SAAs). Some voltage-gated K channels (Kv), but not KvAP, have putative low affinity alcohol-binding sites, and because KvAP structures have been determined in bilayers, KvAP could offer insights into the contribution of bilayer mechanics to SAA actions. We monitored KvAP unitary conductance and macroscopic activation and inactivation kinetics in PE:PG/decane bilayers with and without exposure to classic SAAs (short-chain 1-alkanols, cholesterol, and selected anesthetics: halothane, isoflurane, chloroform). At levels that did not measurably alter membrane specific capacitance, alkanols caused functional changes in KvAP behavior including lowered unitary conductance, modified kinetics, and shifted voltage dependence for activation. A simple explanation is that the site of SAA action on KvAP is its entire lateral interface with the PE:PG/decane bilayer, with SAA-induced changes in surface tension and bilayer packing order combining to modulate the shape and stability of various conformations. The KvAP structural adjustment to diverse bilayer pressure profiles has implications for understanding desirable and undesirable actions of SAA-like drugs and, broadly, predicts that channel gating, conductance and pharmacology may differ when membrane packing order differs, as in raft versus nonraft domains.
Introduction
Voltage-gated channels (VGCs) transform electrical work perpendicular to the plane of the bilayer into mechanical work parallel to that plane (1) and because their voltage sensors extensively contact the lipid bilayer, physical and chemical perturbations of bilayer structure should affect gating. Members of all VGC subclasses (Kv, Cav, HCN, and Nav) are sensitive to stretch (2) and to the chemical make-up of the bilayer (3). Although VGC behavior in lipid subdomains, and in response to surface-active drugs, depends partly on bilayer mechanics (2–4), bilayer mechanics and VGC function have not been systematically examined in any VGC that is known at the atomistic level. KvAP is structurally characterized and reconstitutes into planar bilayers at densities allowing both unitary and macroscopic recordings (5,6). Short-chain 1-alkanols are surface active agents (SAAs) that can be added to membranes to reversibly alter bilayer mechanics. 1-Alkanols modulate VGCs (7) and their bilayer lateral pressure profile (LPP) effects are studied atomistically in silico (Fig. 1, A–C). KvAP and 1-alkanols thus offer an opportunity to examine the bilayer mechanics of VGC function.
Figure 1.
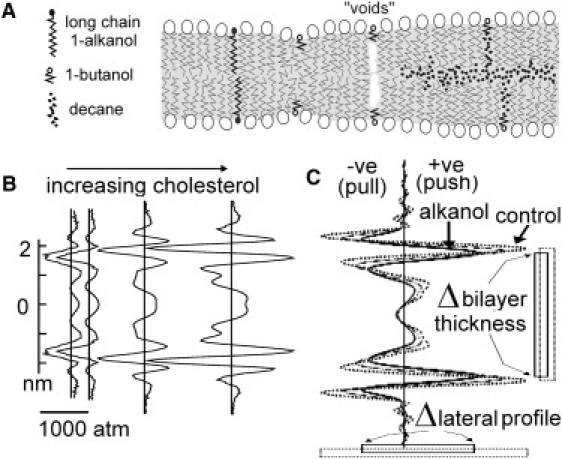
Channels and SAA-induced changes in LPP. (A) Adding SAAs to solvent-free (left) versus alkane-containing (right) phospholipid bilayers: small 1-alkanols like butanol create mid-bilayer voids, long chain 1-alkanols do not (26). Void collapse (solvent-free bilayers) thins bilayers and increases tail disorder. Decane molecules when present, occupy voids and the midplane, ordering hydrocarbon tails and thickening the bilayer. (B) LPPs from simulations of zero-Cho (leftmost) and increasing-Cho bilayers (modified from (54)). LPP Z-direction dimensions and X,Y-pressures are shown. Pressures (due to repulsions and attractions) balance out. At lipid-water interfaces, pressure peaks typically exceed 1000 atm (1 atm ≈ 0.01 kPa). (C) LPPs from a solvent-free bilayer simulation (dotted line) and with two 1-alkanol concentrations (higher solid line) (modified from (55)). Membrane proteins feel structured pressures (pull, push). Small 1-alkanols thin a (solvent-free) bilayer and flatten pressure excursions but long chain alkanols (not shown (56)) act like cholesterol that amplifies local maxima and minima.
In lipid monolayers (8) and bilayers (9), Traube's rule implies that at a given 1-alkanol concentration, each additional CH2 group reduces area compressibility approximately threefold. For solvent-free vesicles with short-chain alkanols, bilayer mechanical properties, including surface and line tensions, thickness, compressibility and bending moduli, area expansion, and lysis tension, all vary as expected if partitioning into the bilayer occurs with increasing ease as acyl chains lengthen (9). To reconstitute VGCs, including KvAP (and KvCa) into planar bilayers, however, lipid/alkane bilayers are generally used (10) and alkane solvents alter the bilayer mechanics of alkanols (Fig. 1 A) and other SAAs. Small alkanols and cholesterol increase and decrease, respectively, alkane partitioning from torus to bilayer (11–13). For the [1-alkanol] range we used, short-chain alkanols in phospholipid/decane bilayers increase hydrocarbon tail packing order because a short alkanol, anchored by its hydroxyl group at the polar interface and by its acyl chain intercalated between phospholipid acyl chains, creates a void normal to the bilayer, into which decane packs (12). Therefore, reversible bilayer mechanical perturbations, induced in our experiments by short-chain 1-alkanols, involved operation of Traube's rule in conjunction with reversible solvent repacking.
If a membrane protein conformation change perturbs the immediate bilayer, then bilayer deformation energy contributes to the conformational free-energy change (14). The combined material properties of the bilayer (thickness, intrinsic lipid curvature, elastic compression, and bending moduli) allosterically modulate the protein (15). Conversely, if bilayer structure is changed by SAAs, protein structure at the protein-lipid interface will alter, with the energy of this deformation contributing to conformational free energy. Bilayer LPPs calculated from molecular dynamic simulations with and without various SAAs (e.g., alkanols, cholesterol; see Fig. 1, B and C, legend for references) imply that localized forces associated with conformation changes, of which Kv tetramers have many, would confront reactive forces dependent on the bilayer structure (16,17).
Kv channels are cruciform homotetramers with peripheral S1–S4 voltage sensors coupled loosely to the S5–S6 central pore (6). Voltage sensor motions entail certain S4 arginine/lipid-phosphate interactions whereas charged residues otherwise avoid contacting hydrocarbons (5,18). Kv channel equilibria adjust to physical or chemical perturbations of bilayer structure; rates of at least three Kv1 channel transitions are stretch-sensitive (activation and slow inactivation accelerate, whereas concerted prepore-opening decelerates (19,20)) and Kv channel gating is modulated by many SAAs including alkanols (16,17) and cholesterol (21) (see reviews 2,4). Substituting wedge-shaped PE with cylindrical PC right-shifts the KvAP activation curve and lessens the apparent gating charge/subunit (5). If this reflects a frustrated spontaneous curvature effect on conformational stability, the attendant free energy change implies a ∼150 Å2 X-sectional expansion on activation (22), comparable to the expansion (∼170 Å2) calculated for Kv1 channels activating (in biomembranes) without and with stretch (23).
To alter KvAP bilayer mechanics, we used 1-alkanols, anesthetics, and cholesterol. Unitary conductance was measured for all agents, whereas activation and inactivation parameters were measured without and with ethanol, propanol and butanol. Finally we asked if ethanol has the same effect on KvAP conductance regardless of the presence of cholesterol in the bilayer.
Materials and Methods
Stock solutions of 1-palmitoyl-2-oleoyl phosphatidylethanolamine (PE; 9.35 mg/mL), phosphatidylglycerol (PG; 3.12 mg/mL), and cholesterol (Cho; 44 mg/mL) (Avanti Polar Lipids, Alabasta, AL) were stored in sealed ampoules at −80°C. Recombinant wild-type KvAP channels reconstituted into vesicles were kindly provided by Dr. R. MacKinnon (Rockefeller University, New York, NY).
Planar bilayer recording
Nylon chambers and Delrin cups (150–250-μm diameter holes; Warner Instruments, New Haven, CT) were used to form vertical planar lipid bilayers. Recordings were in symmetrical [K+] (150 mM KCl, 10 mM Hepes, pH 7). Junction potentials were zeroed before bilayer formation and retested at membrane disruption: drift was <2 mV. Ag-AgCl electrodes (In Vivo Metric, Healdsburg, CA) were connected to cis and trans chambers by salt bridges (2% agar, 3 M KCl, 10 mM EDTA).
KvAP recordings were carried out in bilayers of PE:PG (3:1) in decane. Cholesterol was incorporated into the lipid mixture by mixing chloroform stock solutions in the appropriate ratio (0.7–2 μL of PE:PG was replaced by Cho). Lipid mixtures were dried under nitrogen, washed with pentane, and re-dried before dispersion in decane to final [Cho]s of 0, 8, 11, or 23 mM (0, 0.32, 0.41, 0.60 mol-fraction of Cho in the four-component mixture of PE, PG, Cho, and decane). The resulting decane dispersion was used to form bilayers.
For channel insertion, a 10-μL aliquot of reconstituted vesicles was kept on ice and periodically agitated for 5–10 s in a bath sonicator. A newly formed bilayer was painted from the cis side with ∼0.5 μL of reconstituted vesicles and excess lipid was removed with a clean spatula.
An Axopatch-1B amplifier, a Digidata 1322A interface, and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA) were used for voltage control and data collection by a personal computer. Current traces were filtered at 0.5 or 20 kHz (−3 dB, low pass, 4-pole Bessel filter) and sampled at 5 or 66.7 kHz, respectively. Records were made at room temperature, at −100 and +100 mV to check for channels incorporated in either orientation. In multichannel membranes, unitary current was measured after most channels inactivated. SAA solutions were perfused into both chambers at the specified concentrations (most channel recordings and all capacitance measurements), or concentrated SAA was added directly to both sides of the chamber and stirred. No differences were observed between these methods.
To measure specific capacitances, protein-free bilayers were used in a horizontal chamber on the stage of a compound microscope. For the data reported in Fig. 2 A, single voltage ramp protocols were used to sample total capacitance discretely during the thinning process, and changes in bilayer area were monitored using a video camera. Calibration was checked with a model circuit. ImageJ (NIH, Bethesda, MD) was used for bilayer area measurements. Capacitance (μF) versus area (cm2) plots were fit with a linear regression line, forced through the prethinning blob capacitance, and bilayer specific capacitance (μF/cm2) was given by the slope. For each alkanol concentration, collected data from several experiments were fit; with one exception, the maximum capacitance recorded in a data set corresponded to a bilayer area of >50% of the visible area of the hole. For further data and details, including an alternate protocol, see the Supporting Material.
Figure 2.
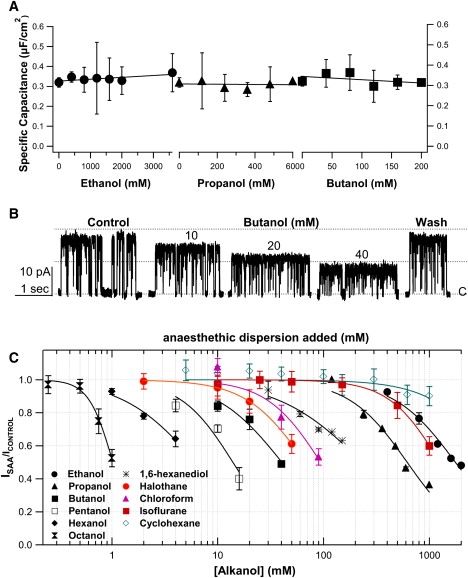
Adding 1-alkanols to bilayers. (A) PE:PG/decane bilayer specific capacitance did not change with ethanol, propanol, or butanol. (B) Reversible reduction of KvAP unitary current amplitude by alkanols. Unitary currents in control conditions, in different butanol concentrations, and after washout. (C) Concentration-response curves from unitary current amplitude in alkanol normalized to control. Mean ± SE of four to seven experiments are plotted.
Data analysis
Single-channel current was estimated from fits to all-point histograms with and without SAAs. Amplitude estimated at 0.5 and 20 kHz were not significantly different.
Half-maximal activation (V1/2) was determined by fitting peak IV plots to the equation
| (1) |
Peak currents, activation, and inactivation time constants (τrise, τinact), were determined by fitting macroscopic currents to an expression of the form:
| (2) |
Summary data are expressed as mean ± SE (n), where n is number of experiments.
Results
Bilayer capacitance
SAA influences on mechanical properties of planar bilayers, including thickness and hence specific capacitance, depend on the hydrocarbon solvent used to disperse the lipids. Adding short-chain alkanols, cholesterol or other SAAs to PE:PG/decane bilayers should have two classes of effects: 1), altered partitioning of decane between torus and bilayer; and 2), altered packing or orientation of bilayer-resident decane molecules (Aagaard et al. (12) and Fig. 1 A). Bilayer capacitance measurements (see the Supporting Material) yielded a specific capacitance for PE:PG/decane bilayers of 0.36 ± 0.06 μF/cm2 (n = 3), low compared to biological membranes, but as expected if decane accumulates in the midplane and thickens the bilayer. Consistent with that interpretation, capacitance was higher (0.65 ± 0.06 μF/cm2, n = 4, p = 0.005) for bilayers formed with hexadecane as solvent, as found in earlier studies (24,25). Hexadecane orients almost exclusively normal to the membrane plane (12,13). Adding short-chain alkanols did not measurably affect specific capacitance of PE:PG bilayers formed with decane or hexadecane (Fig. 2 and Table S1). PG:PE/decane bilayers with cholesterol (23 mM) had a higher specific capacitance (0.56 ± 0.04 μF/cm2, n = 8) than those without (p = 0.03) in agreement with the findings of Coster and Laver (11) who showed that cholesterol causes decane to partition from phospholipid bilayer to torus.
From our capacitance measurements and other findings for planar and vesicular bilayers (11,12,26), we assume: 1), the major 1-alkanol-induced bilayer mechanical change in our phospholipid/decane bilayers was increased packing order; and 2), membranes had greater bilayer packing order with cholesterol than without, but were thinner due to relative exclusion of decane (Table S1).
Alkanols and general anesthetics: KvAP unitary conductance
The principal unitary conductance of KvAP in PE:PG/decane bilayers was 150 ± 26 pS. Fig. 2 B is a representative experiment in control solution, then with increasing [SAA] (1-butanol), and then after washout. Conductance decreased in a reversible, dose-dependent manner as for all SAAs tested (Fig. 3), except for cholesterol that was included in the bilayer mix and could not be removed in the time course of the experiments.
Figure 3.

Various SAAs and KvAP unitary current. Alkanols and anesthetics decreased single channel currents as seen from representative records for 1-alkanols (C2–C8), 1,6-hexanediol, halothane, chloroform, and isoflurane.
With alkanols, unitary conductance decreased to 67% at the following concentrations (IC67 in mM): octanol, 0.84; hexanol, 3.5; pentanol, 9.2; butanol, 24; propanol, 391; and ethanol, 1090 (Fig. 2 C). For 1,6-hexanediol IC67 was 120 mM. For the volatile anesthetic SAAs, and chloroform, aqueous solubility constraints mean that the IC67 values (62, 42, and 850 mM, for chloroform, halothane, and isoflurane respectively; Fig. 2 C) are meaningful only for chloroform (for which 70 mM is the limit of aqueous solubility). Halothane and isoflurane values refer not to concentrations but to mmol/L of material dispersed in saline in the open-system bath. The large isoflurane amount likely reflects faster off-gassing (its blood-gas partition coefficient being ∼0.5 that for halothane). We include these data to show that, like chloroform and alkanols, volatile anesthetics caused reversible and dose-dependent reductions of KvAP unitary conductance.
If KvAP conductance was alkanol-modulated strictly by a Traube mechanism, there would be no optimum alkanol chain length for inhibition; IC67 would simply drop approximately threefold per carbon. Instead, from propanol to butanol, conductance dropped more than expected on that basis, and from hexanol to octanol, less than expected. IC67 values went as octanol < hexanol < pentanol < butanol < 1,6 hexanediol < propanol < ethanol. To express IC67 in terms of bilayer concentrations, aqueous values were multiplied by standard alkanol membrane/buffer partition coefficients, Pm/b (27) (octanol, 189; hexanol, 21.4; pentanol, 5.02; butanol, 1.52; propanol, 0.438; ethanol, 0.096; and 1,6-hexanediol, 0.957) giving an IC67 ranking of: butanol < pentanol < hexanol < ethanol ≈ 1,6 hexanediol < octanol < propanol (i.e., IC67 values for bilayer [alkanol] in mM: 36, 46, 75, 105, 115, 159, 171). Thus, intermediate-sized 1-alkanols (C4–C5) most strongly decreased KvAP conductance, whereas 1,6-hexanediol and ethanol were equally effective.
Mostly outward K+ current was monitored, but unitary inward current also diminished with alkanols (Fig. 4, A–C). Because 1-alkanols (C2–C8), a 1,6-alkanol, and three halogenated anesthetics all produced the same reversible effect on conductance, SAAs in PE:PG/decane bilayers must affect channel regions responsible for K+ throughput, including the selectivity filter and/or the cavity on its intracellular-side. The underlying mechanism(s) is smoothly dose-dependent at the single channel level for all the SAAs, as if progressive bilayer changes progressively distorted the open KvAP protein. Alternately, SAAs might have increased the rate of unresolved flickery transitions to lower conductance state(s). That could account for our data only if flicker rates were >20 kHz, given that Gaussian filtering of alkanol-inhibited channel records at corner frequencies 0.5 and 20 kHz yielded the same apparent unitary channel amplitude. Although the inhibition mechanism is uncertain, our data provide multiple constraints. Two examples: 1), 1,6 hexanediol and ethanol were equally effective, based on calculated bilayer concentrations; and 2), a bilayer hexanol molecule was several-fold more effective than a bilayer chloroform molecule, based on relative octanol/water partition coefficients. A third possibility, that SAAs acted by partially occluding the permeation pathway, is unlikely, requiring that 0.75 mM octanol (aqueous), 800 mM ethanol, 20 mM butanol, and 40 mM chloroform all achieve equivalent occlusions, with higher and lower concentrations all producing smoothly graded unitary current inhibition.
Figure 4.
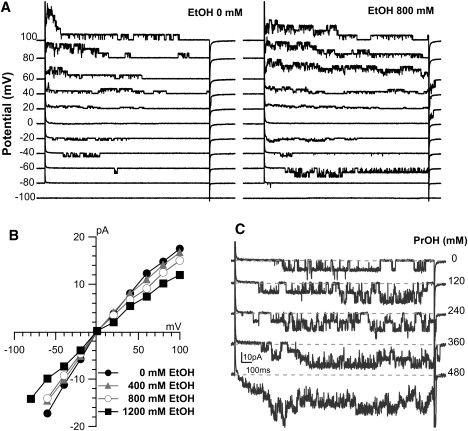
Unitary KvAP channel currents with increasing [ethanol] or [propanol]. (A) Response to 2-s steps between −100 and +100mV in control and 800 mM ethanol. Increased channel activity at −60 mV in 800 mM ethanol reflects a leftward shift of activation (see Fig. 5D). Slowed inactivation is apparent in 800 mM ethanol traces (see Fig. 5A). (B) Shape of the unitary IV relationship did not noticeably change with ethanol. (C) Increasing [propanol] elicited more unitary fluctuations (step from V = −100 to −60 mV).
Alkanols: KvAP gating kinetics
Macroscopic KvAP current families with and without propanol, plus corresponding IV curves, are shown in Fig. 5, A and B. Ethanol was tested at several concentrations (Fig. 5 C) with increasing [ethanol] shifting the IV negative slope region toward more hyperpolarized potentials, as indicated by activation midpoints (Fig. 5 D). Control V1/2 was −34 ± 2 mV whereas at 800 mM ethanol it was −58 ± 3 mV, a ∼−24 mV shift (n = 3–5; p < 0.001). Alkanol-induced left shifts caused notable current increases at negative voltages (Fig. 5, B and C), sometimes accentuated by an increased Gmax (Fig. 5 C) that, with ethanol, could be as much as fourfold (not shown) presumably reflecting increased KvAP recruitment into the bilayer with alkanol.
Figure 5.
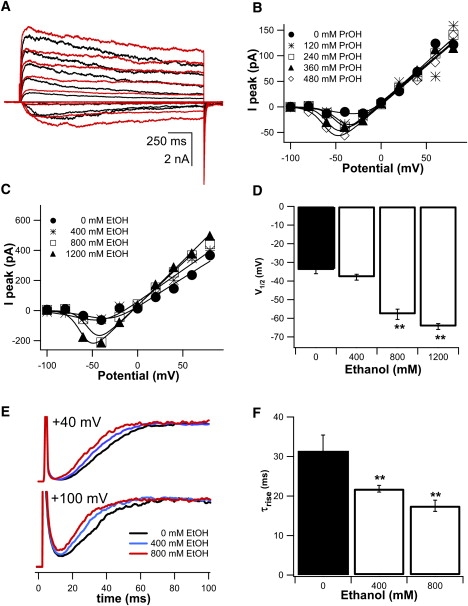
Alkanols shifted KvAP activation to more negative potentials. From Vhold = −100 mV, channels were activated at 45-s intervals by 2-s steps from −60 mV to +100 mV in 20 mV increments. Solutions were symmetric, both in terms of ionic content and alkanol. (A) Propanol effects. Macroscopic current traces for control (black) and 120 mM propanol (red). (B) Peak current IVs for control and increasing propanol; smooth curves are fits to Eq. 1. (C) Ethanol effects. IVs for control and increasing ethanol; smooth curves as in B. (D) Half-maximal activation, from Eq. 1 fits, shifted in the hyperpolarizing direction (comparisons of V1/2 with control values: p = 0.17, 0.0004 and 0.0001 for 400, 800 and 1200 mM ethanol, respectively, n = 3–5). (E) For increasing ethanol, early currents at two voltages normalized to peak and superimposed. (F) Average activation time constants from Hodgkin-Huxley type fits (Eq. 2) to the rising phase during steps to +100 mV (comparisons of τrise with control values: p = 0.01 and 0.008 for 800 and 1200 mM ethanol, respectively, n = 12). ∗∗Denotes significant difference from control (p < 0.01).
KvAP activation was significantly faster with alkanols as illustrated by normalized and superimposed traces (400 and 800 mM ethanol; Fig. 5 E) for macroscopic KvAP currents elicited by steps to +40 mV and +100 mV. At +100 mV, the KvAP activation time constant (τrise) was 31 ± 4 ms (n = 12), with 800 mM ethanol it was 18 ± 1 ms (n = 12; p < 0.01; Fig. 5 F).
KvAP inactivation, a slow C-type process, slowed with alkanol addition, as seen in Fig. 5 A for families of currents with and without propanol. Fig. 6, A–C, shows representative traces before and during exposure to alkanols, with normalized insets showing the robustly inhibited KvAP inactivation. Inactivation speed at +100 mV was quantified by the time constant of mono-exponential fits to 10 s of current decay. Fig. 6, D and E, shows the first 2.5 s of a set of KvAP currents (plus fits) for control, increasing [ethanol], and after washout. Inactivation time constants increased, relative to control, by up to 2.5-fold for hexanol, up to 4.5-fold for butanol, and to 6.5-fold for ethanol; all plotted ratios represent significant differences from controls (Fig. 6 F; n ≥ 3; p < 0.001). Based on the plotted regressions and the partition coefficients listed earlier, the estimated bilayer [alkanol] required to slow inactivation e-fold (2.7-fold) for hexanol, butanol and ethanol were 43 mM, 18 mM, and 48 mM (corresponding to aqueous 2, 12, and 500), yielding bilayer efficacy in slowing inactivation: butanol > hexanol ≅ ethanol. Thus, as with unitary conductance, alkanol inhibition of inactivation was dose-dependent and reversible. The two actions occurred in about the same concentration range, with butanol showing greatest bilayer efficacy for both.
Figure 6.
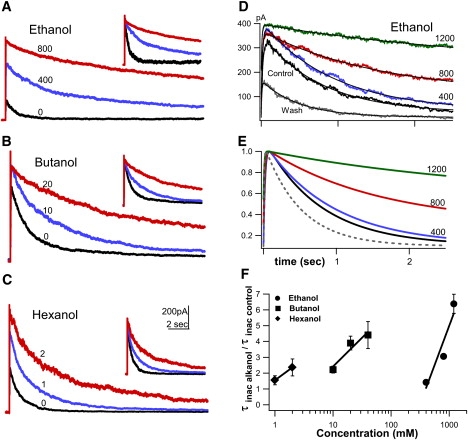
Alkanols slowed KvAP inactivation. Currents during 15-s steps to +100 mV (Vhold = −100 mV) with and without (in mM, adjacent to traces): (A) ethanol, (B) butanol, and (C), hexanol. Insets are superimposed peak-normalized traces. (D) Superimposed KvAP currents, increasing ethanol. Fits to Eq. 2 are superposed. (E) Exponential product fits to Eq. 2, from B, normalized to peak, showing the increased τinact with ethanol. Solid dark lines, control; dashed lines, wash. (F) Inactivation time constants at +100 mV. τinact in alkanol divided by τinact control (0.94 ± 0.17 s) plotted versus [alkanol] (n = 3–6 experiments).
Bilayer cholesterol: KvAP unitary conductances and cholesterol/ethanol interactions
Including cholesterol with PE:PG/decane significantly decreased KvAP unitary conductance (compared to zero-Cho bilayers) at all three levels tested (Fig. 7, A and B). With 8 mM and 11 mM Cho, the drop was slight, but with 23 mM, KvAP unitary conductance fell sharply to ∼50% of that seen in zero-Cho bilayers (Fig. 7, A and B, left).
Figure 7.
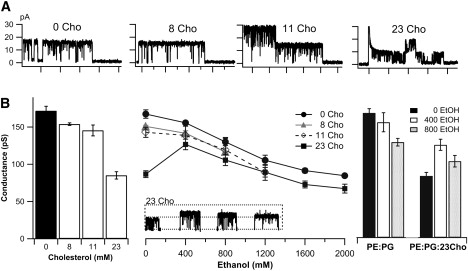
Bilayer cholesterol content affected KvAP unitary conductance. [Cho] (mM) in the bilayer-forming decane dispersion, indicated. (A) Unitary current decreased with increasing [Cho] in PE:PG/decane bilayers. (B, left) Unitary conductance decreased with increasing [Cho]. (B, middle) Effect of ethanol on unitary conductance depended on [Cho]: without cholesterol, progressive addition of ethanol (aqueous, mM) monotonically decreased conductance but for 23 mM Cho bilayers, conductance increased at 400 mM ethanol then decreased with further increases in ethanol. (B, right) Summary of ethanol-associated changes in KvAP unitary conductance, from bilayers with/without cholesterol (ethanol concentrations shown as mM EtOH).
The effect of ethanol on unitary conductance was examined in these bilayers. With 8 or 11 mM Cho, ethanol at increasing concentrations lowered the conductance, paralleling the effect of ethanol on Cho-free bilayers (Fig. 7 B). For the most cholesterol-rich (23 mM) bilayers, however, the lowest [ethanol] tested, 400 mM, sharply increased unitary conductance. Further elevation of [ethanol] then acted as for Cho-free bilayers and progressively reduced conductance.
Difficulty of incorporating large numbers of channels into cholesterol bilayers precluded testing for ethanol-Cho kinetic interactions. For a single case in which it was briefly possible to observe macroscopic KvAP current in a low-Cho bilayer (8 mM), the KvAP current response to ethanol resembled that of channels in the zero-Cho bilayers.
Discussion
Overview
The molecular science of alkanol and anesthetic action is contentious (28,29). VGCs are implicated in many alkanol and anesthetic actions (30–32) but it is unclear whether SAAs bind at saturable protein sites or act as bilayer mechanical reagents (33,34). KvAP responses to SAA-induced bilayer mechanical perturbations are of interest because KvAP can be used to explore structural underpinnings of SAA actions in ways not presently feasible for VGCs for which crystal structures are unavailable. Specific capacitance measurements of bilayers with decane or hexadecane and (for the decane-containing bilayers) with and without different SAAs, confirmed the crucial role of repartitioning and reorientation of decane in the bilayer mechanical (i.e., bilayer structural) changes elicited by SAAs. We used short-chain 1-alkanols, cholesterol, and volatile anesthetics. Bilayer efficacy for inhibiting unitary conductance (from calculated bilayer [alkanol]) went as butanol > pentanol > hexanol > ethanol > octanol > propanol. Alkanols slowed KvAP inactivation, with bilayer efficacy butanol > hexanol ≈ ethanol. Alkanols accelerated KvAP activation and IV relations shifted in the hyperpolarizing direction. The anesthetics and 1,6-hexanediol also diminished unitary conductance as did inclusion of cholesterol in the bilayer. Ethanol (400 mM) increased KvAP unitary conductance at a high mole fraction of Cho but decreased it in zero-Cho bilayers (Fig. 6).
KvAP and bilayer mechanics
As itemized below, our data suggest KvAP channels, together with their immediate bilayer surround, adjust to SAA-induced changes in bilayer structure. First, 11 SAAs, with quite different structures, altered KvAP unitary conductance with smoothly graded dose-dependent effects, and where tested, concomitant gating changes occurred at roughly the same alkanol levels. These parallel outcomes seem incompatible with binding site-mediated actions, but easier to reconcile with effects of lipid-mediated stress.
Second, alkanols in the C4–C6 range are most potent at generating collapsing voids in phospholipids bilayers (12). In our experiments, increased bilayer packing order would result as voids filled with oriented decane (Fig. 1 A, and Coster and Laver (11)), and C4 was optimal for inhibiting KvAP conductance. Alkanol chain-length cutoffs for modulation of Kv3-Shaw2 and KvCa channels (C11 and C8, respectively, in biomembranes) (35) were formerly seen as binding site calipers, but lateral stress explanations now seem more plausible (30,34,35). Likewise, we do not invoke steric constraints of a putative KvAP alkanol-binding site to explain the C4 (butanol) optimum for altered conductance and inactivation. Butanol plus decane presumably generates more bilayer order than decane plus shorter or longer alkanols (12). In cases where butanol increased mid-plane disorder and thinned the bilayer (e.g., solvent-free bilayers), qualitatively different KvAP responses would be expected. Attempts at mechanistic interpretation of our functional studies based on earlier bilayer experiments raised difficulties, motivating our own measurements of bilayer specific capacitance to evaluate possible changes in membrane thickness induced by the alkanols (Fig. 2 A and the Supporting Material). This prompts a cautionary note. If measurements of bilayer properties, such as specific capacitance, are to assist in interpretation of functional data, it is important that they be carried out under conditions matching those used for the functional studies. An examination of careful, earlier studies of parameters including specific capacitance and interfacial tension (11,26,36), and comparison with more recent experimental and theoretical analyses (9,12), suggests the difficulty of extrapolating mechanistic conclusions among studies in different systems. Factors including lipid composition, presence and nature of a solvent or dispersant, general structure and size of the SAA under test, and the ionic strength of the bathing solutions, should all be considered as potentially significant determinants of membrane properties, including membrane thickness, and hence as likely modulators of membrane-protein interactions.
Third, increased [SAA]s diminished K+ throughput as if progressively deforming a variable series resistance in the KvAP permeation path (selectivity filter, and/or inner cavity). Consistent with increased-packing-order-induced deformation, C4 alkanol was more effective (per bilayer molecule) than C2 or C8. Packing effects could also underlie cholesterol-inhibited conductance. In solvent-free bilayers, cholesterol and small alkanols would not act similarly (see their opposing LPP effects; Fig. 1, B and C), but with decane present, packing order increases with both (11,12). Volatile anesthetics also alter bilayer LPPs. For example, halothane prefers the headgroup region, reducing hydrocarbon tilt angles and decreasing peptide-lipid contacts, by lingering near anchoring tryptophan residues (28,37,38). Like small alkanols, halothane in phospholipid/decane bilayers should augment order. If so, comparable packing-induced deformation of the KvAP permeation path by alkanols, anesthetics and cholesterol is intuitively reasonable.
Fourth, at high-Cho, 400 mM ethanol increased KvAP conductance but the opposite response (decreased conductance) was obtained in zero-Cho bilayers. At all Cho levels, ethanol ≥800 mM decreased KvAP conductance. This is understandable if ethanol exerts two distinct structural effects on PE:PG/Cho/decane bilayers: i), decane recruitment into mid-plane voids; and ii), dispersion of high-packing order cholesterol meta-structures (35) that, as discussed previously, diminish KvAP conductance. The biphasic response to ethanol in high-Cho bilayers suggests that from 0–400 mM ethanol, effect ii) dominates, whereas for ≥800 mM ethanol, effect i) dominates. Reconciling this pattern with direct ethanol-channel interactions, such as partial pore occlusion or binding allosterism from beyond the conducting path, would be difficult.
KvAP binding versus LPP perturbations
Near KvAP/bilayer lateral interfaces, SAAs could be constrained yet not bound. For example, butanol aligns its −OH moiety near the lipid-water interface, packing its acyl chain against those of phospholipids, altering protein/lipid interactions. Such LPP actions are distinct from that of a high affinity bilayer-active voltage sensor toxin (39–41) that right-shifts Kv activation by binding to bilayer-embedded S3 residues: regular and enantiomeric toxins both partition into the bilayer, but crucially, enantiomers neither bind S3 nor inhibit gating (40). These toxins do not alter Kv activation via perturbation of bilayer LPP.
It is argued for Kv3 channels that alkanol inhibition involves a discrete alcohol-binding pocket loosely related to LUSH, a crystallized motif from a Drosophila ethanol detector protein (42). Although KvAP primary sequence reveals no hint of this motif, butanol efficacy on KvAP matches that on Kv3 channels, whose putative alkanol-binding site butanol KD is 19 mM (7). For KvAP, 20 mM butanol reduced KvAP unitary conductance by ∼1/e, whereas 10 mM butanol doubled τinactivation. In addition, KvCa channels, also lacking a LUSH motif, are almost as effective at detecting ethanol as LUSH that has an EC50 for ethanol of 107 mM (43). Thus, it seems likely that KvAP, KvCa (44,45), and other Kv channels, respond allosterically to alkanols and anesthetics based on induced changes in lateral pressure.
Possible coupling of LPP perturbations to KvAP structure
KvAP channels are derived from extremophiles whose native membranes are made rigid (46) by bolaform lipids. Because the intracellular cavity electrostatically tunes K channel conductances to specific physiological requirements (18), the striking graded SAA-susceptibility of KvAP unitary conductance might signify graded cavity deformation in a channel adapted to function in a deformation-resistant membrane.
Because physical bilayer deformation by stretching modulates multiple Kv gating transitions (46), modulation of multiple KvAP functions by chemical, SAA-mediated deformation is not unprecedented. Alkanols modulated KvAP unitary conductance, slow inactivation and activation kinetics. Accelerated left-shifted KvAP activation (alkanol-induced) signifies a lowered barrier for voltage sensor activation transitions. Given that alkanols in PE:PG/decane bilayers increase global bilayer order, the free energy component lowering the barrier to activation might be entropic. Voltage sensor activation restructures a high-curvature locally thinned region of bilayer (18); bilayer-resident short-chain alkanols might facilitate the needed bilayer deformation by increasing the number of bilayer-structural degrees of freedom.
For slow (C-type) inactivation, residues near the external vestibule rearrange, occluding the nonrigid (47) K-selectivity filter. LPP changes might simultaneously influence KvAP conductance and inactivation because interacting amino acids in the external vestibule and pore helix control selectivity filter stability. KvAP and KcsA structures align almost perfectly for KcsA residues controlling conductance and slow inactivation. In KcsA channels, slow inactivation depends on E71-D80 interactions (48). We infer that direct access by alkanols to this interaction zone is unlikely from the apparent inaccessibility of E71C channels to small reagents plus the ability of E71S channels to accommodate only one water (48). Certainly butanol could not fit between KvAP V192-D201 (equivalent to E71-D80 in KcsA). Interestingly, the noninactivating KcsA mutant, E71A, shows reduced conductance (48). This is reminiscent of KvAP in PE:PG/decane membranes, for which alkanols both slow inactivation and reduce conductance.
Response switching
For KvCa, ethanol increases open probability in Cho-free, but not high-Cho bilayers (44). With KvAP, 400 mM ethanol increased unitary conductance in high-Cho bilayers, yet decreased it in the absence of Cho. In Kv3-Shaw channels, whose current is stretch-potentiated (44), a mutation reverses butanol potentiation (WT) to inhibition (P410A), a switch thought to imply that P410 interacts with a discrete alkanol-binding site (49). But if alkanols act via bilayer mechanics, different Kv shapes, with different LPPs for WT versus mutant, could explain such a switch. In this spirit, mutations in a Kv (WT versus ILT Shaker) switch the stretch response from potentiation to inhibition (44) without the mutated residues being construed as components of a stretch-sensor site. As for KvCa, KvAP responses to SAAs are not solely specified by the target protein; bilayer properties matter. Diverse in vivo arrangements of cholesterol and other lipids predict a panoply of LPPs for channels within and among cells. Native cells expressing apparently identical channels might therefore be expected to show otherwise baffling differences, including opposite polarity of responses to SAAs.
SAAs and protein-lipid interactions
Phospholipid-phosphates contact outer S4 arginines in KvAP, an interaction mandatory for accessing the open state (5) and other Kv channels have precise lipid requirements for gating (50). Because 1-alkanol hydroxyl groups bind phospholipid phosphates (51), SAA-perturbed KvAP-phospholipid interactions might contribute to our results. Kv1.2 simulations in an open-like, possibly inactivated, state (52) show the intracellular bilayer leaflet precipitously thinned, by ∼0.5 nm, where it meets protein, and headgroup phosphates interact with multiple basic residues (18). Mutations affecting protein structure where lipid, protein, and water converge have been proposed to identify SAA-binding pockets (7,53), but they would alter the interfacial LPP, and therefore responses to SAAs. Except where SAAs show saturable, high affinity actions and/or pronounced selectivity between stereo-isomers it seems appropriate to favor the hypothesis that an SAA exerts an allosteric action via lateral pressure rather than through a binding pocket. Given the physiological importance of cholesterol-rich bilayer domains to VGC function, a fuller investigation of the effect of SAAs on KvAP conductance and structure, in bilayers containing cholesterol and/or other solvents, seems warranted.
Supporting Material
One table and two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01733-0.
Supporting Material
Acknowledgments
We thank R. MacKinnon for recombinant KvAP channels reconstituted in lipid vesicles, M. Colicos for the loan of a video camera, R. Latorre, A. M. Correa, and O. Andersen for helpful discussions and comments, and anonymous reviewers for constructive critiques.
This work was supported by grants from the Heart and Stroke Foundation of Canada (C.E.M.), and from the Canadian Institutes of Health Research (MOP-10053, R.J.F.; MOP-49517, C.E.M.).
Contributor Information
Robert J. French, Email: french@ucalgary.ca.
Catherine E. Morris, Email: cmorris@uottawa.ca.
References
- 1.Yifrach O., MacKinnon R. Energetics of pore opening in a voltage-gated K(+) channel. Cell. 2002;111:231–239. doi: 10.1016/s0092-8674(02)01013-9. [DOI] [PubMed] [Google Scholar]
- 2.Morris C.E., Juranka P.F. Lipid stress at play: mechanosensitivity of voltage-gated channels. Curr. Top. Membr. 2007;59:297–337. doi: 10.1016/S1063-5823(06)59011-8. [DOI] [PubMed] [Google Scholar]
- 3.Maguy A., Hebert T.E., Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc. Res. 2006;69:798–807. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Tillman T.S., Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem. Biophys. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt D., Jiang Q.X., MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.Y., Lee A., MacKinnon R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci. USA. 2005;102:15441–15446. doi: 10.1073/pnas.0507651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharji A., Kaplan B., Covarrubias M. The concerted contribution of the S4-S5 linker and the S6 segment to the modulation of a Kv channel by 1-alkanols. Mol. Pharmacol. 2006;70:1542–1554. doi: 10.1124/mol.106.026187. [DOI] [PubMed] [Google Scholar]
- 8.Traube I. Ueber die capillaritatsconstanten organischer stoffe in wasseriger losungen. Ann. Chem. Liebigs. 1891;265:27–55. [Google Scholar]
- 9.Ly H.V., Longo M.L. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 2004;87:1013–1033. doi: 10.1529/biophysj.103.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt D., Cross S.R., MacKinnon R. A gating model for the archeal voltage-dependent K(+) channel KvAP in DPhPC and POPE:POPG decane lipid bilayers. J. Mol. Biol. 2009;390:902–912. doi: 10.1016/j.jmb.2009.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coster H.G., Laver D.R. The effect of benzyl alcohol and cholesterol on the acyl chain order and alkane solubility of bimolecular phosphatidylcholine membranes. Biochim. Biophys. Acta. 1986;861:406–412. doi: 10.1016/0005-2736(86)90448-7. [DOI] [PubMed] [Google Scholar]
- 12.Aagaard T.H., Kristensen M.N., Westh P. Packing properties of 1-alkanols and alkanes in a phospholipid membrane. Biophys. Chem. 2006;119:61–68. doi: 10.1016/j.bpc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh T.J., Simon S.A., MacDonald R.C. The organization of n-alkanes in lipid bilayers. Biochim. Biophys. Acta. 1980;597:445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- 14.Wiggins P., Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys. J. 2005;88:880–902. doi: 10.1529/biophysj.104.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen O.S., Koeppe R.E. Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 16.Cantor R.S. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gullingsrud J., Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys. J. 2004;86:3496–3509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jogini V., Roux B. Dynamics of the Kv1.2 voltage-gated K+ channel in a membrane environment. Biophys. J. 2007;93:3070–3082. doi: 10.1529/biophysj.107.112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laitko U., Morris C.E. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a Shaker channel. J. Gen. Physiol. 2004;123:135–154. doi: 10.1085/jgp.200308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laitko U., Juranka P.F., Morris C.E. Membrane stretch slows the concerted step prior to opening in a Kv channel. J. Gen. Physiol. 2006;127:687–701. doi: 10.1085/jgp.200509394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajdú P., Varga Z., Gáspár R. Cholesterol modifies the gating of Kv1.3 in human T lymphocytes. Pflugers Arch. 2003;445:674–682. doi: 10.1007/s00424-002-0974-y. [DOI] [PubMed] [Google Scholar]
- 22.Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 2007;93:3884–3899. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabarean I.V., Morris C.E. Membrane stretch accelerates activation and slow inactivation in Shaker channels with S3-S4 linker deletions. Biophys. J. 2002;82:2982–2994. doi: 10.1016/S0006-3495(02)75639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dilger J.P., Benz R. Optical and electrical properties of thin mono olein lipid bilayers. J. Membr. Biol. 1985;85:181–189. doi: 10.1007/BF01871270. [DOI] [PubMed] [Google Scholar]
- 25.Fettiplace R., Andrews D.M., Haydon D.A. The thickness, composition, and structure of some lipid bilayers and natural membranes. J. Membr. Biol. 1971;5:277–296. doi: 10.1007/BF01870555. [DOI] [PubMed] [Google Scholar]
- 26.Elliott J.R., Haydon D.A. The influence of n-alkanols on the capacity per unit area of planar lipid bilayers. Biochim. Biophys. Acta. 1984;773:165–168. doi: 10.1016/0005-2736(84)90561-3. [DOI] [PubMed] [Google Scholar]
- 27.McCreery M.J., Hunt W.A. Physico-chemical correlates of alcohol intoxication. Neuropharmacology. 1978;17:451–461. doi: 10.1016/0028-3908(78)90050-3. [DOI] [PubMed] [Google Scholar]
- 28.Tang P., Xu Y. Large-scale molecular dynamics simulations of general anesthetic effects on the ion channel in the fully hydrated membrane: the implication of molecular mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA. 2002;99:16035–16040. doi: 10.1073/pnas.252522299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmings H.C., Akabas M.H., Harrison N.L. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Asuncion-Chin M., Dopico A.M. CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels. Nat. Neurosci. 2006;9:41–49. doi: 10.1038/nn1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ying S.W., Abbas S.Y., Goldstein P.A. Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur. J. Neurosci. 2006;23:465–480. doi: 10.1111/j.1460-9568.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey J.A., Hamming K.S., Nash H.A. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr. Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 33.Correa A.M. Gating kinetics of Shaker K+ channels are differentially modified by general anesthetics. Am. J. Physiol. 1998;275:C1009–C1021. doi: 10.1152/ajpcell.1998.275.4.C1009. [DOI] [PubMed] [Google Scholar]
- 34.Yuan C., O'Connell R.J., Treistman S.N. Regulation of the gating of BKCa channel by lipid bilayer thickness. J. Biol. Chem. 2007;282:7276–7286. doi: 10.1074/jbc.M607593200. [DOI] [PubMed] [Google Scholar]
- 35.Chu B., Treistman S.N., Treistman S.N. Modulation of two cloned potassium channels by 1-alkanols demonstrates different cutoffs. Alcohol. Clin. Exp. Res. 1997;21:1103–1107. [PubMed] [Google Scholar]
- 36.Elliott J.R., Haydon D.A. The interaction of n-octanol with black lipid bilayer membranes. Biochim. Biophys. Acta. 1979;557:259–263. doi: 10.1016/0005-2736(79)90108-1. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Xu Y., Tang P. Molecular dynamics simulations of C2F6 effects on gramicidin A: implications of the mechanisms of general anesthesia. Biophys. J. 2005;88:3784–3791. doi: 10.1529/biophysj.104.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vemparala S., Saiz L., Klein M.L. Partitioning of anesthetics into a lipid bilayer and their interaction with membrane-bound peptide bundles. Biophys. J. 2006;91:2815–2825. doi: 10.1529/biophysj.106.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruta V., MacKinnon R. Localization of the voltage-sensor toxin receptor on KvAP. Biochemistry. 2004;43:10071–10079. doi: 10.1021/bi049463y. [DOI] [PubMed] [Google Scholar]
- 40.Milescu M., Vobecky J., Swartz K.J. Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 2007;130:497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt D., MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc. Natl. Acad. Sci. USA. 2008;105:19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bucci B.K., Kruse S.W., Jones D.N. Effect of n-alcohols on the structure and stability of the Drosophila odorant binding protein LUSH. Biochemistry. 2006;45:1693–1701. doi: 10.1021/bi0516576. [DOI] [PubMed] [Google Scholar]
- 43.Thode A.B., Kruse S.W., Jones D.N. The role of multiple hydrogen-bonding groups in specific alcohol binding sites in proteins: insights from structural studies of LUSH. J. Mol. Biol. 2008;376:1360–1376. doi: 10.1016/j.jmb.2007.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowley J.J., Treistman S.N., Dopico A.M. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol. Pharmacol. 2003;64:365–372. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- 45.Yuan C., O'Connell R.J., Treistman S.N. Acute alcohol tolerance is intrinsic to the BKCa protein, but is modulated by the lipid environment. J. Biol. Chem. 2008;283:5090–5098. doi: 10.1074/jbc.M708214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Vossenberg J.L., Driessen A.J., Konings W.N. The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles. 1998;2:163–170. doi: 10.1007/s007920050056. [DOI] [PubMed] [Google Scholar]
- 47.Noskov S.Y., Bernèche S., Roux B. Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature. 2004;431:830–834. doi: 10.1038/nature02943. [DOI] [PubMed] [Google Scholar]
- 48.Cordero-Morales J.F., Jogini V., Perozo E. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 2007;14:1062–1069. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 49.Harris T., Graber A.R., Covarrubias M. Allosteric modulation of a neuronal K+ channel by 1-alkanols is linked to a key residue in the activation gate. Am. J. Physiol. Cell Physiol. 2003;285:C788–C796. doi: 10.1152/ajpcell.00113.2003. [DOI] [PubMed] [Google Scholar]
- 50.Swartz K.J. Greasing the gears of potassium channels. Nat. Chem. Biol. 2006;2:401–402. doi: 10.1038/nchembio0806-401. [DOI] [PubMed] [Google Scholar]
- 51.Feller S.E., Brown C.A., Gawrisch K. Nuclear Overhauser enhancement spectroscopy cross-relaxation rates and ethanol distribution across membranes. Biophys. J. 2002;82:1396–1404. doi: 10.1016/S0006-3495(02)75494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villalba-Galea C.A., Sandtner W., Bezanilla F. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA. 2008;105:17600–17607. doi: 10.1073/pnas.0807387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andres-Enguix I., Caley A., Franks N.P. Determinants of the anesthetic sensitivity of two-pore domain acid-sensitive potassium channels: molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J. Biol. Chem. 2007;282:20977–20990. doi: 10.1074/jbc.M610692200. [DOI] [PubMed] [Google Scholar]
- 54.Patra M. Lateral pressure profiles in cholesterol-DPPC bilayers. Eur. Biophys. J. 2005;35:79–88. doi: 10.1007/s00249-005-0011-0. [DOI] [PubMed] [Google Scholar]
- 55.Frischknecht A.L., Frink L.J. Alcohols reduce lateral membrane pressures: predictions from molecular theory. Biophys. J. 2006;91:4081–4090. doi: 10.1529/biophysj.106.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griepernau B., Böckmann R.A. The influence of 1-alkanols and external pressure on the lateral pressure profiles of lipid bilayers. Biophys. J. 2008;95:5766–5778. doi: 10.1529/biophysj.108.142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


