Abstract
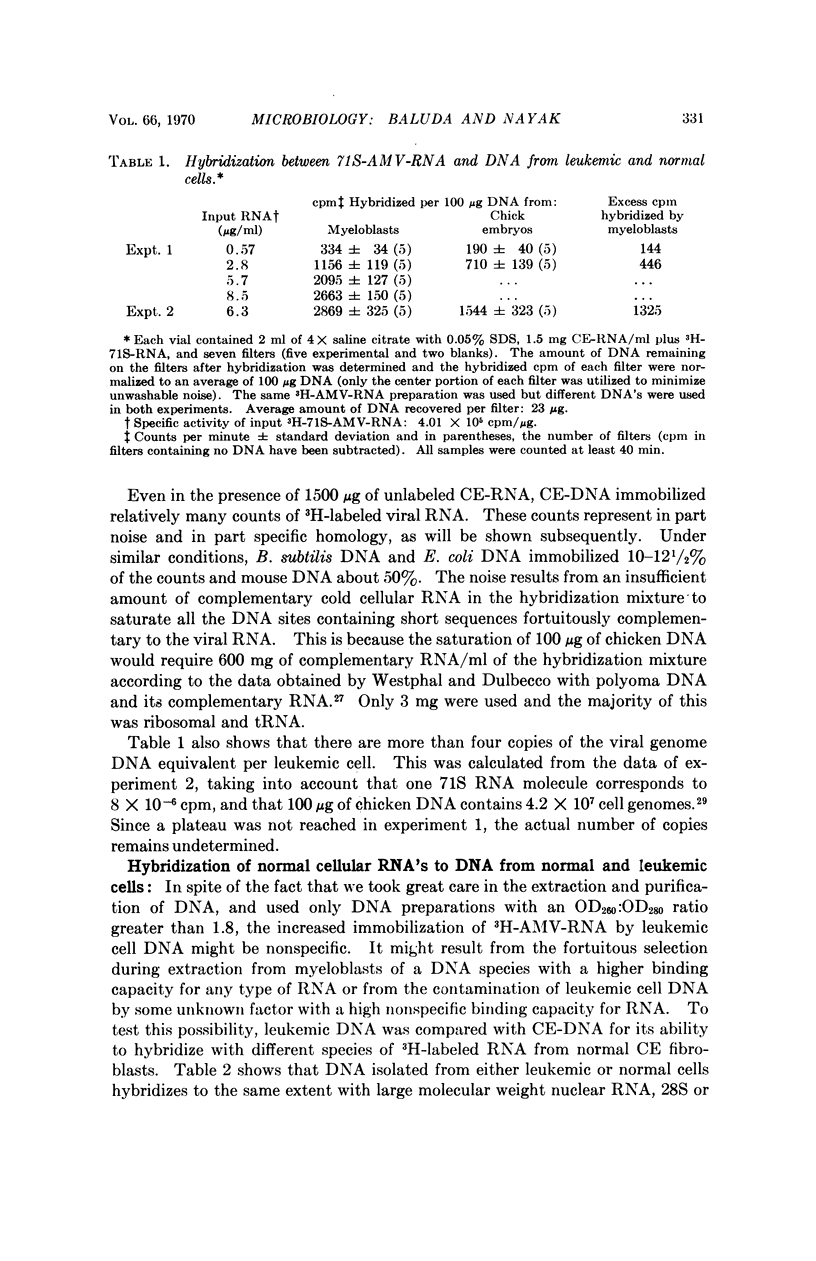
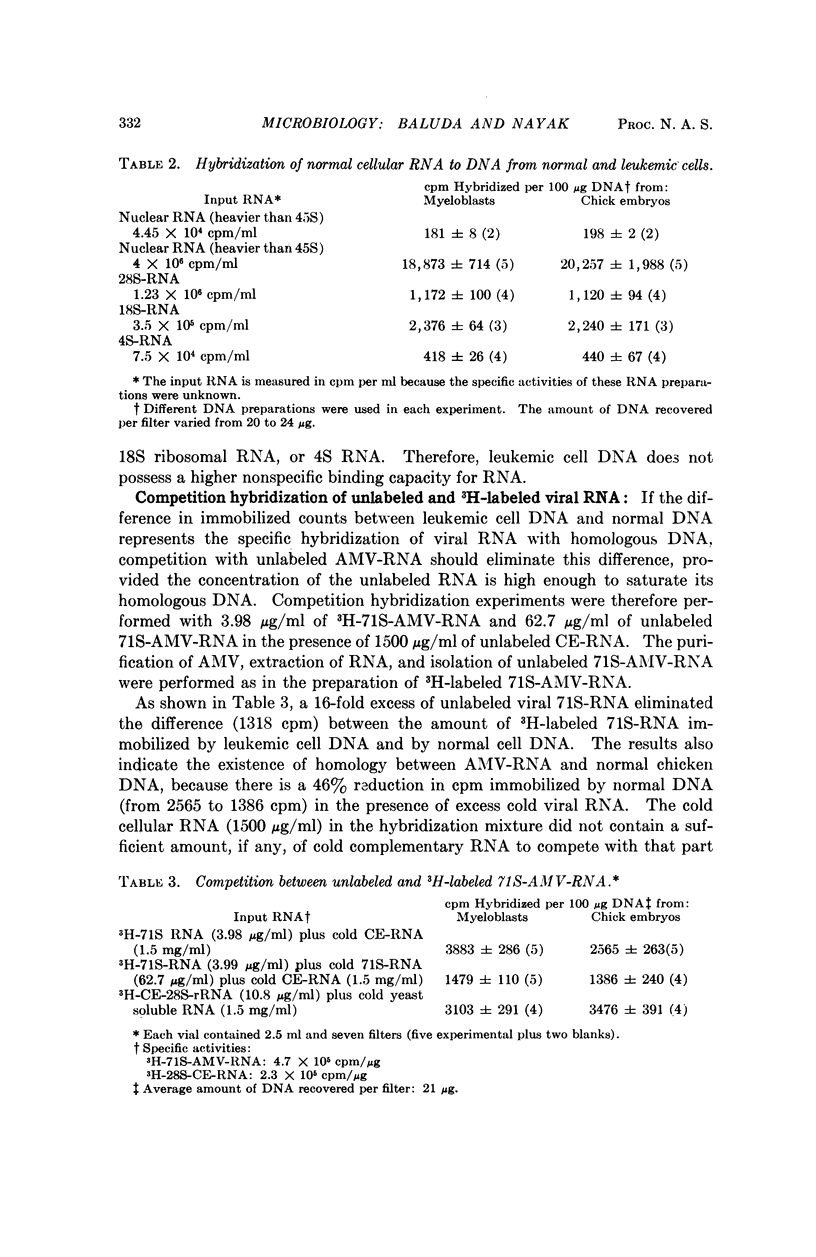
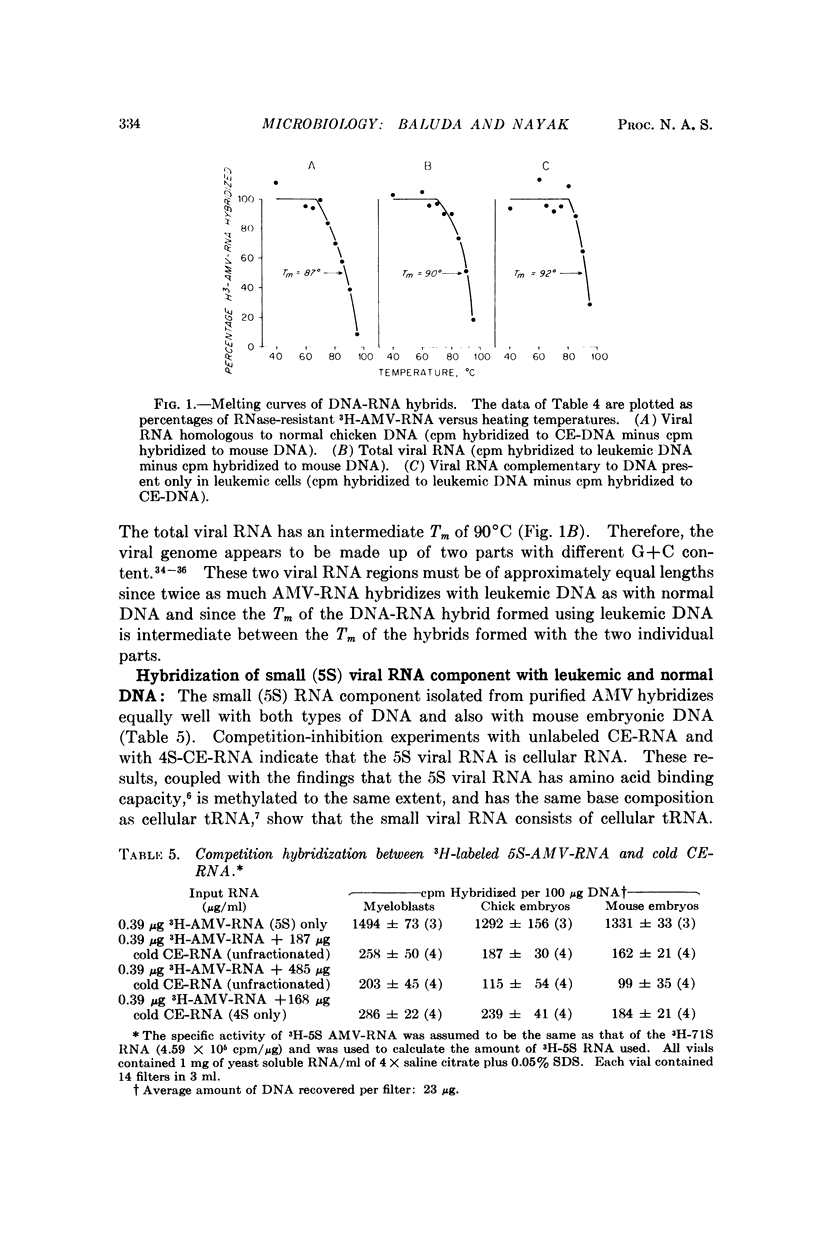
Nucleic acid hybridization studies were made between 71S-AMV-RNA and DNA from leukemic myeloblasts and from normal chicken cells. There was homology between the viral RNA and chicken cell DNA and to a greater extent between viral RNA and leukemic cell DNA. Leukemic cell DNA hybridized approximately twice as much viral RNA as did normal chicken DNA. Thermal melting studies showed that the viral RNA bound to normal and leukemic cell DNA consists of long polynucleotides (Tm = 87° and 92°C, respectively, in 2× saline citrate). This suggests that the leukemic cells contain a DNA template of the viral RNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE REQUIREMENT FOR DNA SYNTHESIS IN THE GROWTH OF ROUS SARCOMA AND ROUS-ASSOCIATED VIRUSES. Virology. 1965 Jun;26:253–261. doi: 10.1016/0042-6822(65)90272-2. [DOI] [PubMed] [Google Scholar]
- BADER J. P. THE ROLE OF DEOXYRIBONUCLEIC ACID IN THE SYNTHESIS OF ROUS SARCOMA VIRUS. Virology. 1964 Apr;22:462–468. doi: 10.1016/0042-6822(64)90067-4. [DOI] [PubMed] [Google Scholar]
- BOLTON E. T., MCCARTHY B. J. FRACTIONATION OF COMPLEMENTARY RNA. J Mol Biol. 1964 Feb;8:201–209. doi: 10.1016/s0022-2836(64)80129-7. [DOI] [PubMed] [Google Scholar]
- BONAR R. A., PURCELL R. H., BEARD D., BEARD J. W. VIRUS OF AVIAN MYELOBLASTOSIS (BAI STRAIN A). XXIV. NUCLEOTIDE COMPOSITION OF THE PENTOSENUCLEIC ACID AND COMPARISON WITH STRAIN R (ERYTHROBLASTOSIS). J Natl Cancer Inst. 1963 Sep;31:705–716. [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. The role of the bursa-dependent lymphoid tissue in oncogenesis by avian myeloblastosis virus. Virology. 1967 Jul;32(3):428–437. doi: 10.1016/0042-6822(67)90294-2. [DOI] [PubMed] [Google Scholar]
- Biswal N., Benyesh-Melnick M. Complementary nuclear RNA's of murine sarcoma-leukemia virus complex in transformed cells. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1372–1379. doi: 10.1073/pnas.64.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R. TRANSFORMATION OF CELLS IN VITRO BY VIRUSES. Science. 1963 Nov 15;142(3594):932–936. doi: 10.1126/science.142.3594.932. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Harel J., Huppert J., Lacour F., Harel L. Mise en évidence d'un acide ribonucléique de très haut poids moléculaire dans le virus de la myéloblastose aviaire. C R Acad Sci Hebd Seances Acad Sci D. 1965 Sep 13;261(11):2266–2268. [PubMed] [Google Scholar]
- Harel L., Harel J., Lacour F., Huppert J. Homologie entre génome du virus de la myéloblastose aviare (AMV) et génome cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1966 Aug 8;263(6):616–619. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Brodetsky A. M., Baluda M. A. Transient inhibition of avian myeloblastosis virus reproduction by amethopterin and fluorodeoxyuridine. J Virol. 1967 Dec;1(6):1150–1157. doi: 10.1128/jvi.1.6.1150-1157.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Isolation and partial characterization of nucleic acid of influenza virus. J Virol. 1967 Dec;1(6):1217–1223. doi: 10.1128/jvi.1.6.1217-1223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi S. K. The influence of chain length and base composition on the specific association of oligoribonucleotides with denatured deoxyribonucleic acid. J Biol Chem. 1969 Mar 25;244(6):1576–1581. [PubMed] [Google Scholar]
- Niyogi S. K., Thomas C. A., Jr The specific association of ribooligonucleotides of known chain length with denatured DNA. Biochem Biophys Res Commun. 1967 Jan 10;26(1):51–57. doi: 10.1016/0006-291x(67)90251-3. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSHEIMER R. L., LAWRENCE M. IN VITRO SYNTHESIS AND PROPERTIES OF A PHI-X DNA-RNA HYBRID. J Mol Biol. 1964 Feb;8:289–296. doi: 10.1016/s0022-2836(64)80138-8. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. HOMOLOGY BETWEEN RNA FROM ROUS SARCOMA VIROUS AND DNA FROM ROUS SARCOMA VIRUS-INFECTED CELLS. Proc Natl Acad Sci U S A. 1964 Aug;52:323–329. doi: 10.1073/pnas.52.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- VIGIER P., GOLDE A. EFFECTS OF ACTINOMYCIN D AND OF MITOMYCIN C ON THE DEVELOPMENT OF ROUS SARCOMA VIRUS. Virology. 1964 Aug;23:511–519. doi: 10.1016/0042-6822(64)90235-1. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. ATTEMPTS TO DETECT AN INTEGRATED POLYOMA GENOME BY NUCLEIC ACID HYBRIDIZATION. I. "RECONSTRUCTION" EXPERIMENTS AND COMPLEMENTARITY TESTS BETWEEN SYNTHETIC POLYOMA RNA AND POLYOMA TUMOR DNA. Virology. 1965 Feb;25:276–288. doi: 10.1016/0042-6822(65)90206-0. [DOI] [PubMed] [Google Scholar]
- Watson K. F., Beaudreau G. S. Isolation of a RNA-dependent RNA polymerase from virus infected myeloblasts. Biochem Biophys Res Commun. 1969 Dec 4;37(6):925–932. doi: 10.1016/0006-291x(69)90219-8. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. G., Bader J. P. Viral ribonucleic acid polymerase: chick-embryo cells infected with vesicular stomatitis virus or Rous-associated virus. Biochim Biophys Acta. 1965 Aug 10;103(4):549–557. doi: 10.1016/0005-2787(65)90076-6. [DOI] [PubMed] [Google Scholar]



