Abstract
To date, 5 different human dental stem/progenitor cells have been isolated and characterized: dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), stem cells from apical papilla (SCAP), and dental follicle progenitor cells (DFPCs). These post-natal populations have mesenchymal-stem-cell-like (MSC) qualities, including the capacity for self-renewal and multilineage differentiation potential. MSCs derived from bone marrow (BMMSCs) are capable of giving rise to various lineages of cells, such as osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic cells. The dental-tissue-derived stem cells are isolated from specialized tissue with potent capacities to differentiate into odontogenic cells. However, they also have the ability to give rise to other cell lineages similar to, but different in potency from, that of BMMSCs. This article will review the isolation and characterization of the properties of different dental MSC-like populations in comparison with those of other MSCs, such as BMMSCs. Important issues in stem cell biology, such as stem cell niche, homing, and immunoregulation, will also be discussed.
Keywords: MSCs, DPSCs, SHED, SCAP, PDLSCs, DFPCs, stem cell niche, apical papilla, stem cell homing, tissue regeneration
Introduction
Stem cell biology has become an important field for the understanding of tissue regeneration and implementation of regenerative medicine. Since the discovery and characterization of multipotent mesenchymal stem cells (MSCs) from bone marrow (BM), MSC-like populations from other tissues have now been characterized based on the ‘gold standard’ criteria established for BMMSCs (Friedenstein et al., 1976; Caplan, 1991; Prockop, 1997; Pittenger et al., 1999; Gronthos et al., 2003). Of those, MSC-like populations from adipose tissues and umbilical cord blood have been shown to be promising alternative multipotent MSC sources (Mareschi et al., 2001; Zuk et al., 2001). These MSCs are capable of giving rise to at least 3 cell lineages: osteogenic, chondrogenic, and adipogenic. Other lineages, such as myogenic, neurogenic, and tenogenic, may also be derived from BMMSCs. The search for MSC-like cells in specific tissues has led to the discovery of a variety of stem cells in every organ and tissue in the body in the past decades (reviewed by Baksh et al., 2004; Porada et al., 2006; Kolf et al., 2007). Dental-tissue-derived MSC-like populations are among many other stem cells residing in specialized tissues that have been isolated and characterized. The first type of dental stem cell was isolated from the human pulp tissue and termed ‘post-natal dental pulp stem cells’ (DPSCs) (Gronthos et al., 2000). Subsequently, 3 more types of dental-MSC-like populations were isolated and characterized: stem cells from exfoliated deciduous teeth (SHED) (Miura et al., 2003), periodontal ligament stem cells (PDLSCs) (Seo et al., 2004), and stem cells from apical papilla (SCAP) (Sonoyama et al., 2006, 2008). Recent studies have identified a fifth dental-tissue-derived progenitor cell population, referred to as ‘dental follicle precursor cells’ (DFPCs) (Morsczeck et al., 2005). However, the precise relationship among these different stem cell populations remains unclear.
During the characterization of these newly identified dental stem cells, certain aspects of their properties have been compared with those of BMMSCs. Dental stem cells display multidifferentiation potential, with the capacity to give rise to at least 3 distinct cell lineages: osteo/odontogenic, adipogenic, and neurogenic. Differences have been noted between the dental stem cell populations and BMMSCs, where dental stem cells appear to be more committed to odontogenic rather than osteogenic development. To date, dental-tissue-derived stem/progenitor cells have been used for tissue-engineering studies in large animals to assess their potential in pre-clinical applications (Sonoyama et al., 2006; Liu et al., 2008). A greater understanding of the biology of these dental stem cell populations is a prerequisite to understanding the extent of their efficacy for regenerative medicine. This article will review the current understanding of different dental stem cells in relation to MSC-like populations derived from other tissues.
Characteristics of MSCs
Among stem cells of mesenchymal origin, BMMSCs or BM-derived stromal stem cells (BMSSCs) are the most studied stromal stem cell populations (Caplan, 1991; Prockop, 1997; Pittenger et al., 1999). In mice, MSC-like populations reside in all post-natal organs and tissues (Meirelles et al., 2006). A rare population (0.02%) of very small embryonic-like (VSEL) Sca-1+lin-CD45-CXCR4+ SSEA-1+Oct-4+ stem cells has been identified in adult murine BM. It was hypothesized that VSEL stem cells are deposited early during development in BM (Kucia et al., 2006; Ratajczak et al., 2008). To relate the potency of dental stem cells, the characteristics and the multipotentiality of the better-known BMMSCs should be first reviewed.
BMMSCs
Bone-marrow-derived MSCs have been described as colony-forming unit-fibroblasts (CFU-Fs) in vitro which were able to commit to osteogenic differentiation (Cohnheim, 1867; Friedenstein et al., 1976). Their capacity to form clonogenic colonies is characteristic of other somatic stem cells, such as hematopoietic stem cells, and defines their ability to undergo self-renewal. However, BMMSCs are limited to a growth potential of 30 to approximately 50 population-doublings (PDs) following ex vivo expansion (Bruder et al., 1997; Bianco et al., 2001).
Morphologically, BMMSCs are a heterogeneous population of cells (Colter et al., 2000). Additionally, these expanded cells contain mixed populations of cells in terms of the stage of cell immaturity along differentiation pathways (Gronthos et al., 1999; Stewart et al., 1999). More recently, various cell-surface markers have been used in attempts to identify putative MSCs. Markers that are more consistently reported are STRO-1, CD73, CD90, CD105, CD146, Oct4, Nanog, beta2 integrin positive, and CD14, CD34, CD45, and HLA-DR negative expression (Gronthos et al., 2003; Shi and Gronthos, 2003; Miura et al., 2005; Dominici et al., 2006; Battula et al., 2007; Greco et al., 2007; Gronthos and Zannettino, 2008). A purified population has gene and protein expression different from that of the cultured heterogeneous MSCs. At a clonal level, only a minor proportion of individual colonies can undergo extensive proliferation (> 20 PD) and completely regenerate a bone/marrow organ in vivo. This heterogeneity in colony morphology, growth, and function supports the stromal hierarchy of cellular differentiation (Owen and Friedenstein, 1988) and thus makes it difficult to identify the exact “phenotypic fingerprint” of a stromal stem cell (Bianco et al., 2001).
BMMSCs are capable of differentiating into multiple cell lineages when grown in defined conditions in vitro, including osteogenic, chondrogenic, adipogenic, myelosupportive stroma, myogenic, and neurogenic lineages. The first 4 cell types are considered the essential lineages for defining multipotent MSCs (Tuli et al., 2003; Baksh et al., 2004). Some studies have reported the myogenic potential of MSCs, following the delivery of BMMSCs into muscle tissues, especially the myocardium, in animal models (Ferrari et al., 1998; Orlic et al., 2001; Barbash et al., 2003; Gojo et al., 2003). Numerous clinical trials with various human stem/progenitor cell delivery methods to the heart have reported various levels of success (Segers and Lee, 2008). Reported clinical trials with BMMSCs are limited, yet the intracoronary transplantation of autologous BMMSCs for ischemic cardiomyopathy has shown promising results (Chen et al., 2006a). However, the benefits seen in the majority of cardiac studies are probably due to the paracrine effects of MSCs, rather than their capacity to develop into functional cardiomyocytes.
In rodent systems, BMMSCs have been shown to differentiate into astrocytes and, in some instances, neurons after transplantation into the mouse brain (Kopen et al., 1999), or to facilitate functional recovery of damaged brain or spinal cord in rats (Chen et al., 2001; Hofstetter et al., 2002). Clinical trials where granulocyte macrophage colony-stimulating factors (GM-CSF) were administered along with BMMSCs appeared to improve the conditions for acute and subacute spinal cord injuries, but not chronic cases (Yoon et al., 2007). While BMMSCs from rats and humans can be induced to differentiate into neurons in vitro (Woodbury et al., 2000), the neurogenic potential is weaker when compared with that of stem cells derived from neural tissues (Raedt et al., 2007; Song et al., 2007).
Potent MSCs from Other Sources
Due to certain shortcomings of obtaining the BMMSCs, including pain, morbidity, and low cell number upon harvest, alternate sources for MSCs have been sought. MSCs derived from adipose tissue obtained by suction-assisted lipectomy (liposuction) (Zuk et al., 2001; Mizuno et al., 2002) and MSC-like populations from umbilical cord blood (UCB) have been isolated and characterized (Mareschi et al., 2001). MSCs from BM, adipose tissue, and umbilical cord blood are morphologically and immunophenotypically similar, but not identical (Kern et al., 2006). UCB-derived MSCs form the fewest colonies and show the highest proliferative capacity, whereas adipose-tissue-derived MSCs form the greatest number of colonies, and BMMSCs have the lowest proliferative capacity. MSCs from adipose tissues and especially UCB (Ballen et al., 2008) may gain more popularity, because these tissues are versatile and possess great potential for many clinical applications. Most importantly, they are discarded if not used.
Dental MSCs
Dental tissues are specialized tissues that do not undergo continuous remodeling as shown in bony tissue; therefore, dental-tissue-derived stem/progenitor cells may be more committed or restricted in their differentiation potency in comparison with BMMSCs. Additionally, dental mesenchyme is termed ‘ectomesenchyme’ due to its earlier interaction with the neural crest. From this perspective, ectomesenchyme-derived dental stem cells may possess different characteristics akin to those of neural crest cells.
Dental Pulp Stem Cells (DPSCs)
Isolation of Heterogeneous Populations of DPSCs
One important feature of pulp cells is their odontoblastic differentiation potential. Human pulp cells can be induced in vitro to differentiate into cells of odontoblastic phenotype, characterized by polarized cell bodies and accumulation of mineralized nodules (Tsukamoto et al., 1992; About et al., 2000; Couble et al., 2000). DPSCs isolated with enzyme treatment of pulp tissues form CFU-Fs with various characteristics (Gronthos et al., 2000; Huang et al., 2006a). There are different cell densities of the colonies, suggesting that each cell clone may have a different growth rate, as reported for BMMSCs (Gronthos et al., 2002). Within the same colony, different cell morphologies and sizes may be observed. If seeded onto dentin, some DPSCs convert into odontoblast-like cells with a polarized cell body and a cell process extending into the existing dentinal tubules (Huang et al., 2006a,b).
In vitro Characterization of DPSCs—Multilineage Differentiation
In addition to their dentinogenic potential, subpopulations of hDPSCs also possess adipogenic and neurogenic differentiation capacities by exhibiting adipocyte- and neuronal-like cell morphologies and expressing respective gene markers (Gronthos et al., 2002). More recently, DPSCs were also found to undergo osteogenic, chondrogenic and myogenic differentiation in vitro (summarized in Table 1) (Laino et al., 2005; Zhang et al., 2006; d’Aquino et al., 2007).
Table 1.
Properties of Human Dental MSCs
|
In vitro Analysis |
In vivo Analysis |
|||
|---|---|---|---|---|
| Cell Type | PD* | Multipotentiality | Ectopic tissue formation | |
| DPSCs | 60 - > 120 | Osteo/Dentinogenic | + | Dentin-pulp-like complex |
| Adipogenic | + | Odontoblast-like cells | ||
| Chondrogenic | + | Bone-like tissue | ||
| Myogenic | + | |||
| Neurogenic | + | |||
| SHED | > 140 | Dentinogenic | + | Dentin-pulp-like tissue |
| Adipogenic | + | Odontoblast-like cells | ||
| Chondrogenic | + | No dentin-pulp complex formation | ||
| Myogenic | + | Bone formation | ||
| Neurogenic | + | |||
| Osteo-inductive | + | |||
| SCAP | > 70 | Dentinogenic | + | Dentin-pulp-like complex |
| Adipogenic | + | Odontoblast-like cells | ||
| Chondrogenic | ND | |||
| Myogenic | ND | |||
| Neurogenic | + | |||
| PDLSCs | ND | Osteo/Cementogenic | + | Cementum-like |
| Adipogenic | + | PDL-like formation | ||
| Chondrogenic | + | |||
| Myogenic | ND | |||
| Neurogenic | + | |||
| DFPCs | ND | Cementogenic | + | PDL-like formation |
| Odontogenic | + | Cementum matrix formation | ||
| Adipogenic-Chondrogenic | + | |||
| Myogenic | + | |||
| Neurogenic | ND | |||
| ND | ||||
| BMMSCs | 30 - > 50 | Odontogenic | − | BMMSCs have shown both ectopic and orthotopic tissue formation: |
| Osteogenic | + | Bone and bone marrow-like, cartilage, muscle, and neuronal cell/tissue formation | ||
| Adipogenic-Chondrogenic | + | |||
| MyogenicNeurogenic | + | |||
| + | ||||
| + | ||||
PD, population doubling; ND, not determined.
In vivo Characterization of DPSCs—Ectopic Formation of Dentin-Pulp-like Complex
Transplanted ex vivo expanded DPSCs mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) form ectopic pulp-dentin-like tissue complexes in immunocompromised mice (Gronthos et al., 2000; Batouli et al., 2003) (Figs. 1A-1C). These pools of heterogeneous DPSCs form vascularized pulp-like tissue and are surrounded by a layer of odontoblast-like cells expressing dentin sialophosphoprotein (DSPP), which produces dentin containing dentinal tubules similar to those in natural dentin. Over time, the amount of dentin thickened (Batouli et al., 2003). When DPSCs are seeded onto human dentin surfaces and implanted into immunocompromised mice, reparative dentin-like structure is deposited on the dentin surface (Batouli et al., 2003) (Figs. 1D-1F).
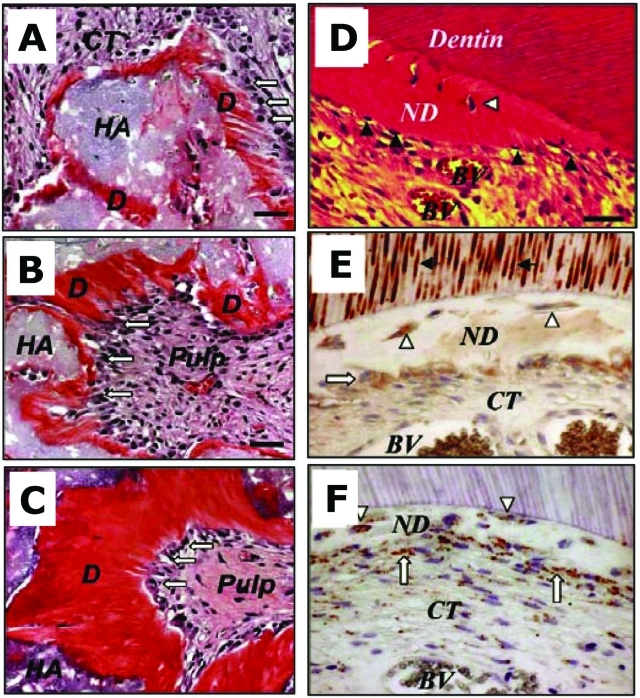
Figure 1.
Subcutaneous DPSC transplants in immunocompromised mice (A-C) and characterization of DPSC-mediated dentinogenesis in vivo (D-F). (A) Four wks after transplantation, DPSCs differentiated into odontoblasts (open arrows) responsible for new dentin (D) formation on the surface of the HA/TCP (HA). (B,C) At 8 and 16 wks post-transplantation, respectively. (D) Newly formed reparative dentin-like structure (ND) attached to the surfaces of human dentin in DPSC/dentin transplants. BV, blood vessels; CT, connective tissue; dentinogenic cells (black arrowheads). DPSCs formed reparative dentin-like structure containing entrapped cells (open arrowheads). (E) In DPSC/dentin transplants, dentinogenic cells (open arrows) and trapped cells (open arrowheads) within the newly formed reparative dentin-like structure (ND) were immunoreactive to human DSP antibody, as was the pre-existing dentin (black arrows). (F) Staining of human-specific anti-mitochondria antibody, showing the human origin of DPSCs (open arrows). Bar, 40 µm in A-C, 20 µm in D-F (adapted from Batouli et al., 2003).
While multiple-colony-derived DPSCs can have a PD of more than 120, single-colony-derived strains of DPSCs proliferate 10-20 PDs, and some are able to pass 20 PD to generate enough cells to form a dentin-pulp-like complex. Approximately two-thirds of the single-colony-derived hDPSCs were able to form the same amount of dentin as multi-colony hDPSCs. The other one-third generated only a limited amount of dentin (Gronthos et al., 2002).
Carinci and his colleagues identified a subpopulation of stem cells from human dental pulp with osteogenic potential forming bone-like tissue in vivo. They termed these cells ‘osteoblasts derived from human pulpar stem cells’ (ODHPSCs) and used microarrays to compare the genetic profiles of these cells with those of normal osteoblasts. They identified a long list of genes that are down-regulated in ODHPSCs vs. normal osteoblasts, which may explain the differences observed in the histological characteristics of the bone-like tissue formed by ODHPSCs compared with normal osteoblasts (Carinci et al., 2008).
Stem Cells from Human Exfoliated Deciduous Teeth (SHED)
In vitro Characterization of SHED—Sphere-like Cluster Formation
SHED proliferate faster with greater PDs than DPSCs and BMMSCs (SHED > DPSCs > BMMSCs). SHED form sphere-like clusters when cultured in neurogenic medium. This is due to the highly proliferative cells, which aggregate in clusters that either adhere to the culture dish or float freely in the culture medium. The sphere-like clusters can be dissociated by passage through needles and subsequently grown on 0.1% gelatin-coated dishes as individual fibroblastic cells. This phenomenon suggests a high proliferative capacity analogous to that of neural stem cells (Miura et al., 2003).
Investigators subsequently also isolated SHED and termed the cells ‘immature DPSCs’ (IDPSCs) (Kerkis et al., 2006). Besides confirming the findings described above, they found that IDPSCs express the embryonic stem (ES) cell markers Oct4, Nanog, stage-specific embryonic antigens (SSEA-3, SSEA-4), and tumor recognition antigens (TRA-1-60 and TRA-1-81) (Kerkis et al., 2006).
In vitro Multilineage Differentiation
As reported for DPSCs, SHED showed the capacity to undergo osteogenic and adipogenic differentiation (Miura et al., 2003). Furthermore, cultured SHED readily express a variety of neural cell markers (Table 2). If stimulated with neurogenic medium, expression of βIII-tubulin, GAD, and NeuN is increased, whereas the other neural markers remain unchanged. Under neurogenic conditions, SHED also exhibit multicytoplasmic processes instead of the typical fibroblastic morphology (Figs. 2A-2D) (Miura et al., 2003). Myogenic and chondrogenic potentials have also been demonstrated (Kerkis et al., 2006).
Table 2.
In vitro Phenotypic Characteristics#
| Gene | BMMSCs | SHED | DPSCs* | SCAP* | PDLSCs | DFPCs |
|---|---|---|---|---|---|---|
| STRO-1 | + (5-10%) | + (9%) | + (5-10%) | + (> 18%) | + | + |
| CD + (selected) | CD44 | CD146 | CD13 | CD13 | CD13 | CD13CD29 |
| CD73 | CD29 | CD44 | CD29 | |||
| CD105 | CD44 | CD24 | CD44 | CD44 | ||
| CD106 | CD59 | CD29 | CD59 | CD59 | ||
| CD73 | CD73 | CD90 | CD73 | |||
| CD90 | CD90 | CD105 | CD90 | |||
| CD105 | CD105 | CD105 | ||||
| CD146 | CD106 | |||||
| CD146 | ||||||
| CD- (selected) | CD14 | CD14 | CD18 | CD45 | ||
| CD34 | CD24 | CD34 | ||||
| CD45 | CD34 | CD45 | ||||
| CD45 | CD150 | |||||
| Oct4 | + | + | + | |||
| Nanog | + | + | ||||
| Survivin | *– | *++ | ||||
| hTERT | − | *+ | *++ | |||
| Notch-1 | + | |||||
| TGFβRI | + | + | ||||
| TGFβRII | + | *+ | *+/– | |||
| Endostatin | + | *++ | *++ | |||
| bFGF | + | *++ | *++ | |||
| FGFR3 | + | *+++ | *+ | + | ||
| Flt-1 (VEGF receptor 1), | + | *+ | *+/– | |||
| Flg (FGFR1) | *+++ | *++ | ||||
| MUC18 (melanoma-associated glycoprotein, CD146) | + | + | *+++ | *++ | + | |
| Collagen type I | + | + | + | |||
| Collagen type III | + | + | ||||
| Scleraxis | + | + | + | |||
| Osteo/odontogenic | ||||||
| ALP | + | + | *+++ | *++ | + | |
| BSP | +/– | *+ | *+ | + | + | |
| DSP | + | *+++ | *++ | |||
| MEPE (matrix extracellular phosphoglycoprotein) | + | *+++ | *++ | + | ||
| Cbfa1/Runx2 | + | *+ | *+ | |||
| Osteocalcin (OCN) | +/– | + | + | + | ||
| Osteonectin | +/– | + | ||||
| Osterix | + | + | + | |||
| Neurogenic | ||||||
| Nestin | *+ | *+ | + | |||
| βIII tubulin | + | + | ||||
| Tau | + | |||||
| MAP2 (microtubule-associated protein) | (+) | |||||
| GAD (glutamic acid decarboxylase), | + | (+) | ||||
| NeuN (Neuronal nuclear antigen), | + | (+) | ||||
| NFM (Neurofilament M) | − | + | *+/– | *+/– (+) | ||
| NSE (neuron-specific enolase) | + | |||||
| CNPase (glial markers 2’,3’-cyclic nucleotide 3’-phosphodiesterase) | + | + | ||||
| GFAP (glial fibrillary acidic protein), | + | + | + |
Comparison of relative expression levels of genes between DPSCs and SCAP. For other cells, + or—indicates only positive or negative expression; +, expression without stimulation; (+) expression after induction.
Markers listed do not exclude others not listed herein.
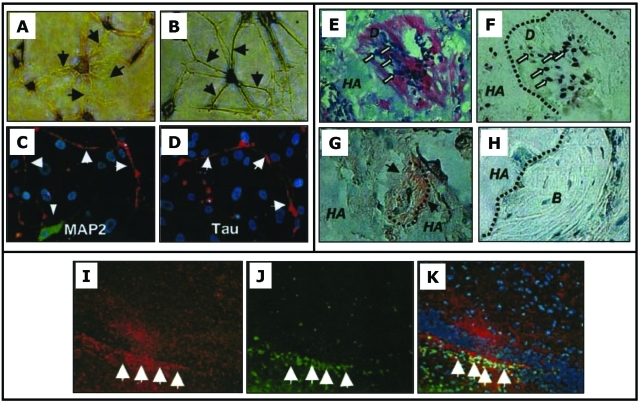
Figure 2.
In vitro neurogenesis of SHED (A-D), transplanted SHED into immunocompromised mice (E-H) and into the mouse brain (I-K). (A,B) Toluidine blue staining of the altered morphology of SHED after neurogenic induction. (C,D) Immunopositive staining for MAP2 and Tau on dendrites and axons (arrows), respectively. (E,F) Eight wks after transplantation into the subcutaneous space, SHED differentiate into odontoblasts (open arrows) and form dentin-like structure (D) on the surfaces of HA. The same field is shown for human-specific alu in situ hybridization, indicating the human origin of odontoblasts (open arrows in F). (G) Immunohistochemical staining of DSPP on the regenerated dentin (black arrows). (H) Newly generated bone (B) by host cells in the same SHED transplant shows no reactivity to the DSPP antibody. (I-K) Neurogenically induced SHED injected into the dentate gyrus of the hippocampus of immunocompromised mice for 10 days. (I) NFM (red) and (J) human-specific anti-mitochondrial antibody (green) and (K) merged images showing co-localization of the two (adapted from Miura et al., 2003, with permission).
In vivo characterization of SHED—Production of dentin-pulp-like structures but without a complex formation
Ex vivo-expanded SHED transplanted into immunocompromised mice yield human-specific odontoblast-like cells directly associated with a dentin-like structure. The regenerated dentin expresses dentin-specific DSPP. However, unlike DPSCs, SHED are unable to regenerate a complete dentin-pulp-like complex in vivo (Figs. 2E-2H) (Miura et al., 2003).
Osteo-inductive capacity
One striking feature of SHED is that they are capable of inducing recipient murine cells to differentiate into bone-forming cells, which is not a property attributed to DPSCs following transplantation in vivo. When single-colony-derived SHED clones were transplanted into immunocompromised mice, only one-fourth of the clones had the potential to generate ectopic dentin-like tissue equivalent to that generated by multicolony-derived SHED (Miura et al., 2003). However, all single-colony-derived SHED clones tested are capable of inducing bone formation in immunocompromised mice. While SHED could not differentiate directly into osteoblasts, they appeared to induce new bone formation by forming an osteoinductive template to recruit murine host osteogenic cells (Miura et al., 2003). With the osteo-inductive potential, SHED can repair critical-sized calvarial defects in mice with substantial bone formation (Seo et al., 2008). These findings imply that deciduous teeth may not only provide guidance for the eruption of permanent teeth, as generally assumed, but may also be involved in inducing bone formation during the eruption of permanent teeth.
In vivo neurogenesis in mouse brain
Neural developmental potential was studied by the injection of SHED into the dentate gyrus of the hippocampus of immunocompromised mice (Miura et al., 2003). SHED can survive for more than 10 days inside the mouse brain microenvironment and express neural markers such as neurofilament M (NFM) (Figs. 2I-2K). This finding is similar to what was demonstrated for BMMSCs, which are capable of differentiating into neural-like cells after in vivo transplantation into the rat brain (Azizi et al., 1998). SHED appear to represent a population of multipotent stem cells that are perhaps more immature than other post-natal stromal stem-cell populations. SHED express neuronal and glial cell markers, which may be related to the neural-crest-cell origin of the dental pulp (Chai et al., 2000).
In vivo engraftment into different tissues
Three months following the injection of IDPSCs into the intraperitoneal space of nude mice, IDPSCs can be traced in various tissues and organs, including liver, spleen, and kidney, suggesting their potent differentiation plasticity (Kerkis et al., 2006).
Stem Cells from Apical Papilla (SCAP)
Apical papilla refers to the soft tissue at the apices of developing permanent teeth (Sonoyama et al., 2006, 2008). Apical papilla is more apical to the epithelial diaphragm, and there is an apical cell-rich zone lying between the apical papilla and the pulp (Fig. 3) (Rubio et al., 2005).
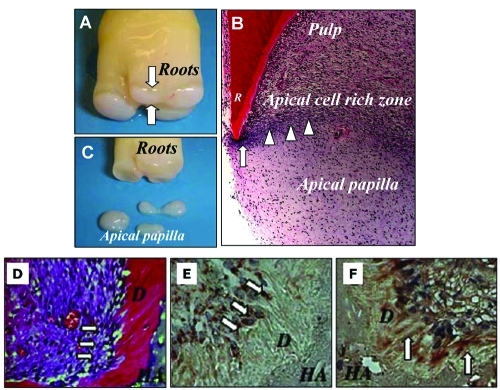
Figure 3.
The anatomy of the human apical papilla (A-C) and dentinogenesis of SCAP in immunocompromised mice (D-F). (A) An extracted human third molar depicting root attached to the root apical papilla (open arrows) at the developmental stage. (B) Hematoxylin and eosin staining of human developing root (R) depicting epithelial diaphragm (open arrows) and apical cell-rich zone (open arrowheads). (C) Harvested root apical papilla for stem cell isolation. (D) Eight wks after transplantation, SCAP differentiated into odontoblasts (arrows) that formed dentin (D) on the surfaces of a HA carrier. (E) SCAP differentiated into odontoblasts (arrows) are positive for anti-human specific mitochondria antibody staining. (F) Immunohistochemical staining of SCAP-generated dentin (D) showing positive anti-DSP antibody staining (arrows) (adapted from Sonoyama et al., 2006, 2008, with permission).
In vitro Characterization of SCAP—Multilineage Differentiation Potential
Similar to DPSCs and SHED, ex vivo expanded SCAP can undergo odontogenic differentiation in vitro. SCAP express lower levels of DSP, matrix extracellular phosphoglycoprotein (MEPE), transforming growth factor β receptor II (TGFβRII), FGFR3, Flt-1 (VEGF receptor 1), Flg (FGFR1), and melanoma-associated glycoprotein (MUC18) in comparison with DPSCs. Significantly, CD24 is expressed by SCAP which is not detected on DPSCs or BMMSCs. The expression of CD24 by SCAP is down-regulated in response to osteogenic stimulation. However, the biological significance of this finding requires further investigation.
SCAP also demonstrate the capacity to undergo adipogenic differentiation following induction in vitro (Sonoyama et al., 2006; Abe et al., 2007). Interestingly, without neurogenic stimulation, cultured SCAP show positive staining for several neural markers (Abe et al., 2007). After stimulation, additional neural markers are also expressed by SCAP, including glutamic acid decarboxylase (GAD), neuronal nuclear antigen (NeuN), neurofilament M (NFM), neuron-specific enolase (NSE), and glial markers 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNPas) (Sonoyama et al., 2008).
DPSCs vs. SCAP
The distinction between dental pulp and apical papilla is that apical papilla is the precursor tissue of the radicular pulp. From this perspective, it may be speculated that SCAP are similar to stem cells residing in the dental papilla that gives rise to the coronal dentin-producing odontoblasts. Once the apical papilla turns into pulp, whether the SCAP convert into DPSCs or the latter are derived from a different stem cell pool is currently unclear. Nonetheless, our previous studies showed that when SCAP and DPSCs are compared in vitro, there are some differences (Table 2). Overall, SCAP are derived from a developing tissue that may represent a population of early stem/progenitor cells which may be a superior cell source for tissue regeneration. Additionally, these cells also highlight an important fact that developing tissues may contain stem cells distinctive from those of mature tissues.
In vivo Characterization of SCAP—Formation of Dentin-Pulp-like Complex
The capacity of SCAP to differentiate into functional dentinogenic cells has been verified by the same approaches as for the above-mentioned dental stem cells. A typical dentin-pulp-like complex is generated when SCAP are transplanted into immuno-compromised mice in an appropriate carrier matrix (Figs. 3D-3F) as described for DPSCs.
Potential Role of SCAP in Continued Root Formation
As described above, SCAP show characteristics similar to, but different from, those of DPSCs. SCAP appear to be the source of primary odontoblasts that are responsible for the formation of root dentin, whereas DPSCs are likely the source of replacement odontoblasts that form reparative dentin. The role of apical papilla in root formation has been discussed previously (Huang et al., 2008). In a pilot study with minipigs as a model, the surgical removal of the root apical papilla at an early developing stage halted root development, despite the pulp tissue being intact, whereas other roots of the tooth, containing apical papilla, maintained normal growth and development.
Periodontal Ligament Stem Cells (PDLSCs)
Earlier evidence has shown that PDL contains cell populations that can differentiate into either cementum-forming cells (cementoblasts) or bone-forming cells (osteoblasts) (McCulloch and Bordin, 1991; Isaka et al., 2001). The presence of multiple cell types within PDL suggests that this tissue contains progenitor cells that maintain tissue homeostasis and regeneration of periodontal tissue. Enzyme digestion treatment of PDL releases a population of clonogenic cells with characteristics of post-natal stem cells (Seo et al., 2004). The successful isolation and characterization of PDLSCs have led to the identification of tendon MSCs by the same approaches (Bi et al., 2007).
In vitro Characterization of PDLSCs—Multilineage Differentiation Potential
PDLSCs express the MSC-associated markers STRO-1, CDs, and scleraxis (Table 2)—a tendon-specific transcription factor, which is expressed at higher levels in PDLSCs than in BMMSCs and DPSCs. Immunohistochemical staining and Western blot analysis showed that cultured PDLSCs expressed an array of cementoblastic/osteoblastic markers (Seo et al., 2004). Similar to the other dental stem cells described above, PDLSCs exhibit osteogenic, adipogenic, and chondrogenic characteristics under defined culture conditions (Gay et al., 2007; Lindroos et al., 2008; Xu et al., 2009).
In vivo Characterization of PDLSCs—Formation of Cementum- and pdl-like Tissue
Typical cementum/PDL-like structure can be regenerated after transplantation of ex vivo-expanded PDLSCs into immunocompromised mice. A thin layer of cementum-like tissue is formed along with condensed collagen fibers with sparse cells resembling PDL structures. The cementum/PDL-like structures are totally different from typical bone/marrow structures generated by BMMSCs and dentin/pulp-like structures generated by DPSCs.
Transplanted human PDLSCs form a dense type I collagen-positive PDL-like tissue within the transplants. More importantly, collagen fibers generated in vivo were able to connect with newly formed cementum-like structures that mimicked physiological attachment of Sharpey’s fibers (Figs. 4A, 4B), responsible for the functional attachment of cementum/PDL structures. From these findings, one can infer that PDLSCs may contain a subpopulation of cells capable of differentiating into cementoblasts/cementocytes and collagen-forming cells in vivo. After transplantation of hPDLSCs into the periodontal defects of immunocompromised mice, PDL-like tissue was regenerated, and these human stem cells were also identified to be closely associated with the trabecular bone next to the regenerated PDL, suggesting their involvement in alveolar bone regeneration (Figs. 4C-4E) (Seo et al., 2004).
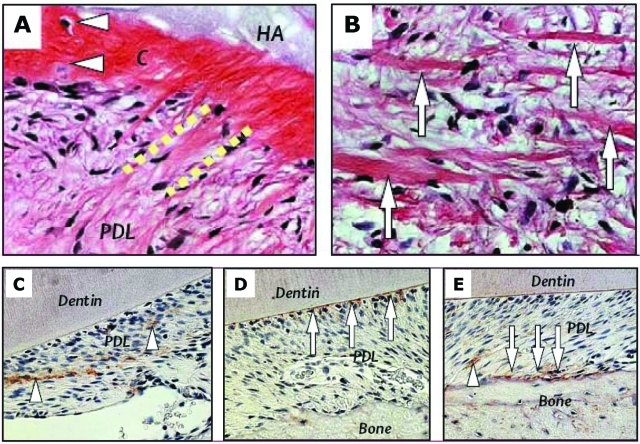
Figure 4.
Generation of cementum-like structure and collagen fibers by PDLSCs in immunocompromised mice (A,B) and PDLSCs in periodontal tissue repair in immunocompromised rats (C-E). (A,B) Transplanted PDLSCs formed cementum-like structures (C) that connected to newly formed collagen fibers (dashed lines), similar to the structure of Sharpey’s fibers, and generated a substantial amount of collagen fibers (arrows in B). (C-E) Staining of human-specific anti-mitochondria antibody showing human PDLSCs located in the PDL compartment (arrowheads in C), involved in the attachment of PDL to the tooth surface (arrows in D), and participating in the repair of alveolar bone (arrows in E) and PDL (arrowhead in E) (adapted from Seo et al., 2004, with permission).
Dental Follicle Precursor Cells (DFPCs)
Dental follicle is an ectomesenchymal tissue surrounding the enamel organ and the dental papilla of the developing tooth germ prior to eruption. This tissue contains progenitor cells that form the periodontium, i.e., cementum, PDL, and alveolar bone. Precursor cells have been isolated from human dental follicles of impacted third molars. Similar to other dental stem cells, these cells form low numbers of adherent clonogenic colonies when released from the tissue following enzymatic digestion (Morsczeck et al., 2005).
In vitro Characterization
Cells in dental follicles express markers such as Notch-1 and Nestin, suggesting the presence of undifferentiated cells. After cells are released from the tissue, only a small number of single dental follicle cells are attached onto the plastic surface and form CFU-F. DFPCs show a typical fibroblast-like morphology and express Nestin, Notch-1, collagen type I, bone sialoprotein (BSP), osteocalcin (OCN), and fibroblast growth factor receptor (FGFR)1-IIIC (Morsczeck et al., 2005). DFPCs demonstrate osteogenic differentiation capacity in vitro after induction. A membrane-like structure forms in DFPC cultures after 5 wks of stimulation with dexamethasone. It has been reported that STRO-1 and BMP receptors (BMPR) are expressed in dental follicles in vivo (Kemoun et al., 2007). Incubation with rhBMP-2 and rhBMP-7 or enamel matrix derivatives (EMD) for 24 hrs increases the expression of BMP-2 and BMP-7 by DFPCs. Expression of cementum attachment protein and cementum protein-23 (CP-23), two putative cementoblast markers, has been detected in EMD-stimulated whole dental follicle and in cultured DFPCs stimulated with EMD or BMP-2 and BMP-7 (Kémoun et al., 2007).
In vivo Characterization
Transplantation of DFPCs by the same methods as described for other dental stem cells generates a structure comprised of fibrous or rigid tissue. These transplants expressed human-specific transcripts for BSP, OCN, and collagen type I. Gene expression was increased more than 100 times for BSP and OCN and was decreased for collagen type I transcripts after transplantation into immunocompromised mice. However, there was no dentin, cementum, or bone formation observed in the transplant in vivo. The authors explained that it could be due to the low number of cells in the original cultures (Morsczeck et al., 2005, 2008).
Dental MSCs vs. BMMSCs
Immunophenotype
Although there has been no systematic comparison between dental stem cells and BMMSCs, certain differences between some dental MSCs and BMMSCs have been compared. Immunophenotypic analysis is presented in Table 2. Collectively, current evidence suggests that biochemical pathways involved in the differentiation of DPSCs into functional odontoblasts are similar to differentiation pathways of BMMSCs into osteoblasts (Shi et al., 2001). DPSCs do share a similar pattern of protein expression with BMMSCs in vitro.
Gene Expression Profile
A similar level of gene expression between DPSCs and BMMSCs was found for more than 4000 known human genes, except a few differentially expressed genes, including collagen type XVIII alpha1, insulin-like growth factor-2 (IGF-2), discordin domain tyrosine kinase 2, NAD(P)H menadione oxidoreductase, homolog 2 of Drosophila large disk, and cyclin-dependent kinase 6, which are highly expressed in DPSCs, whereas insulin-like growth factor binding protein-7 (IGFBP-7) and collagen type I α2 are more highly expressed in BMMSCs (Shi et al., 2001).
In a cDNA microarray system consisting of 12,814 genes, a clustering algorithm was applied to characterize and compare the expression profiles and functional classifications of odontogenic and osteogenic human stem cell populations between DPSCs and BMMSCs (Yamada et al., 2006a). These investigators discovered the high expression levels of the ALP gene, DSPP, and DMP-1 in DPSCs after osteoinduction compared with levels in BMMSCs. When they focused on differences between induced DPSCs and BMMSCs on a cluster that contains genes which are up-regulated in DPSCs and down-regulated in MSCs after induction, they found a notable feature of this cluster to be the cooperative regulation of genes for cell signaling, cell communication, or metabolism (Yamada et al., 2006a).
Multilineage Differentiation Potential
Although DPSCs and BMMSCs are regulated by similar factors, and share a common protein expression profile, these populations differ significantly in their proliferative ability and developmental potentials in vitro, and, more importantly, in their ability to develop into distinct tissues representative of the micro-environments from which they were derived in vivo. BMMSCs formed only bone tissue in the mouse model when treated in the same manner (Gronthos et al., 2000; Batouli et al., 2003). The chondrogenic potential of DPSCs appears weak, and both DPSCs and SCAP are weaker in adipogenesis in comparison with BMMSCs (Zhang et al., 2006; Sonoyama et al., 2008). Conversely, the neurogenicity of dental stem cells may be more potent than that of BMMSCs, most probably due to their neural crest origin.
MSC NICHE
The stem cell niche concept was first proposed as a specialized micro-environment needed for cells to retain their ‘stemness’ (Schofield, 1978). The niche is considered a fixed compartment of a three-dimensional structure containing elements that participate in the regulation of stem cell proliferation, control the fate of stem cell progeny, and prevent the stem cells from exhaustion or death (Scadden, 2006; Jones and Wagers, 2008). The bone marrow micro-environment is a major site of MSC niche in the body, in which a complex cellular and non-cellular interaction occurs among hematopoietic stem cells (HSCs). HSCs are known to reside in two different niches: endosteal and perivascular niches. The endosteal niche is thought to maintain HSC quiescence over the long term, whereas the perivascular niche is to maintain HSC proliferation and mediate circulation (Mitsiadis et al., 2007). Little is known regarding the niche for the BMMSCs. Findings from previous studies suggested that BMMSCs and adipose-derived MSC ‘harbor’ in the perivascular areas of BM (Shi and Gronthos, 2003; Zannettino et al., 2008).
The DPSC niche in human dental pulp was identified by antibodies against STRO-1, CD146, and pericyte-associated antigen (3G5) and was found to be localized in the perivascular and perineural sheath regions (Shi and Gronthos, 2003). These STRO-1+/CD146+ DPSCs form a dentin-pulp-like complex in vivo, similar to the multiple-colony-derived DPSCs. The STRO-1-positive region in the pulp of deciduous teeth is similar to that of permanent teeth, also in the perivascular regions. STRO-1/CD146/CD44 staining of the PDL has shown that it is located mainly in the perivascular region, with small clusters of cells in the extravascular region (Chen et al., 2006b), suggesting that these are the niches of PDLSCs. STRO-1 staining of apical papilla has shown that the positive stain is located in the perivascular region as well as other regions scattered in the tissue (Sonoyama et al., 2006). Thus, it appears that dental stem cells and BMMSCs secure at least one niche in the perivascular region. It is speculated that the MSC compartment extends through the whole post-natal organism as a result of its perivascular location (Meirelles et al., 2006). Currently, it is not known if tissue-specific MSCs originate from the local mesenchymal tissues and later migrate toward the ingrown vasculature, or if they are derived from the vasculature and then influenced by the local signals to acquire their tissue specificity.
Msc Homing
Circulating, adherent clonogenic cells in post-natal human blood are very rare—found in three of 66 individuals (Kuznetsov et al., 2007). The blood from the three donors yielded only 1-2 colony-forming cells each, and these cells displayed immunophenotype characteristics of fibroblastic/smooth muscle/weakly osteogenic cells and adipogenic conversion in vitro. Chondrogenic potential could not be tested due to insufficient cell numbers (Kuznetsov et al., 2007). Other investigators applied a two-step method that included an enrichment of mononuclear cells followed by depletion of unwanted cells, and were able to obtain 4-6 CFU-Fs from peripheral blood of all 14 donors, showing osteogenic and adipogenic potential (Valenti et al., 2008). It seems that the number of circulating MSCs in human blood is low under steady-state conditions. However, if ex vivo-expanded MSCs are injected into the blood stream, they show some limited capacity to home into various tissues and organs. Systemic delivery of ex vivo-expanded BMMSCs through intravenous infusion led to lodging of these MSCs mainly in the lungs, with significantly smaller amounts in the liver, heart, and spleen (Barbash et al., 2003). Intravenous injection of BMMSCs to rats significantly improved functional neurologic recovery (Li et al., 2005). These injected BMMSCs showed some capacity to migrate into damaged areas of brain tissue when administered at an early stage after the onset of ischemia. The signals that guided BMMSCs to the sites of injury may have been the stromal-cell-derived factor-1 (SDF-1), since its expression was up-regulated in the ischemic boundary zone of the brain. Additionally, these BMMSCs expressed CXCR4, the specific receptor of SDF-1, suggesting that the interaction of SDF-1 with CXCR4 mediated the trafficking of these stem cells to the impaired site (Kortesidis et al., 2005). However, BMMSCs improving cerebral recovery by becoming brain cells is highly unlikely because of the small number of donor cells found in the brain (Shen et al., 2006). Therefore, the beneficial effect of these stem cells on cerebral recovery is likely to be their ability to induce neurogenesis (Chopp and Li, 2002). Currently, no evidence has been shown that BMMSCs administered intravenously migrate to orofacial or dental organs.
Immunomodulation of Mscs
The immunoregulation of MSCs can be viewed from two perspectives: (i) immunosuppressive effects of allogeneic MSCs, and (ii) the effects of inflammatory cytokines on MSC activity and differentiation. Due to interest in the allogeneic or xenogeneic MSC supply to compensate for the paucity and time constraint of autogeneic MSC sources, there has been considerable progress in the understanding of the MSC immunosuppressive effect. While xenogeneic MSCs are rejected by the host after transplantation (Grinnemo et al., 2004), allogeneic MSCs are well-tolerated by the recipient hosts. Many in vivo studies have confirmed the immunosuppressive effects of MSC (Chen et al., 2006). The potential mechanisms underlying this immunosuppression are the capacity of MSCs to down-modulate immune reactions executed by T-, dendritic, NK, and B-cells. MSCs may potentially be used in vivo for enhancing the engraftment of other tissues (e.g., hematopoietic stem cells), for prophylactic prevention, and even possibly as a treatment of graft-vs.-host-disease or autoimmune diseases such as rheumatoid arthritis (Jorgensen et al., 2003a,b), to prevent rejection, and to promote transplant tolerance and patient survival. DPSCs immunosuppression has also been shown (Pierdomenico et al., 2005). Other dental stem cells, including SCAP and PDLSCs, also showed immunosuppressive properties in vitro (our unpublished observations).
Relatively limited information is available on the effects of pro-inflammatory cytokines on MSCs. A preliminary study in porcine MSCs showed that interferon may act to differentiate MSCs into osteoblasts (Abukawa et al., 2006). In the context of autologous implantation for chondrogenesis, a study has shown that inflammatory reactions against scaffold materials and serum components led to the production of cytokines such as IL-1α that may inhibit cartilage tissue formation (Rotter et al., 2005).
Dental Msc-Based Therapy For Regenerative Medicine
Stem-cell-based tooth tissue engineering has been a much-discussed subject, because cell-based therapy for the regeneration of tissue is considered a promising mode of future medicine (Morsczeck et al., 2008; Yen and Sharpe, 2008). To engineer and regenerate a whole tooth, the cell source may have to come from tooth buds in which all the needed cell types are retained. To repair partly lost tooth tissues such as PDL, dentin, and pulp, one or two particular types of dental stem cells may be sufficient to fulfill the need.
SCAP and PDLSCs for Bio-root Engineering
In the past decade, dental implants have become more practical and reliable for restoring the dentition. However, the fundamental pitfalls of dental implants are the lack of a natural contour and structural relationship with the alveolar bone, i.e., the artificial cylindrical shape and absence of the PDL. These shortcomings have led to the search for other alternatives. Experimental models of tooth regeneration have been tested on the ectopic formation of tooth-like structures in vivo, with cells isolated from tooth buds and seeded onto scaffolds (Young et al., 2002; Duailibi et al., 2004; Nakao et al., 2007). A few reports (discussed below) have demonstrated the orthotopic regeneration of engineered teeth. In larger animal studies, single cells from dog tooth buds at the bell stage were directly seeded onto scaffolds and transplanted back to the original tooth sockets. Dentin structure regeneration was observed, but not enamel or root formation (Honda et al., 2006). In a swine model, ex vivo-expanded tooth bud cells (from bell stage) were cultured onto cylindrical scaffolds and autografted back to the original alveolar sockets (Kuo et al., 2007). This group was able to observe tooth formation with root structures along with periodontium (Kuo et al., 2007). Overall, tooth regeneration still faces many obstacles: (i) lack of formation of normal tooth size, (ii) lack of consistent root formation, and (iii) no evidence of complete eruption into functional occlusion.
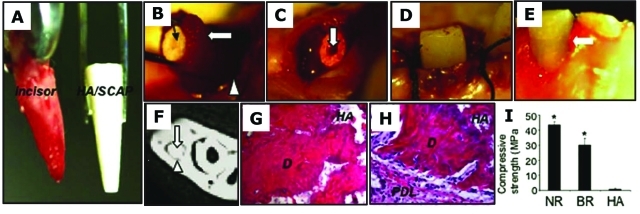
Instead of attempting to form an entire tooth, Sonoyama et al. demonstrated that by utilizing SCAP along with the PDLSCs, they were able to generate a bio-root with periodontal ligament tissues. A mini-swine model was used, and the autologous SCAP and PDLSCs were then loaded onto HA/TCP and gelfoam scaffolds, respectively, and implanted into sockets of the lower jaw. Three months later, the bio-root was formed in the porcine jaw and was exposed for the insertion of post and a porcelain crown (Fig. 5) (Sonoyama et al., 2006). The bio-root structure was comprised of dentin randomly deposited by the SCAP. The bio-root was encircled with periodontal ligament tissue and appeared to have a natural relationship with the surrounding bone. However, the presence of residual HA in the newly generated dentin formed a structure different from that of the naturally formed dentin. This led to a reduced mechanical strength of the bio-root (approximately two-thirds of a natural tooth), where the integrity of this tissue remains to be assessed in long-term studies.
Figure 5.
Swine SCAP/PDLSC-mediated bio-root engineering. (A) Extracted minipig lower incisor and root-shaped HA/TCP carrier loaded with SCAP. (B) Gelfoam containing PDLSCs (open arrow) to cover the HA/SCAP (black arrow) and implanted into the lower incisor socket (open arrowhead). (C) HA/SCAP-Gelfoam/PDLSCs were implanted into a newly extracted incisor socket. A post channel was pre-created inside the root-shaped HA carrier (arrow). (D) Three months after implantation, the bio-root was exposed and a porcelain crown inserted. (E) Four wks after fixation, the porcelain crown was retained after normal tooth use. (F) After 3 months’ implantation, the HA/SCAP-Gelfoam/PDLSC implant formed a hard root structure (open arrows) in the mandibular incisor area, as shown by CT scan image. A clear PDL space was found between the implant and surrounding bony tissue (arrowhead). (G, H) H&E staining showed that implanted HA/SCAP-Gelfoam/PDLSC contains newly regenerated dentin (D) and PDL tissue (PDL) on the outside of the implant. (I) Compressive strength measurement showed that newly formed bio-roots have compressive strength much higher than that of the original HA/TCP carrier (*P = 0.0002), but lower than that in natural swine root dentin (*P = 0.003) (NR, natural minipig root; BR, newly formed bio-root; HA, original HA carrier) (adapted from Sonoyama et al., 2006, with permission).
Regeneration of Periodontal Defects with PDLSCs
The use of processed acellular allogenic bone grafts to facilitate the repair of periodontal defects has been a common clinical practice. The beneficial effects of PDGF (platelet-derived growth factor) and IGF (insulin-derived growth factor) on periodontal repair were evidenced from animal studies as well as in human clinical trials (Lynch et al., 1989; Giannobile et al., 1994; Howell et al., 1997; Camelo et al., 2003; Nevins et al., 2003, 2005; McGuire et al., 2006). Platelet-rich plasma (PRP) has also been shown to improve periodontal healing and promote bone regeneration, and the preparation of PRP from patients has become part of the periodontal practice (Tozum and Demiralp, 2003).
Cell-based regenerative periodontal therapy has gained attention since the isolation of mesenchymal stem cells from various tissues. A clinical case report has demonstrated that using ex vivo-expanded autologous BMMSCs facilitated the repair of periodontal defects (Yamada et al., 2006b). To repair periodontal defects, it has been considered that regenerating the PDL is as important as repairing the bone. Focusing only on bone regeneration using the recombinant human bone morphogenetic protein-2 (rhBMP-2) can stimulate clinically significant regeneration of alveolar bone and cementum, but not a functionally oriented PDL, which frequently results in ankylosis between the teeth and the newly formed bone in the coronal aspect of the supra-alveolar defect (Selvig et al., 2002). PDLSCs may be an ideal cellular source for regeneration of the PDL. A recent report on a minipig model has shown that periodontal defects may be repaired by the application of PDLSCs (Liu et al., 2008). This PDSC-mediated treatment resulted in a regeneration of PDL and the recovery of the heights of alveolar bone. This is the first report demonstrating the application of autologous PDLSCs to regenerate PDL and alveolar bone heights in a large animal model.
Stem Cells for Pulp Tissue Engineering and Regeneration
Attempts to induce tissue regeneration in the pulp space have been a long quest. It has previously been proposed to induce hemorrhage and form blood clots in the canal space of mature teeth in the hope of guiding tissue repair in the canal (Ostby, 1961). As a result, attempts were made to regenerate dental pulp with a blood clot filling the canal (Myers and Fountain, 1974). However, the connective tissue that grew into the canal space was limited (0.1-1.0 mm from the foramen) and was not pulp tissue. More recently, with the emergence of tissue-engineering sciences, dental pulp tissue regeneration has been explored with the use of various biomaterials, where pulp cells grown on polyglycolic acid (PGA) formed pulp-like tissue in both in vitro and in vivo models (Gu et al., 1996; Mooney et al., 1996; Buurma et al., 1999).
Since the isolation and characterization of DPSCs, SHED, and SCAP, the use of these stem cells for dentin/pulp tissue regeneration has been investigated (Huang et al., 2006a, 2008; Murray et al., 2007; Prescott et al., 2008). In a tooth slice model (horizontal section, 1 mm thick), it was shown that SHED seeded onto synthetic scaffolds seated into the pulp chamber space formed odontoblast-like cells that localized against the existing dentin surface (Cordeiro et al., 2008). However, no orthotopic regeneration of pulp-like tissues in the pulp space has been reported with this approach. One concern is that implanting stem cells/scaffolds into root canals that have a blood supply only from the apical end may compromise vascularization to support the vitality of the implanted cells in the scaffolds. It has been proposed that, because of the concern over vascularization, a stepwise insertion of engineered pulp may have to be implemented clinically to achieve the desired pulp tissue regeneration (Huang et al., 2008).
There has been speculation that the undifferentiated mesenchymal cells residing in the periapical tissue or the BMMSCs in the alveolar bone of the jaws can be introduced into the root canal space via the formation of blood clots, to allow for pulp-like tissue regeneration and the formation of new odontoblasts (Myers and Fountain, 1974). From our understanding of the characteristics of PDLSCs, DPSCs, and SCAP, it is unlikely that odontoblasts can be derived from PDL or periapical bone. As described above, when BMMSCs and DPSCs are transplanted into the subcutaneous space of immunocompromised mice, the two types of stem cells form BM-like and dentin-pulp-like complexes, respectively (Gronthos et al., 2000). Although some reports showed that DPSCs have osteogenic potential and may form bone-like structure in vitro and in vivo (Laino et al., 2005, 2006; d’Aquino et al., 2007), there has been no evidence demonstrating that BMMSCs can give rise to functional odontoblasts and generate dentin. One report showed that crude bone marrow cells rarely give rise to dental cells, and only c-kit+-enriched bone marrow cells can acquire the characteristics of odontoblasts. Nonetheless, this phenomenon requires interactions between oral epithelial cells and enriched BM cells (Hu et al., 2006).
Future Prospects
There are several main objectives that need to be addressed before the development of effective cellular-based therapies for regenerative medicine:
Understanding the mechanisms of self-renewal will allow us to regulate adult stem cell growth in vitro to generate sufficient cell numbers needed for different applications. One alternative is embryonic stem cells (ESCs) by way of nuclear transfer technologies. However, this process involves the use of unfertilized donor eggs and discarded embryos. Another approach is the in vitro manipulation of stem cells to allow for the maintenance of their ‘stemness’. Recent demonstrations of the reprogramming of somatic cells to revert to ES-like cells by introducing only 3-4 factors shed light on the possibility of manipulating cells into pluripotent stem cells for a wide variety of applications (Takahashi et al., 2007; Yu et al., 2007; Nakagawa et al., 2008).
Understanding the regulation of stem cells during differentiation and specific tissue production. Certain tissues require the production of specialized extracellular materials such as bone, dentin, cartilage, and tendon. The production of the extracellular matrix and its maturation into specialized tissues involve a sequential activation of cascades of signals. Controlling and providing these signals artificially at a particular step may facilitate the desired tissue regeneration (Kolf et al., 2007).
Understanding the interactions between stem cells and the immune system. Immunosuppressive allogenic MSCs may present an abundant cell source for clinical applications. However, immune responses should be noted, as shown by some in vivo studies (Poncelet et al., 2007). Further research is needed to determine whether allogenic dental MSCs may suppress recipient host short- and long-term immunorejection.
Controlling and preventing ex vivo-expanded MSCs from transformation. Careful monitoring and observation of this possibility are of paramount important, since evidence has shown that adipose-derived MSCs lost genetic stability over time and are prone to tumor formation (Rubio et al., 2005).
Footnotes
This work was supported by grants from the National Institutes of Health: R01 DE019156-01 (G.T.-J.H.), RO1 DE17449 (S.S.), and R21 DE017632 (S.S.).
References
- Abe S, Yamaguchi S, Amagasa T. (2007). Multilineage cells from apical pulp of human tooth with immature apex. Oral Sci Int 4:45-58 [Google Scholar]
- About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. (2000). Human dentin production in vitro. Exp Cell Res 258:33-41 [DOI] [PubMed] [Google Scholar]
- Abukawa H, Kaban LB, Williams WB, Terada S, Vacanti JP, Troulis MJ. (2006). Effect of interferon-alpha-2b on porcine mesenchymal stem cells. J Oral Maxillofac Surg 64:1214-1220 [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA 95:3908-3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh D, Song L, Tuan RS. (2004). Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8:301-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballen KK, Barker JN, Stewart SK, Greene MF, Lane TA. (2008). Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant 14:356-363 [DOI] [PubMed] [Google Scholar]
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. (2003). Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108:863-868 [DOI] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. (2003). Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82:976-981 [DOI] [PubMed] [Google Scholar]
- Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, et al. (2007). Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 75:279-291 [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219-1227 [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180-192 [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. (1997). Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64:278-294 [DOI] [PubMed] [Google Scholar]
- Buurma B, Gu K, Rutherford RB. (1999). Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci 107:282-289 [DOI] [PubMed] [Google Scholar]
- Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. (2003). Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent 23:213-225 [PubMed] [Google Scholar]
- Caplan AI. (1991). Mesenchymal stem cells. J Orthop Res 9:641-650 [DOI] [PubMed] [Google Scholar]
- Carinci F, Papaccio G, Laino G, Palmieri A, Brunelli G, D’Aquino R, et al. (2008). Comparison between genetic portraits of osteoblasts derived from primary cultures and osteoblasts obtained from human pulpar stem cells. J Craniofac Surg 19:616-625 [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127:1671-1679 [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. (2001). Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 189:49-57 [DOI] [PubMed] [Google Scholar]
- Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, et al. (2006a). Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol 18:552-556 [PubMed] [Google Scholar]
- Chen SC, Marino V, Gronthos S, Bartold PM. (2006b). Location of putative stem cells in human periodontal ligament. J Periodontal Res 41:547-553 [DOI] [PubMed] [Google Scholar]
- Chen X, Armstrong MA, Li G. (2006). Mesenchymal stem cells in immunoregulation. Immunol Cell Biol 84:413-421 [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. (2002). Treatment of neural injury with marrow stromal cells. Lancet Neurol 1:92-100 [DOI] [PubMed] [Google Scholar]
- Cohnheim J. (1867). Ueber entzündung und eiterung. Virchows Arch Path Anat Physiol Klin Med 40:1-79 [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. (2000). Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 97:3213-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969 [DOI] [PubMed] [Google Scholar]
- Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. (2000). Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int 66:129-138 [DOI] [PubMed] [Google Scholar]
- d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. (2007). Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14:1162-1171 [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315-317 [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. (2004). Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83:523-528 [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. (1998). Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528-1530; erratum in Science 281:923, 1998 [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267-274 [PubMed] [Google Scholar]
- Gay I, Chen S, MacDougall M. (2007). Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res 10:149-160 [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Finkelman RD, Lynch SE. (1994). Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol 65:1158-1168 [DOI] [PubMed] [Google Scholar]
- Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, et al. (2003). In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res 288:51-59 [DOI] [PubMed] [Google Scholar]
- Greco SJ, Liu K, Rameshwar P. (2007). Functional similarities among genes regulated by Oct4 in human mesenchymal and embryonic stem cells. Stem Cells 25:3143-3154 [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Mansson A, Dellgren G, Klingberg D, Wardell E, Drvota V, et al. (2004). Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg 127:1293-1300 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino ACW. (2008). A method to isolate and purify human bone marrow stromal stem cells. In: Mesenchymal stem cells: methods and protocols. Prockop DJ, Bunnell BA, Phinney DG, editors. Totowa, NJ: Humana Press, pp. 45-57 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. (1999). Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res 14:47-56 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625-13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. (2002). Stem cell properties of human dental pulp stem cells. J Dent Res 81:531-535 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. (2003). Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116:1827-1835 [DOI] [PubMed] [Google Scholar]
- Gu K, Smoke RH, Rutherford RB. (1996). Expression of genes for bone morphogenetic proteins and receptors in human dental pulp. Arch Oral Biol 41:919-923 [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. (2002). Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 99:2199-2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda MJ, Ohara T, Sumita Y, Ogaeri T, Kagami H, Ueda M. (2006). Preliminary study of tissue-engineered odontogenesis in the canine jaw. J Oral Maxillofac Surg 64:283-289 [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. (1997). A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol 68:1186-1193 [DOI] [PubMed] [Google Scholar]
- Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. (2006). Bone marrow cells can give rise to ameloblast-like cells. J Dent Res 85:416-421 [DOI] [PubMed] [Google Scholar]
- Huang G, Sonoyama W, Chen J, Park S. (2006a). In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 324:225-236 [DOI] [PubMed] [Google Scholar]
- Huang GT, Shagramanova K, Chan SW. (2006b). Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod 32:1066-1073 [DOI] [PubMed] [Google Scholar]
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. (2008). The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka J, Ohazama A, Kobayashi M, Nagashima C, Takiguchi T, Kawasaki H, et al. (2001). Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72:314-323 [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. (2008). No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 9:11-21 [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Djouad F, Apparailly F, Noel D. (2003a). Engineering mesenchymal stem cells for immunotherapy. Gene Ther 10:928-931 [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Djouad F, Fritz V, Apparailly F, Plence P, Noel D. (2003b). Mesenchymal stem cells and rheumatoid arthritis. Joint Bone Spine 70:483-485 [DOI] [PubMed] [Google Scholar]
- Kémoun P, Laurencin-Dalicieux S, Rue J, Farges J-C, Gennero I, Conte-Auriol F, et al. (2007). Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res 329:283-294 [DOI] [PubMed] [Google Scholar]
- Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, et al. (2006). Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184:105-116 [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294-1301 [DOI] [PubMed] [Google Scholar]
- Kolf C, Cho E, Tuan R. (2007). Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. (1999). Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 96:10711-10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. (2005). Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 105:3793-3801 [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. (2006). A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia 20:857-869 [DOI] [PubMed] [Google Scholar]
- Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, et al. (2007). Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A 86:1062-1068 [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Mankani MH, Leet AI, Ziran N, Gronthos S, Robey PG. (2007). Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells 25:1830-1839 [DOI] [PubMed] [Google Scholar]
- Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. (2005). A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res 20:1394-1402 [DOI] [PubMed] [Google Scholar]
- Laino G, Carinci F, Graziano A, d’Aquino R, Lanza V, De Rosa A, et al. (2006). In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg 17:511-515 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, et al. (2005). Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49:407-417 [DOI] [PubMed] [Google Scholar]
- Lindroos B, Mäenpää K, Ylikomi T, Oja H, Suuronen R, Miettinen S. (2008). Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun 368:329-335 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. (2008). Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26:1065-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, et al. (1989). A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol 16:545-548 [DOI] [PubMed] [Google Scholar]
- Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. (2001). Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica 86:1099-1100 [PubMed] [Google Scholar]
- McCulloch CA, Bordin S. (1991). Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res 26(3 Pt 1):144-154 [DOI] [PubMed] [Google Scholar]
- McGuire MK, Kao RT, Nevins M, Lynch SE. (2006). rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int J Periodontics Restorative Dent 26:223-231; erratum in Int J Periodontics Restorative Dent 27:88, 2007 [PubMed] [Google Scholar]
- Meirelles LdS, Chagastelles PC, Nardi NB. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119 (Pt 11):2204-2213 [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. (2007). Stem cell niches in mammals. Exp Cell Res 313:3377-3385 [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807-5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Miura M, Gronthos S, Allen MR, Cao C, Uveges TE, et al. (2005). Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci USA 102:14022-14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. (2002). Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg 109:199-209 [DOI] [PubMed] [Google Scholar]
- Mooney DJ, Powell C, Piana J, Rutherford B. (1996). Engineering dental pulp-like tissue in vitro. Biotechnol Prog 12:865-868 [DOI] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, et al. (2005). Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24:155-165 [DOI] [PubMed] [Google Scholar]
- Morsczeck C, Schmalz G, Reichert T, Völlner F, Galler K, Driemel O. (2008). Somatic stem cells for regenerative dentistry. Clin Oral Investig 12:113-118 [DOI] [PubMed] [Google Scholar]
- Murray PE, Garcia-Godoy F, Hargreaves KM. (2007). Regenerative endodontics: a review of current status and a call for action. J Endod 33:377-390 [DOI] [PubMed] [Google Scholar]
- Myers WC, Fountain SB. (1974). Dental pulp regeneration aided by blood and blood substitutes after experimentally induced periapical infection. Oral Surg Oral Med Oral Pathol 37:441-450 [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101-106 [DOI] [PubMed] [Google Scholar]
- Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. (2007). The development of a bioengineered organ germ method. Nat Methods 4:227-230 [DOI] [PubMed] [Google Scholar]
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. (2003). Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol 74:1282-1292 [DOI] [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. (2005). Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol 76:2205-2215 [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. (2001). Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann NY Acad Sci 938:221-229 [DOI] [PubMed] [Google Scholar]
- Ostby BN. (1961). The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand 19:324-353 [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. (1988). Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42-60 [DOI] [PubMed] [Google Scholar]
- Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. (2005). Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836-842 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147 [DOI] [PubMed] [Google Scholar]
- Poncelet AJ, Vercruysse J, Saliez A, Gianello P. (2007). Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro intracardiac injection elicits an immune response in vivo. Transplantation 83:783-790 [DOI] [PubMed] [Google Scholar]
- Porada CD, Zanjani ED, Almeida-Porad G. (2006). Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther 1:365-369 [DOI] [PubMed] [Google Scholar]
- Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. (2008). In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod 34:421-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71-74 [DOI] [PubMed] [Google Scholar]
- Raedt R, Pinxteren J, Van Dycke A, Waeytens A, Craeye D, Timmermans F, et al. (2007). Differentiation assays of bone marrow-derived Multipotent Adult Progenitor Cell (MAPC)-like cells towards neural cells cannot depend on morphology and a limited set of neural markers. Exp Neurol 203:542-554 [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. (2008). Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol 36:742-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter N, Ung F, Roy AK, Vacanti M, Eavey RD, Vacanti CA, et al. (2005). Role for interleukin 1alpha in the inhibition of chondrogenesis in autologous implants using polyglycolic acid-polylactic acid scaffolds. Tissue Eng 11:192-200 [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. (2005). Spontaneous human adult stem cell transformation. Cancer Res 65:3035-3039; erratum in Cancer Res 65:4969, 2005 [DOI] [PubMed] [Google Scholar]
- Scadden DT. (2006). The stem-cell niche as an entity of action. Nature 441:1075-1079 [DOI] [PubMed] [Google Scholar]
- Schofield R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4:7-25 [PubMed] [Google Scholar]
- Segers VF, Lee RT. (2008). Stem-cell therapy for cardiac disease. Nature 451:937-942 [DOI] [PubMed] [Google Scholar]
- Selvig KA, Sorensen RG, Wozney JM, Wikesjö UM. (2002). Bone repair following recombinant human bone morphogenetic protein-2 stimulated periodontal regeneration. J Periodontol 73:1020-1029 [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155 [DOI] [PubMed] [Google Scholar]
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. (2008). SHED repair critical-size calvarial defects in mice. Oral Dis 14:428-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. (2006). Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab 27(1):6-13 [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696-704 [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. (2001). Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29:532-539 [DOI] [PubMed] [Google Scholar]
- Song S, Song S, Zhang H, Cuevas J, Sanchez-Ramos J. (2007). Comparison of neuron-like cells derived from bone marrow stem cells to those differentiated from adult brain neural stem cells. Stem Cells Dev 16:747-756 [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. (2008). Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K, Walsh S, Screen J, Jefferiss CM, Chainey J, Jordan GR, et al. (1999). Further characterization of cells expressing STRO-1 in cultures of adult human bone marrow stromal cells. J Bone Miner Res 14:1345-1356 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861-872 [DOI] [PubMed] [Google Scholar]
- Tozum TF, Demiralp B. (2003). Platelet-rich plasma: a promising innovation in dentistry. J Can Dent Assoc 69:664. [PubMed] [Google Scholar]
- Tsukamoto Y, Fukutani S, Shin-Ike T, Kubota T, Sato S, Suzuki Y, et al. (1992). Mineralized nodule formation by cultures of human dental pulp-derived fibroblasts. Arch Oral Biol 37:1045-1055 [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, et al. (2003). Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells 21:681-693 [DOI] [PubMed] [Google Scholar]
- Valenti MT, Dalla Carbonare L, Donatelli L, Bertoldo F, Zanatta M, Lo Cascio V. (2008). Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone 43:1084-1092 [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. (2000). Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364-370 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang W, Kapila Y, Lotz J, Kapila S. (2009). Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev 18:487-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. (2006a). Cluster analysis and gene expression profiles: a cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 27:3766-3781 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ueda M, Hibi H, Baba S. (2006b). A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: a clinical case report. Int J Periodontics Restorative Dent 26:363-369 [PubMed] [Google Scholar]
- Yen A, Sharpe P. (2008). Stem cells and tooth tissue engineering. Cell Tissue Res 331:359-372 [DOI] [PubMed] [Google Scholar]
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, et al. (2007). Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: phase I/II clinical trial. Stem Cells 25:2066-2073 [DOI] [PubMed] [Google Scholar]
- Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. (2002). Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 81:695-700 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917-1920 [DOI] [PubMed] [Google Scholar]
- Zannettino ACW, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, et al. (2008). Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214:413-421 [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. (2006). Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 12:2813-2823 [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211-228 [DOI] [PubMed] [Google Scholar]