Abstract
Objective
Abnormalities in the morphology and function of two gray matter structures central to emotional processing, the perigenual anterior cingulate cortex (pACC) and amygdala, have consistently been reported in bipolar disorder (BD). Evidence implicates abnormalities in their connectivity in BD. This study investigates the potential disruptions in pACC-amygdala functional connectivity and associated abnormalities in white matter that provides structural connections between the two brain regions, in BD.
Methods
Thirty-three individuals with BD and 31 healthy comparison participants (HC) participated in a scanning session during which functional magnetic resonance imaging (fMRI) during processing of face stimuli and diffusion tensor imaging (DTI) were performed. The strength of pACC-amygdala functional connections was compared between BD and HC groups, and associations between these functional connectivity measures from the fMRI scans and regional fractional anisotropy (FA) from the DTI scans were assessed.
Results
Functional connectivity was decreased between the pACC and amygdala in the BD group, compared to HC group, during the processing of fearful and happy faces (p<0.005). Moreover, a significant positive association between pACC-amygdala functional coupling and FA in ventrofrontal white matter including the region of the uncinate fasciculus was identified (p<0.005).
Conclusion
This study provides evidence for abnormalities in pACC-amygdala functional connectivity during emotional processing in BD. The significant association between pACC-amygdala functional connectivity and the structural integrity of white matter that contains pACC-amygdala connections suggest that disruptions in white matter connectivity may contribute to disturbances in the coordinated responses of the pACC and amygdala during emotional processing in BD.
The perigenual anterior cingulate cortex (pACC) and the amygdala play key roles in the emotional processes disrupted in BD thereby implicating them in the disorder. Convergent evidence suggests that the pACC shares extensive connections with the amygdala (1, 2) and provides regulation of the amygdala response, essential to emotional processing (3). Morphometric magnetic resonance imaging (MRI) and conventional functional MRI (fMRI) analyses provide convergent evidence of morphological and functional abnormalities within the pACC and amygdala in BD (4-10). During emotional processing in persons with BD, dysregulated pACC responses are accompanied by excessive amygdala responses (4, 5), suggesting a disruption in pACC-amygdala functional connections. However, conventional fMRI activation analyses do not assess the functional connections between brain structures. Herein we assessed the strength of the correlation in time between the blood oxygen level dependent (BOLD) responses in the pACC and amygdala during the processing of emotional stimuli. This technique has been used previously as a measure of the strength of pACC-amygdala functional connectivity during emotional processing (11). We hypothesized that there would be reduced pACC-amygdala functional connectivity during emotional processing in BD.
Increasing evidence implicates abnormalities in the structural integrity of frontal white matter in BD. This includes recent evidence for susceptibility in individuals with BD to genetic variations with potential to adversely influence the development of white matter connectivity (12, 13), as well as for decreases in the density of glia including oligodendrocytes and expression of oligodendrocyte- and myelination-related factors in postmortem studies of frontal cortex in BD (14-17). MRI studies provide further evidence for white matter abnormalities in BD including abnormalities in the volume and structural integrity of frontal white matter (18-20).
Diffusion tensor imaging (DTI) presents the opportunity to measure the organization and coherence of white matter fibers (21). Fractional anisotropy (FA) is a DTI measure of white matter organization that provides an index of the coordinated directionality of fibers within a white matter region (22). In the study of neurological disorders, functional connectivity and DTI methods have been combined to show abnormalities in both the functional connectivity and white matter connections between brain regions (23, 24), suggesting that structural abnormalities might contribute to functional connectivity abnormalities in the disorders. A combination of functional and structural connectivity techniques was applied herein to test the second hypothesis of this study that there would be an association between the pACC-amygdala functional connections and the structural integrity of the pACC-amygdala white matter connections, including the uncinate fasciculus which provides major white matter connections between these structures (25, 26).
Methods
Subjects
The Structured Clinical Interview for DSM-IV Axis I Disorders Version 2.0 (27) confirmed the presence or absence of Axis I Disorders and mood state at scanning for the 33 BD and 31 healthy comparison (HC) participants. Symptoms were assessed using the Hamilton Depression Rating Scale (28) and Clinician-Administered Rating Scale for Mania (29). No subject had a history of neurological illness, head trauma with loss of consciousness over 5 minutes or major medical disorder with the exception of 3 female BD participants with treated hypothyroidism. The HC participants did not have a history of Axis I disorder themselves or a history of a mood, psychotic, anxiety or substance related disorder in their first-degree family members. Tables 1 and 2 provide sample details. After a complete description of the study, written informed consent was obtained from all participants in accordance with the human investigation committees of the Yale School of Medicine and the Department of Veterans Affairs.
Table 1.
Sample demographics and behavioral measures
| HC | BD | |
|---|---|---|
| Number (Male) | 31 (14) | 33 (10) |
| Age (years, mean±SD) | 30.4±10.8 | 31.8±9.6 |
| HAMD (mean±SD)* | 0.7±1.2 | 10.8±12.8 |
| CARS-M (mean±SD)* | 0.2±0.5 | 4.9±6.2 |
| Current Tobacco Use (yes:no)* | 2:29 | 11:22 |
| Reaction Time (msecs, mean±SD) | ||
| Fear | 848.7±170.8 | 862.6±161.4 |
| Happy | 819.4±144.5 | 864.9±150.9 |
| Neutral | 812.0±153.3 | 858.8±158.5 |
| Response Accuracy (% correct, mean±SD) | ||
| Fear | 97.3±4.6 | 98.6±3.0 |
| Happy | 97.3±4.1 | 98.3±3.2 |
| Neutral | 97.0±4.9 | 98.1±3.4 |
Notes: HC, healthy control subjects; BD, bipolar disorder patients; SD, standard deviation; HAMD, Hamilton Depression Rating Scale; CARS-M, Clinician-Administered Rating Scale for Mania.
Group comparisons revealed significant differences (all Ps<.05), with increased HAMD, CARS-M, and tobacco usagein the participants with BD relative to the HCs. For all other factors listed in this table, group comparisons were not significant (all Ps>.10). HAM-D scores as Mean±SD for the different mood states: depressed, 28.14±7.1; euthymic, 7.12±6.7; manic/mixed or hypomanic, 4.60±4.6; CARS-M scores as Mean±SD for the different mood states: depressed, 5.00±3.3; euthymic, 3.38±3.4; manic/mixed or hypomanic, 7.10±10.1.
Table 2.
Comparisons of functional connectivity and diffusion tensor imaging indices in participants with bipolar disorder
| Characteristics of Participants with Bipolar Disorder | number (percent) | p values | ||
|---|---|---|---|---|
| fear FC* | happy FC* | ventrofrontal FA* | ||
| Mood state | ||||
| manic/mixed or hypomanic | 10 (30%) | |||
| depressive | 7 (21%) | 0.883 | 0.820 | 0.104 |
| euthymic | 16 (48%) | |||
| Rapid cycling | 19 (58%) | 0.166 | 0.395 | 0.999 |
| History of substance related disorders** | 17 (51%) | 0.390 | 0.440 | 0.095 |
| Medication | ||||
| unmedicated | 6 (18%) | 0.518 | 0.244 | 0.993 |
| lithium carbonate | 9 (27%) | 0.406 | 0.734 | 0.073 |
| anticonvulsants | 16 (48%) | 0.279 | 0.513 | 0.885 |
| atypical antipsychotics | 14 (42%) | 0.956 | 0.791 | 0.679 |
| antidepressants | 12 (36%) | 0.510 | 0.416 | 0.398 |
| benzodiazepines | 7 (21%) | 0.112 | 0.135 | 0.166 |
Group comparisons were conducted for perigenual anterior cingulate cortex-amygdala functional connectivity (FC) during fearful and happy face processing and for ventrofrontal fractional anisotropy (FA). Comparisons were conducted across the three mood states and for the presence/absence of rapid cycling, history of substance related disorders, medications overall and each medication class at the time of scanning.
This included 13 (39%) with a history of alcohol abuse or dependence, 9 (27%) of whom also had a history of other substance abuse or dependence, and an additional 4 (12%) BD participants who had a history of other substance abuse or dependence.
MRI Data Acquisition
FMRI and DTI data were acquired in the same scanning session for each subject with a 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany). FMRI data were acquired with a single-shot echo planar imaging (EPI) sequence in alignment with the anterior commissure-posterior commissure plane with the parameters TR=2000ms; TE=25ms; matrix=64×64; FOV=240×240mm2 and 32 three-mm slices without gap. DTI data were acquired in alignment with the fMRI data with diffusion sensitizing gradients applied along 32 non-collinear directions with b-value=1000 s/mm2, together with an acquisition without diffusion weighting (b-value=0) (TR=7400ms; TE=115ms; matrix=128×128; FOV=256×256mm2 and 40 three-mm slices without gap).
Emotional Face Paradigm
During the fMRI runs, an event-related emotional face task was completed by each participant. Participants viewed faces from the Ekman series depicting fearful, happy or neutral expressions and were instructed to press a button to make a male-female determination, as described previously (30).
Functional Connectivity Processing
Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm) was used for BOLD fMRI pre-processing, as described previously (30). Briefly, images were realigned, spatially normalized to a standard EPI template from the Montreal Neurological Institute (MNI), and spatially smoothed.
The pACC seed region of interest (ROI) was defined with the WFU Pick Atlas Tool (http://www.fmri.wfubmc.edu/download.htm) based on its ACC region excluding the dorsal component (31). For each subject, a mean time series for the pACC seed ROI was calculated by averaging the time series for all voxels within the pACC ROI. Correlational analyses were then performed between the pACC time series and the time series for each brain voxel (32, 33), resulting in a correlation map for each subject that contained the correlation coefficient for each voxel with that of the pACC ROI. For further statistical analysis, the correlation coefficients were transformed to Z values using Fisher r-to-z transformation (34).
DTI Data Processing
Diffusion-weighted data were first interpolated to 1.5 mm isotropic voxels and then denoised by a three-dimensional isotropic Gaussian kernel with sigma 2 mm full-width-at-half-maximum (FWHM) Gaussian kernel. After diagonalization of the DTI data, diffusion eigenvectors and corresponding eigenvalues (λ1, λ2, λ3) were acquired. FA was then calculated according to the methodology of Basser et al (35):
Whole brain FA maps were normalized with SPM5 to the standard MNI space using a tissue probability map of white matter as a template. The FA maps were resampled to 2mm×2mm×2mm during the normalization. Further, each FA map was spatially smoothed by a 10 mm FWHM. FA values at each brain voxel were submitted to a linear-regression analysis in SPM (see below) in order to determine the relationship between FA values throughout the entire brain and pACC-amygdala functional connectivity.
Statistical Analyses
In order to test the primary study hypothesis, group differences were analyzed using two-sample t-tests in SPM with the functional connectivity correlation coefficients (Z scores) from the pACC to all brain voxels as the dependent variables for each face condition (fearful, happy and neutral face expressions). Findings were considered significant for P<0.005 (uncorrected) and for clusters>640 mm3 (10 voxels).
In order to test the second study hypothesis regarding the relationship between functional and structural pACC-amygdala connectivity, correlation coefficients (Z scores) for each face condition in which group differences in pACC-amygdala connectivity were detected were extracted from the amygdala [defined with the MarsBar toolbox(http://marsbar.sourceforge.net)]. Whole-brain linear regression analysis was performed in SPM to investigate the association between these pACC-amygdala correlation coefficients (exploratory variables) and FA values (dependent variables) at all brain voxels for all participants. Findings were considered significant for P<0.005 (uncorrected) and for clusters>640 mm3 (80 voxels). Finally, group differences in FA values from the point of highest association between FA and pACC-amygdala functional connectivity correlation coefficients were analyzed using two-sample t-tests.
Results
There were no significant group differences in mean reaction time or response accuracy to the different face types (Table 1). We observed decreased pACC-amygdala functional connectivity in the BD group, compared to the HC group (P<0.005), during fearful (Figure 1a and c) and happy (Figure 1b and d) face processing. No significant group differences in functional connectivity were detected during neutral face processing. Whole brain analyses revealed additional group differences in functional connectivity from the pACC to the ventral caudate and nucleus accumbens during fearful and happy processing, as well as decreased connectivity from the pACC to the right inferior temporal gyrus during fearful processing. Each of the amygdala findings remained significant after Bonferroni correction to account for the three face conditions considered; however, the additional findings in the striatum and cortex did not survive this correction.
Figure 1.
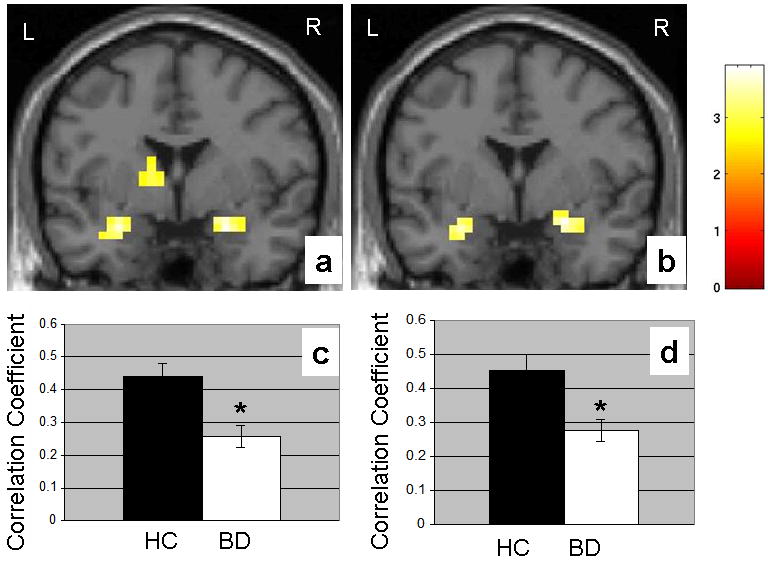
The coronal images (MNI coordinates y=0 mm) display the amygdala and ventral striatal regions where functional coupling to the perigenual anterior cingulate cortex (pACC) is decreased in the 33 participants with bipolar disorder (BD), compared to the 31 healthy comparison (HC) participants during (a) fearful face processing (MNI coordinates for the maximal point of difference in the amygdala: x=-28mm, y=0mm, z=-20mm, 22 voxels, T=3.63, P<0.001 uncorrected; x=24mm, y=-4mm, z=-20mm, 39 voxels, T=3.9, P<0.001 uncorrected) and (b) happy face processing (MNI coordinates for the maximal point of difference in the amygdala: x=-28mm, y=0mm, z=-20mm, 13 voxels, T=3.17, P=0.001 uncorrected; x=24mm, y=0mm, z=-16mm, 28 voxels, T=3.46, P<0.001 uncorrected). The color bar represents the range of T values. The findings are displayed on a T1 image. L: left brain; R: right brain. The graph shows the mean correlation coefficients from the pACC to the amygdala and standard errors for the BD group (n = 33) and the HC (n = 31) group during (c) fearful face processing and (d) happy face processing.
*denotes significant decreases
Within the entire sample, regression analyses revealed a significant positive association between the pACC-amygdala functional coupling during fearful and happy face processing and FA values in the region of the uncinate fasciculus, and neighboring ventrofrontal white matter (P<0.005) (Figure 2). These findings remained significant when Bonferroni correction was applied to account for analyses performed in the two emotional face conditions. Significant positive associations between pACC-amygdala functional connectivity and FA were also detected in other distributed white matter regions including regions of fronto-thalamo-striatal projections, as well as frontotemporal projections to posterior association cortex. The association between pACC-amygdala functional coupling and FA in the uncinate fasciculus region found in the entire sample was also present within the BD group alone (Fear: r=0.308,P=0.041; Happy: r=0.3,P=0.045), as well as the HC group alone (Fear: r=0.401,P=0.013; Happy: r=0.371,P=0.02). FA values were significantly decreased (P=0.04) in the BD group (mean±SD=0.28±0.08), relative to the HC group (mean±SD=0.32±0.06), in the region where the functional-structural connectivity associations were detected (x=-18mm,y=18mm,z=-10mm; sphere of radius=2mm).
Figure 2.
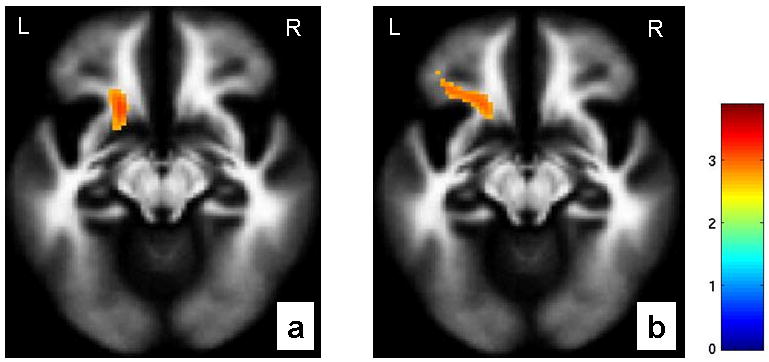
The axial-oblique images (MNI coordinates z=-12mm) display the regions in ventrofrontal white matter in which fractional anisotropy showed a positive association with functional coupling from perigenual anterior cingulate cortex to amygdala during (a) fearful processing (MNI coordinates for the point of maximal association: x= -18mm, y= 18mm, z= -10mm, 107 voxels, T=3.32, P=0.001 uncorrected) and (b) happy processing (MNI coordinates for the point of maximal association: x= -18mm, y= 20mm, z= -10mm, 157 voxels, T=2.88, P=0.002 uncorrected) in the entire cohort. The color bar represents the range of T values. The findings are displayed on a tissue probability map of white matter. L: left brain; R: right brain.
Post-hoc exploratory analyses performed in patients did not reveal any significant effects of mood state, rapid cycling, history of substance related disorders or medication status on pACC-amygdala functional connectivity or ventrofrontal FA (Table 2).
Discussion
We detected disturbances in pACC-amygdala functional connectivity during the processing of faces depicting positive and negative emotions in BD. Moreover, we found an association between pACC-amygdala functional connectivity measurements and the structural integrity of ventrofrontal white matter, including the uncinate fasciculus where FA was also significantly decreased in the BD group. Taken together, these data provide some of the first evidence that abnormalities in the structural integrity of white matter may contribute to disruptions in the coordinated response of the pACC and amygdala to emotional stimuli in BD.
The pACC and amygdala are critical components of emotional processing circuitry and share extensive interconnections (1, 2). Evidence has suggested that the pACC activates local inhibitory circuits in the amydala, thereby regulating amygdala response (36, 37). The decrease in pACC-amygdala functional connectivity in BD could reflect a reduction in the pACC's inhibitory control over the amygdala. Disrupted structural integrity of white matter bundles that contain pACC-amygdala connections, as reported herein, might contribute to pACC-amygdala functional connectivity deficits. PACC structural abnormalities (6, 7) have also been reported in BD and may contribute to the circuitry dysfunction. Finally, abnormalities in a third structure with connectivity to both pACC and amygdala might play a role in the reduced functional connectivity between the regions. Therefore, further study of gray-white matter relationships and their associations to pACC-amygdala functional connectivity disruptions, and exploration of abnormalities in other regions with connectivity to pACC and amygdala, are required to advance understanding of the interconnections between the pACC and amygdala. Of note, results from conventional functional activation analyses show pACC activation decreases in the BD group compared to the HC group, during fearful and happy face processing (Supplementary Materials). No group differences were observed in amygdala activation. The absence of group differences in amygdala activation, despite the significant decreases in pACC-amygdala connectivity, is unclear. A possible explanation is that our imaging methods were more sensitive to detecting connectivity abnormalities than within-region amygdala abnormalities. Alternatively, connectivity abnormalities may have been especially prominent in this sample or factors such as medication might have blunted amygdala differences (4). Other research groups that used similar functional connectivity methods to study disorders in which frontotemporal connectivity disturbances are implicated, such as schizophrenia, have similarly detected connectivity differences in the absence of activation differences within the connected brain regions (38).
White matter fibers that course through the ventral prefrontal cortex, including the uncinate fasciculus, are thought to provide the structural framework for the functional connections between the pACC and amygdala (25, 26). We performed structural and functional connectivity measures in the same subjects, during the same scanning session, permitting examination of their relationship. Significant positive associations were identified between FA values in the region of the uncinate fasciculus and the correlation coefficients for functional coupling between the pACC and amygdala, suggesting that abnormalities in ventrofrontal white matter may contribute to disruptions in pACC-amygdala functional connectivity in BD. A recent DTI tractography study reported an increase in the number of fibers connecting the subgenual cingulate and amygdalo-hippocampal complex in BD (39), providing a view of white matter abnormalities in BD complementary to that described herein. However, this study did not detect FA reductions in these fibers. Potential explanations for the divergent results between the two studies may include differences in the specific fibers studied due to the different imaging processing and analysis methodologies and differences in sample characteristics. Future work using complementary DTI imaging methods in a single study, and collaborative studies across imaging centers using comparable methods, could help resolve discrepant results.
The functional connectivity analyses revealed significant decreases in pACC-amygdala functional coupling in BD during the processing of fearful and happy faces, but not during processing of emotionally neutral faces. This is consistent with previous functional neuroimaging studies which showed engagement of this circuitry in response to both fearful and happy faces (40-42) and the efficacy of emotionally-valenced faces as stimuli to study pACC-amygdala interactions in functional connectivity analyses (11). Moreover, it supports the utility of this approach to probe abnormalities in pACC-amygdala functional connectivity in disorders of affective regulation.
Reduced functional coupling from pACC was also detected in the ventral caudate and nucleus accumbens, although these findings did not survive more stringent corrections for multiple comparisons. The ventral caudate and nucleus accumbens are interconnected with pACC and amygdala (25, 43) and are implicated in BD because of their role in motivated behaviors that are dysregulated in the disorder and findings of alterations in their shape, volumes and activation in BD (44-46). In addition to ventrofrontal areas, regions in which the structural integrity of white matter were associated with pACC-amygdala coupling included fronto-thalamo-striatal projections and projections to posterior sensory regions that provide connections within a more widely distributed neural system. This suggests that disruptions in pACC-amygdala connectivity may be associated with abnormalities in the development of a more widely distributed neural system subserving emotional regulation (47).
Participants with BD were in different mood states, and had variable histories of rapid-cycling, comorbidity and medication and substance exposure. While we did not detect significant main effects of these factors on pACC-amygdala functional connectivity or ventrofrontal FA, our ability to detect effects of these factors was potentially limited by inadequate power. We also performed our analyses with current tobacco use as a covariate, yielding equivalent results. Additional studies reporting reduced functional connectivity in manic participants (48) as well as across acute mood states and euthymia (49) suggest that functional connectivity disturbance may be a trait abnormality in BD. The majority of our BD participants reported symptom onset in adolescence/early adulthood. Interestingly, ACC receives a progressive and dramatic growth of fibers originating from the amygdala during adolescence/early adulthood (50), a time period coinciding with this peak in the onset of BD, suggesting that further study in the development of the ACC-amygdala connectivity in adolescence/early adulthood in BD may help to elucidate a neurodevelopmental mechanism contributing to the disorder.
Acknowledgments
The authors were supported by grants from the National Institute of Mental Health R01MH69747 (HPB), R01MH070902 (HPB), T32MH14276 (JHK, LGC), CTSA Grant Number UL1 RR0249139 from the NIH National Center for Research Resources (NCRR), the Department of Veterans Affairs Career Development (HPB), Merit Review (HPB) and Research Enhancement Award Program (REAP) (HPB, LGC) programs, the National Alliance for Research in Schizophrenia and Depression (Great Neck, NY) (HPB, FW, JHK), The Attias Family Foundation (HPB), Marcia Simon Kaplan (JHK), The Ethel F. Donaghue Women's Investigator Program at Yale (New Haven, CT) (HPB), the Klingenstein Foundation (JHK) and a Howard Hughes Medical Institute Fellowship (MPS).
We wish to thank Ms. Kathleen Colonese who was devoted to helping those suffering from psychiatric illnesses and to advancing the field of bipolar disorder research. The authors thank Ralitza Gueorguieva, Ph.D. and Brian Pittman, M.S. for providing their statistical expertise, Cheryl Lacadie, B.S., Karen Martin, R.T.R.M.R., Terry Hickey, R.T.R.M.R. and Hedy Sarofin, R.T.R.M.R. for their technical expertise, Allison McDonough, B.S. and Lindsay Warren for assistance with the study, and the research subjects for their participation.
Footnotes
Financial Disclosures: Dr. Blumberg reports having received lecture honoraria from Eli Lilly and Abbott Laboratories and consultant fees from Pfizer. Drs. Wang, He, Jackowski, Kalmar, Chepenik, Tie, Gong, Constable, Mr. Shah, Ms. Edmiston, Ms. Jones and Ms. Uderman reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 2.Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–30. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 3.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183:308–13. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 5.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–55. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–43. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–6. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 10.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–3. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 11.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2008;13:1054–9. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- 13.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–6. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 16.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 17.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–92. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 18.Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 19.Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–9. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 20.Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 21.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 23.Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–8. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, et al. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136–45. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured interview guide for the Hamilton depression rating scale: seasonal affective disorder version (SIGH-SAD) New York, NY: New York State Psychiatric Institute; 1994. [Google Scholar]
- 29.Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–34. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- 30.Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–10. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 34.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press; 1992. [Google Scholar]
- 35.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 36.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 39.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–10. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 40.Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, et al. Time courses of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp. 2001;12:193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 42.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 43.Fudge JL, Emiliano AB. The extended amygdala and the dopamine system: another piece of the dopamine puzzle. J Neuropsychiatry Clin Neurosci. 2003;15:306–16. doi: 10.1176/jnp.15.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–46. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 45.Hwang J, Lyoo IK, Dager SR, Friedman SD, Oh JS, Lee JY, et al. Basal ganglia shape alterations in bipolar disorder. Am J Psychiatry. 2006;163:276–85. doi: 10.1176/appi.ajp.163.2.276. [DOI] [PubMed] [Google Scholar]
- 46.Beyer JL, Kuchibhatla M, Payne M, Moo-Young M, Cassidy F, MacFall J, et al. Caudate volume measurement in older adults with bipolar disorder. Int J Geriatr Psychiatry. 2004;19:109–14. doi: 10.1002/gps.1030. [DOI] [PubMed] [Google Scholar]
- 47.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 48.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]


