Abstract
Peroxiredoxins are neuroprotective antioxidant enzymes that reduce hydroperoxides and protect neurons against oxidative stress. However, they can be inactivated through hyperoxidation of their active site cysteine, an event that can take place in the brain in response to oxidative insults such as stroke and also normal aging. Synaptic activity promotes the reduction of hyperoxidized peroxiredoxins in neurons, and induces the expression of sulfiredoxin (Srxn1) and sestrin 2 (Sesn2) which have been reported to mediate this. We have investigated the importance of histone acetylation in the regulation of these genes, to understand more about how these genes are regulated by synaptic activity.
We show that the sestrin 2 promoter undergoes activity-dependent histone acetylation, which contributes to its transcriptional activation. In contrast, promoter-proximal histone acetylation is not involved in the activity-dependent induction of sulfiredoxin. Nevertheless, expression of both sestrin 2 and sulfiredoxin can be induced by enhancing histone acetylation through treatment of neurons with the histone deacetylase inhibitor trichostatin A (TSA). Furthermore, protective doses of TSA inhibit the formation of hyperoxidized peroxiredoxins in neurons exposed to oxidative insults. Histone deacetylases are emerging therapeutic targets in neurodegenerative disorders associated with oxidative stress. Our results indicate that manipulating the histone acetylase-deacetylase balance in neurons may mimic the effects of synaptic activity in preventing the oxidative inactivation of peroxiredoxins.
Keywords: neuroprotection, peroxiredoxin, oxidative stress, neurodegeneration, synaptic activity
Introduction
Peroxiredoxins (Prxs) are a ubiquitous family of thiol-based antioxidative enzymes.1-3 The 2-cysteine (2-Cys) Prxs is the predominant Prx subfamily, comprising Prx I-IV.4 Prxs have cytoprotective effects in the face of oxidative insults in a variety of cell types.1,5,6 PrxII protects PC12 cells against trophic deprivation-induced apoptosis7 and protects cortical neurons against Aβ toxicity.8 PrxIII protects hippocampal neurons against excitotoxicity.2 Antisense against PrxII renders neuroblastoma cells vulnerable to oxidative stress,3 and knock-down of PrxII renders cortical neurons vulnerable to MPP+ treatment.9 Overexpression of PrxII protects neurons against ischemia in vitro.10
Prxs contain a peroxidatic cysteine residue at the heart of their active site which catalyses the reduction of hydroperoxides, becoming oxidized to cysteine sulfenic acid (-SOH) in the process. Cys-SOH then forms a disulfide bond with the resolving cysteine, which is in turn reduced by thiol-based reducing enzymes, principally thioredoxin.4 Sometimes, under increased oxidative stress, Prx-SOH is further oxidized by peroxide to sulfinic (-SO2H) acid, causing inactivation of peroxidase activity.11 Prx hyperoxidation to Prx-SO2H takes place in neurons undergoing oxidative death in vitro, and is associated with ischemic brain damage in vivo as well as normal aging.12,13 Prx-SO2H is not a substrate for the resolving cysteine and cannot be reduced by thioredoxin. As such, Prx hyperoxidation to Prx-SO2H was thought to be irreversible. However, it was subsequently found that Prx-SO2H can be reduced back to the catalytically active thiol form by two ATP-dependent reductases, sulfiredoxin (Srxn1)14-16 and sestrin 2 (Sesn2).17 We recently found that both sulfiredoxin and sestrin 2 are inducible genes whose expression is rapidly triggered by synaptic activity.12 Synaptic activity also promoted the reduction of hyperoxidized Prx and protection against peroxide-induced death.12 The strong inducibility of both sestrin 2 and sulfiredoxin essentially means that neuronal Prx-SO2H-reducing capacity can be switched on or off by a variety of signals.12,18 Since knockdown studies causally linked these genes to neuroprotection,12 pharmacological manipulation of their transcription may be of therapeutic benefit in disorders associated with oxidative damage.
The regulation of transcription is a complicated process, but a key level of control is in the post-translational modification of promoter-proximal histones.19,20 It is generally true that an increase in histone acetylation promotes transcriptional activation through the relaxation of chromatin structure.19,21 The degree of histone acetylation at any one locus is controlled by the balance of histone acetyl transferases (HATs) and histone deacetylases (HDACs). Changes in histone acetylation are observed in response to learning paradigms, recreational drug exposure, environmental enrichment and seizure activity.22-25 As well as a role for histone acetylation changes in physiological processes, inappropriate histone acetylation (particularly hypoacetylation) may contribute to several CNS disorders by causing aberrant transcription within neurons.26,27 As a result, histone deacetylase inhibitors are emerging as potential therapies for a number of neurodegenerative diseases and syndromes, including Huntington disease, motor-neuron disease, fragile X syndrome and Friedreich ataxia.27-31 In vitro, inhibition of HDACs prevents oxidative neuronal death of embryonic cortical neurons29 by a mechanism independent of the glutathione pathway.29 It is not clear whether more mature neurons are also protected by HDAC inhibition.
Here we have investigated the role of histone acetylation in regulating the basal and activity-induced expression of sulfiredoxin and sestrin 2, which can reverse the hyperoxidation of Prxs. We find that histone acetylation plays an important role in the activity-dependent induction of sestrin 2, but not sulfiredoxin. Nevertheless, expression of both genes can be induced by elevating global histone acetylation levels by pharmacological inhibition of class I and II histone deacetylases.
Results
Synaptic activity triggers acetylation of histone H4 at the sestrin 2 promoter, but not the sulfiredoxin promoter
To investigate the importance of promoter-proximal histone acetylation in the activity-dependent induction of sestrin 2 and sulfiredoxin expression, we wanted to know whether promoter-specific histone acetylation takes place in response to synaptic activity. We used the established method of network disinhibition to enhance synaptic activity, by applying the GABAA receptor antagonist bicuculline, and the K+ channel antagonist 4-aminopyridine (which enhances burst frequency, hereafter BiC/4-AP32,33). As expected, expression of sulfiredoxin and sestrin 2 were strongly induced by BiC/4-AP treatment (Fig. 1A and B).
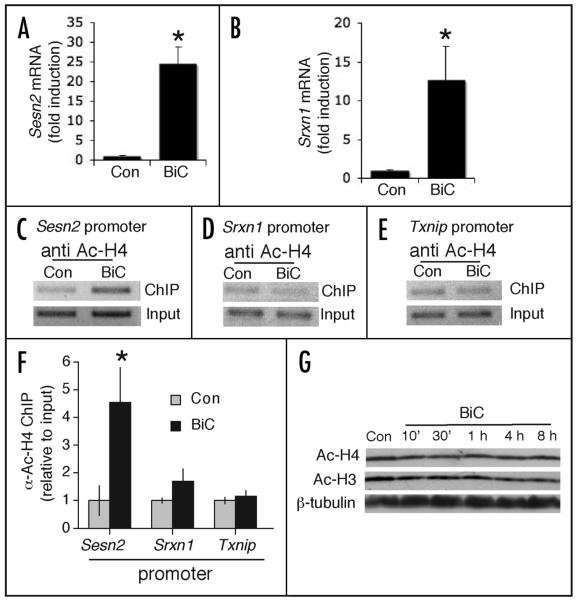
Figure 1.
Synaptic activity triggers acetylation of histone H4 at the sestrin 2 promoter, but not the sulfiredoxin promoter. (A and B) Neuronal cultures were treated with bicuculline (50 μM) plus 4-aminopyridine (250 μM, denoted “BiC”) for 8 h followed by RNA extraction and q-RT-PCR analysis of Sesn2 (A) and Srxn1 mRNA (B) normalized to Gapdh mRNA levels. *p < 0.05 (two-tailed t-test, n = 3). (C–E) Chromatin immunoprecipitation (ChIP) analysis of histone H4 acetylation of gene promoters in either control neurons or those treated with BiC/4-AP. Stained agarose gels are shown of representative PCR reactions using primers spanning the promoters of Sesn2 (C), Srxn1 (D) and Txnip (E). (F) Densitometric analysis of PCR-amplified bands from all independent repeats (4) of the anti-acetyl H4 ChIP. The purpose of this analysis is to demonstrate the reproducibility of the observed activity-dependent Sesn2 promoter H4 acetylation. *p < 0.05 (two-tailed t-test, n = 4). (G) Synaptic activity does not change global levels of histone acetylation. Neurons were treated with BiC/4-AP for the indicated times followed by western blot analysis with antibodies specific for the acetylated forms of histones H3 and H4.
Signal-dependent acetylation of histones normally occurs on histones H3 and H4.19 We decided to study histone H4 acetylation, since increases in this modification are readily detectable in neurons at inducible genes, in response to signalling events.20,34 We performed chromatin-immunoprecipitation (ChIP) with an anti-acetyl H4 antibody on extracts taken from control and BiC/4-AP-treated neurons. We then performed PCR on the immunoprecipitated DNA using primers spanning the promoter regions of sestrin 2, sulfiredoxin and also Txnip, a gene whose expression is suppressed by synaptic activity.12
Using this technique, we found that BiC/4-AP treatment caused a consistently large increase in H4 acetylation at the sestrin 2 promoter in each of four independent experiments (Fig. 1C and F). In contrast, no significant increase was observed at the sulfiredoxin promoter (Fig. 1D and F), nor in the promoter of Txnip (Fig. 1E and F). Thus, sulfiredoxin and sestrin 2, two genes induced to a similar extent by synaptic activity, appear to differ in the degree to which their promoters become acetylated at histone H4. This is consistent with the idea that activity-dependent histone acetylation is a specific, targeted event, rather than a globally induced effect. Indeed, we found that promoting synaptic activity with BiC/4-AP treatment did not cause a global increase in histone H3 or H4 acetylation, as assayed by western blot analysis with anti acetyl H3 and H4 antibodies (Fig. 1G).
Histone acetylation plays a role in the activity-dependent induction of sestrin 2 expression
The above results indicate that synaptic activity triggers promoter-proximal histone acetylation of the sestrin 2 gene which may contribute to its enhanced transcription. To test this specifically, we first investigated whether inducing histone acetylation is sufficient to cause an increase in sestrin 2 expression. Histone acetylation in neurons was induced by inhibition of class I and II histone deacetylases by trichostatin A (TSA). As expected, treatment of cortical neurons with TSA resulted in a global increase in histone H3 and H4 acetylation, as assayed by western blot (Fig. 2A). TSA treatment resulted in significant induction of Sestrin 2 mRNA (Fig. 2B). Thus, elevation of histone acetylation is sufficient to induce sestrin 2 expression.
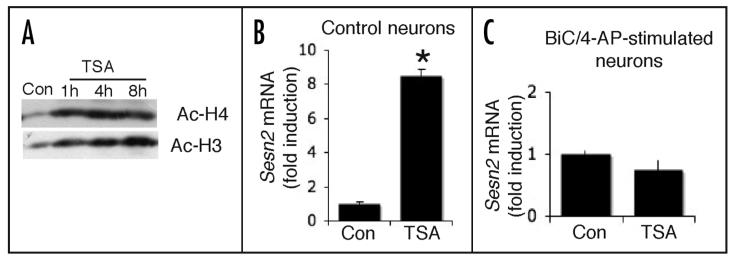
Figure 2.
Histone acetylation plays a role in the activity-dependent induction of sestrin 2 expression. (A) Confirmation that treatment of neurons with TSA induces histone acetylation. Western analysis of acetylation on histones H4 and H3 in neurons treated with TSA (1 μM here and throughout) for the indicated times. (B) TSA treatment is sufficient to induce expression of sestrin 2. qPCR based analysis of Sesn2 mRNA expression in neurons treated with TSA (8 h). *p < 0.05 (two-tailed t-test, n = 3). (C) The effect of TSA in inducing sestrin 2 expression is occluded by synaptic activity. Neurons were treated with BiC/4-AP in the presence or absence of TSA for 8 h followed by RNA extraction and q-RT-PCR analysis of Sesn2 (n = 3).
We next investigated whether increased histone acetylation plays a role in the transcriptional activation of sestrin 2 by synaptic activity. To do this, we determined the extent to which TSA promoted expression of sestrin 2 in neurons experiencing high levels of synaptic activity. We reasoned that if synaptic activity was promoting gene expression by enhancing promoter-proximal histone acetylation, then the effect of TSA would be occluded by the effect of synaptic activity. We found that in BiC/4-AP treated neurons, where sestrin 2 expression is high (Fig. 1A), TSA treatment failed to further induce sestrin 2 expression (Fig. 2C). Thus, the strong induction of sestrin 2 by TSA treatment [observed in electrically quiet neurons (Fig. 2B)] is completely occluded by synaptic activity. This indicates that synaptic activity induces sestrin 2 expression in part by promoting promoter histone acetylation.
Histone acetylation is not involved in the activity-dependent induction of sulfiredoxin expression
The absence of an observed increase in histone H4 acetylation accompanying the activity-dependent induction of sulfiredoxin expression (Fig. 1D) indicated that histone acetylation may not be involved in the induction of this gene by synaptic activity. However, this possibility could not be ruled out. To investigate this further we studied the influence of TSA on sulfiredoxin expression in both electrically quiet and synaptically active neurons. Treatment of neurons with TSA led to a significant induction of sulfiredoxin mRNA (Fig. 3A). This demonstrates that enhancing histone acetylation is sufficient to induce sulfiredoxin expression, just as it is sufficient to induce sestrin 2 expression. However, this induction of sulfiredoxin by TSA treatment was not occluded by synaptic activity: TSA still induced sulfiredoxin expression in BiC/4-AP stimulated neurons (Fig. 3B). This suggests that synaptic activity and TSA induce sulfiredoxin expression by different pathways i.e., that histone acetylation is not involved in the activity-dependent induction of sulfiredoxin expression, consistent with the absence of an increase in histone H4 acetylation following synaptic activity (Fig. 1D).
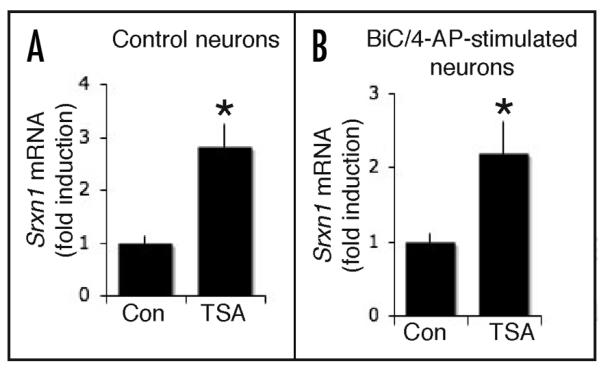
Figure 3.
Histone acetylation is not involved in the activity-dependent induction of sulfiredoxin expression. (A) TSA treatment is sufficient to induce expression of sulfiredoxin. qPCR based analysis of Srxn1 mRNA expression in neurons treated with TSA (8 h, two-tailed t-test, n = 3). (B) The effect of TSA in inducing sulfiredoxin expression is not occluded by synaptic activity. Neurons were treated with BiC/4-AP in the presence or absence of TSA for 8 h followed by RNA extraction and q-RT-PCR analysis of Srxn1 (n = 3).
Protective doses of TSA inhibit the formation of hyperoxidized Prxs
The therapeutic application of histone deacetylase inhibitors has been suggested for a variety of diseases of the nervous system, many of which are associated with oxidative stress.26 Despite a differential role for histone acetylation in the activity-dependent induction of sulfiredoxin and sestrin 2 expression, both genes are induced by TSA. This raises the possibility that the reduction of hyperoxidized Prxs could be promoted by this pharmacological intervention. We first wanted to confirm that the induction of sestrin 2 and sulfiredoxin mRNA by TSA treatment led to an upregulation in protein expression. Western analysis revealed this to be the case: expression of both proteins were induced by TSA (Fig. 4A). We then tested the ability of TSA to inhibit the formation of hyperoxidized Prxs, using western analysis employing an antibody specific for the hyperoxidized form of Prx. We applied a brief dose of H2O2 to induce Prx hyperoxidation and then looking for reduction of this after H2O2 washout. We focussed on a band that we believe to be PrxII, the major neuronal cytosolic Prx.35,36 As reported previously, unstimulated neurons did not exhibit significant reduction in Prx-SO2H levels 16 h after washout, while synaptic activity evoked by BiC/4-AP treatment promotes a reduction of Prx-SO2H levels (Fig. 4B and C; and reviewed in ref. 12). Strikingly, TSA pre-treatment for 8 h also promoted a significant reduction in Prx-SO2H levels following washout (Fig. 4B and C). Further biochemical analysis would be required to determine for sure whether this reflects actual reduction of the Prx-SO2H form of Prx. Moreover, other experiments are required to determine whether induction of sulfiredoxin and/or sestrin 2 is responsible for these effects. Of note, HDAC6 inhibition has recently been reported to induce N-acetylation of Prx I and II, increasing resistance to hyperoxidation.37 This offers an alternative explanation as to why TSA treatment inhibits formation of hyperoxidized Prxs.
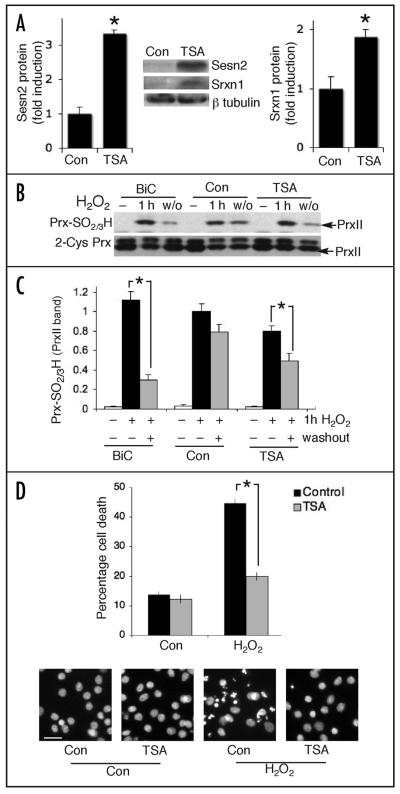
Figure 4.
Inhibiting histone deacetylases may promote the reduction of levels of hyperoxidized Prxs and protect against oxidative insults. (A) TSA treatment is sufficient to induce expression of sestrin 2 and sulfiredoxin protein. Neurons were treated with TSA for 8 h followed by western analysis of sulfiredoxin and sestrin 2 expression. Densitometric analysis of ECL-developed blots is shown for sestrin 2 (left) and sulfiredoxin (right). *p < 0.05 (two-tailed t-test, n = 4). Inset shows an example blot. (B and C) TSA promotes the recovery of hyperoxidized PrxII-SO2/3H following a 1 h exposure to H2O2 (100 μM). Neurons were treated with TSA as indicated for 8 h prior to H2O2 exposure. Washout period was 16 h after which cell lysates were prepared for western analysis of PrxII-SO2/3H levels using an antibody specific for the hyperoxidized form of 2-Cys Prxs. To control for loading, blots were stripped and re-probed with an antibody against 2-Cys Prxs. (B) shows an example blot; (C) shows quantitation of all blots performed (*p < 0.05 compared to pre-washout level, two-tailed t-test, n = 5). (D) TSA treatment protects neurons against peroxide-induced apoptosis. Neurons were pre-treated with TSA or vehicle for 8 h prior to the application of H2O2 (100 μM). Neurons were fixed after a further 24 h and subjected to DAPI staining and cell death quantified blind (see Materials and Methods) and expressed as the number of apoptotic nuclei as a percentage of the total. (*p < 0.05, two-tailed t-test, n = 3). Lower panels show example pictures of DAPI-stained nuclei, scale bar represents 50 μm.
Finally, we wanted to know whether the TSA-induced changes outlined above are associated with reduced neuronal death following challenge with an oxidative insult. TSA has been shown to protect against oxidative neuronal death in embryonic E17 neurons cultured for 1–2 days in vitro.29 However, the neurons used in this study are more mature (E21 cortical neurons cultured for 8–10 days in vitro). We found that 8 h TSA pre-treatment resulted in far lower levels of peroxide-induced apoptosis compared to control (Fig. 4D). Thus, enhancing histone acetylation levels protects neurons against oxidative insults in our system, as well as upregulating expression of sulfiredoxin and sestrin 2.
Discussion
Although synaptic activity promotes the expression of a large number of genes, knowledge of the role of dynamic changes in histone acetylation in the induction of individual genes is incomplete. We have found that two activity-induced genes, one or both of which contribute to enhanced antioxidant defenses, differ markedly in terms of the role of histone acetylation in their induction by synaptic activity.
Activity-dependent regulation of sulfiredoxin via AP-1 is independent of histone acetylation
Sulfiredoxin has demonstrable cytoprotective and antioxidative effects in diverse cells including neurons and lung epithelial cells.12,38,39 A study of the transcriptional regulation of sulfiredoxin in neurons revealed that it could be controlled by AP-1 via two cis-acting AP-1 consensus sites.12 Mutation of each AP-1 site in turn reduced transcriptional activation of sulfiredoxin by synaptic activity, and mutation of both sites abolished induction completely.12 Also, expression of the widely-used inhibitor of AP-1-mediated gene expression, TAM67 (a dominant-negative form of c-Jun40) inhibited synaptic activity-dependent induction of the sulfiredoxin promoter in neurons.18 The direct regulation of sulfiredoxin by AP-1 acting via these two sites was confirmed in the context of mouse epidermal cells. Here it was shown that 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated induction of sulfiredoxin was stimulated by AP-1 acting on these sites.41 Our findings indicate that histone acetylation is not involved in the activity-dependent induction of sulfiredoxin. Activity-evoked signals that result in the activation or expression of AP-1 can clearly promote transcription independent of acetylation. Despite this, TSA is still able to induce sulfiredoxin: this may either be via the direct acetylation of the sulfiredoxin promoter, or conceivably due to the induction of AP-1 transcription factor expression. For example, TSA is known to induce c-fos expression in neurons,42 which could conceivably feed back onto the sulfiredoxin promoter. Alternatively, the induction of sulfiredoxin expression by TSA treatment may be independent of AP-1. Sulfiredoxin is also subject to transcriptional regulation by Nuclear factor erythroid 2-related factor (Nrf2) acting via an antioxidant response element.18,39 Nrf2 acts at these elements as a heterodimer with small Maf proteins.43 Under basal conditions, when Nrf2 is inactive, Maf heterodimerizes with Bach1 and exerts active repression of target genes. Thus, TSA could be interfering with the activity of the corepressor complex recruited by Maf/Bach1 heterodimers.
Histone acetylation-dependent induction of the C/EBPβ target gene sestrin 2
The transcriptional activation of sestrin 2 by synaptic activity is mediated by C/EBPβ, acting via two sites in the promoter. Evidence presented here suggests that this induction mediated by C/EBPβ may involve derepression of the sestrin 2 promoter through enhanced histone acetylation. The strong sensitivity of basal levels of sestrin 2 to changes in histone acetylation suggest that it may ordinarily be subject to active suppression through promoter deacetylation. C/EBPβ is repressed by the corepressor SMRT44 which is known to recruit a number of histone deacetylases to transcription factors with which it interacts,45 indicating that it could be capable of mediating such suppression under basal conditions. Following synaptic activity, however, SMRT is exported from the nucleus46 so any SMRT-mediated repression would be abolished. Treatment of neurons with TSA also causes SMRT redistribution46 and in any case would prevent SMRT-associated HDACs from working. Interestingly, sestrin 2 was recently found to have a novel role in mediating the arrest of cellular proliferation in response to genotoxic insults by preventing mTOR signaling47 and so its induction by HDAC inhibitors may contribute to the antitumour activity of these compounds.48
Promoting histone acetylation as a neuroprotective strategy
Synaptic activity, acting via NMDA receptor (NMDAR) signaling, boosted antioxidant defences through a coordinated pattern of gene expression that included the upregulation of sulfiredoxin and sestrin 2. Both sulfiredoxin and sestrin 2 were induced by synaptic activity, which also promoted the reduction of hyperoxidized Prxs.12 Simultaneous knock-down of sulfiredoxin and sestrin 2 promoted neuronal vulnerability of neurons to oxidative stress.12 However, recent doubt has been cast on the ability of sestrin 2 to catalyze this reaction.49 In the light of this study it may be that induction of sulfiredoxin is more significant with respect to reducing hyperoxidized Prxs than sestrin 2. In any case, both sulfiredoxin and sestrin 2 are induced by inhibiting class I and II HDACs (Figs. 2B and 3A).
The inactivation of Prxs, through their hyperoxidation, is an indicator that the thioredoxin-Prx system has become overwhelmed. Prxs are able to promote the resistance of neurons to a diverse array of oxidative insults, including excitotoxicity and ischemia, as well as exposure to β-amyloid, 1-methyl-4-phenylpyridinium (MPP+), ibotenate and peroxide.2,8-10,50 As such, their oxidative inactivation may have deleterious effects on neurons in a variety of pathological scenarios. In vitro, Prx hyperoxidation is associated with peroxide-induced neuronal death,12 as well as NMDA receptor-dependent excitotoxic cell death.51 The hyperoxidation of Prxs may also take place in the brain in response to a variety of oxidative trauma: we recently found that an episode of ischemia, followed by reperfusion, induces strong hyperoxidation of Prxs in the mouse cortex.12 Increased levels of hyperoxidized Prxs have also been observed during normal aging in the gerbil hippocampus.13
Manipulation of the acetylation-deacetylation balance in neurons to favour acetylation may prevent the oxidative inactivation of Prxs, although this requires further detailed investigation. This effect may act in parallel with other protective effects of TSA treatment, such as upregulation of p21waf1, which may contribute to TSA-induced protection of embryonic neurons.29 Given that TSA has been shown to have neuroprotective effects in stroke models in vivo,52,53 it would be of interest in the future to determine whether ischemia-induced Prx hyperoxidation can be prevented by HDAC inhibition in vivo.
Materials and Methods
Tissue culture and drug treatment
Cortical rat neurons were cultured as described54 from E21 rats except that growth medium contained B27 (Invitrogen). A single dose of anti-mitotic agent (AraC, 4.8 μM) was added to the cultures at DIV4 to minimize glial numbers. Experiments were carried out after neurons were cultured for 8–10 days during which cortical neurons develop a network of processes, express functional NMDA-type and AMPA/kainate-type glutamate receptors, and form synaptic contacts. Experiments were performed after transferring neurons at DIV8 into defined medium lacking trophic support “TMo”.33 Trichostatin A (TSA) was used at a concentration (1 μM) previously shown to have maximal neuroprotective effect,55 a fact confirmed by a dose-response study in our experimental system (data not shown).
Induction of oxidative stress and assessment of cell death
An oxidative insult in the form of H2O2 (100 μM, stabilized solution: Sigma) was applied to control cells or those that had been pre-treated with TSA for 8 h. Neurons were fixed after a further 24 h and subjected to DAPI staining. Pictures were taken (blind) and cell death was quantified using an automated technique employing a macro in ImageJ written by David Stark from Nicholas Bazan's lab. The application of this macro was also performed blind. Briefly, the assessment as to whether a nucleus was apoptotic or healthy is based on the large difference in neuronal area between live and dead cells. Images are thresholded to generate a binary black/white picture of nuclei, from which outlines of the nuclei were obtained. The area from each outlined nucleus was then calculated in ImageJ. The area of an apoptotic nucleus is typically 2–4 fold smaller than a healthy nucleus, enabling nuclei to be scored as live or dead based on a threshold level set by the blind analyser. In cases where multiple cells overlap, generating a large area, this area is divided by a figure approximating to a single healthy nucleus, and the quotient of this figure used to determine the number of healthy nuclei in this clump. Pictures were taken with a 10X objective; at this resolution chromatin fragmentation that is a feature of apoptotic nuclei is not generally thresholded into individual outlines, rather a single outline around the multiple clumps is obtained. A final filter was applied to ensure that tiny spots of fluorescent debris were not falsely counted as an apoptotic nucleus. This technique has been validated in that it generates data that tightly correlate with that obtained with blind manual counting. Using this technique, around 5,000 cells were counted per treatment, across three independent experiments.
Other techniques
For details of western blotting and qPCR and ChIP see.12 For ChIP, the anti-acetyl H4 antibody was from Upstate. The primer sequences used were: Sesn2: forward 5′ CAA ATA TGC GGG TTT TGG TC 3′, reverse 5′ GAC AGC CCA GGG AGT CAT TA 3′. Srxn1: forward 5′ TCT GTG TGT GTG TGT GTG TGG 3′, reverse 5′ GAG GAT GAG TCG GCT CTC AG 3′. Txnip: forward 5′ AGC ACA CAC CCA AAC AAC C 3′, reverse 5′ TCT CCC ATT GGC TAC TGG 3′. Cycle times were optimised in order to ensure that the reaction was stopped during the linear phase of amplification.
Acknowledgements
We thank David Stark and Nicholas Bazan for the macro used to quantitate neuronal apoptosis. This work was supported by the BBSRC, the Royal Society, the Wellcome Trust and the Network of European Neuroscience Institutes.
References
- 1.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 2.Hattori F, Murayama N, Noshita T, Oikawa S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J Neurochem. 2003;86:860–8. doi: 10.1046/j.1471-4159.2003.01918.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Font MF, Sebastia J, Sanfeliu C, Cristofol R, Marfany G, Gonzalez-Duarte R. Peroxiredoxin 2 (PRDX2), an antioxidant enzyme, is under-expressed in Down syndrome fetal brains. Cell Mol Life Sci. 2003;60:1513–23. doi: 10.1007/s00018-003-3048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 5.Simzar S, Ellyin R, Shau H, Sarafian TA. Contrasting antioxidant and cytotoxic effects of peroxiredoxin I and II in PC12 and NIH3T3 cells. Neurochem Res. 2000;25:1613–21. doi: 10.1023/a:1026670620633. [DOI] [PubMed] [Google Scholar]
- 6.Shau H, Merino A, Chen L, Shih CC, Colquhoun SD. Induction of peroxiredoxins in transplanted livers and demonstration of their in vitro cytoprotection activity. Antioxid Redox Signal. 2000;2:347–54. doi: 10.1089/ars.2000.2.2-347. [DOI] [PubMed] [Google Scholar]
- 7.Ichimiya S, Davis JG, O'Rourke DM, Katsumata M, Greene MI. Murine thioredoxin peroxidase delays neuronal apoptosis and is expressed in areas of the brain most susceptible to hypoxic and ischemic injury. DNA Cell Biol. 1997;16:311–21. doi: 10.1089/dna.1997.16.311. [DOI] [PubMed] [Google Scholar]
- 8.Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, et al. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates upregulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007;35:377–82. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–97. doi: 10.1002/jnr.21429. [DOI] [PubMed] [Google Scholar]
- 11.Wagner E, Luche S, Penna L, Chevallet M, Van Dorsselaer A, Leize-Wagner E, Rabilloud T. A method for detection of overoxidation of cysteines: peroxiredoxins are oxidized in vivo at the active-site cysteine during oxidative stress. Biochem J. 2002;366:777–85. doi: 10.1042/BJ20020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo KY, Park OK, Yu J, Yan B, Li H, Lee CH, et al. Expression and Changes of Hyperoxidized Peroxiredoxins in Non-Pyramidal and Polymorphic Cells in the Gerbil Hippocampus During Normal Aging. Cell Mol Neurobiol. 2008 doi: 10.1007/s10571-008-9333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–4. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 15.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–6. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 16.Woo HA, Kang SW, Kim HK, Yang KS, Chae HZ, Rhee SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–4. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 17.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 18.Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, et al. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–43. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ooi L, Wood IC. Regulation of gene expression in the nervous system. Biochem J. 2008;414:327–41. doi: 10.1042/BJ20080963. [DOI] [PubMed] [Google Scholar]
- 21.Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Sweatt JD. Experience-Dependent Epigenetic Modifications in the Central Nervous System. Biological psychiatry. 2008 doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–68. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 27.Butler R, Bates GP. Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci. 2006;7:784–96. doi: 10.1038/nrn1989. [DOI] [PubMed] [Google Scholar]
- 28.Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Human molecular genetics. 1999;8:2317–23. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- 29.Langley B, D'Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–76. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahnen E, Eyupoglu IY, Brichta L, Haastert K, Trankle C, Siebzehnrubl FA, et al. In vitro and ex vivo evaluation of second-generation histone deacetylase inhibitors for the treatment of spinal muscular atrophy. J Neurochem. 2006;98:193–202. doi: 10.1111/j.1471-4159.2006.03868.x. [DOI] [PubMed] [Google Scholar]
- 31.Rai M, Soragni E, Jenssen K, Burnett R, Herman D, Coppola G, et al. HDAC inhibitors correct frataxin deficiency in a Friedreich ataxia mouse model. PLoS ONE. 2008;3:1958. doi: 10.1371/journal.pone.0001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–7. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 33.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–87. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–5. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 35.Jin MH, Lee YH, Kim JM, Sun HN, Moon EY, Shong MH, et al. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett. 2005;381:252–7. doi: 10.1016/j.neulet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 36.Sarafian TA, Verity MA, Vinters HV, Shih CC, Shi L, Ji XD, et al. Differential expression of peroxiredoxin subtypes in human brain cell types. J Neurosci Res. 1999;56:206–12. [PubMed] [Google Scholar]
- 37.Parmigiani RB, Xu WS, Venta-Perez G, Erdjument-Bromage H, Yaneva M, Tempst P, Marks PA. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc Natl Acad Sci USA. 2008;105:9633–8. doi: 10.1073/pnas.0803749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae SH, Woo HA, Sung SH, Lee HE, Lee SK, Kil IS, Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2008.2325. [Epub ahead of print], PMID: 19086807. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Ling G, Suhasini AN, Zhang P, Yamamoto M, Navas-Acien A, et al. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic Biol Med. 2009;46:376–86. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–86. [PubMed] [Google Scholar]
- 41.Wei Q, Jiang H, Matthews CP, Colburn NH. Sulfiredoxin is an AP-1 target gene that is required for transformation and shows elevated expression in human skin malignancies. Proc Natl Acad Sci USA. 2008;105:19738–43. doi: 10.1073/pnas.0810676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sng JC, Taniura H, Yoneda Y. Inhibition of histone deacetylation by trichostatin A intensifies the transcriptions of neuronal c-fos and c-jun genes after kainate stimulation. Neurosci Lett. 2005;386:150–5. doi: 10.1016/j.neulet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 44.Ki SH, Cho IJ, Choi DW, Kim SG. Glucocorticoid receptor (GR)-associated SMRT binding to C/EBPbeta TAD and Nrf2 Neh4/5: role of SMRT recruited to GR in GSTA2 gene repression. Mol Cell Biol. 2005;25:4150–65. doi: 10.1128/MCB.25.10.4150-4165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci. 2002;115:689–98. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 46.Mckenzie GJ, Stephenson P, Ward G, Papadia S, Bading H, Chawla S, et al. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J Neurochem. 2005;93:171–85. doi: 10.1111/j.1471-4159.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- 47.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Iglesias O, Ruiz-Llorente L, Sanchez-Martinez R, Garcia L, Zambrano A, Aranda A. Histone deacetylase inhibitors: mechanism of action and therapeutic use in cancer. Clin Transl Oncol. 2008;10:395–8. doi: 10.1007/s12094-008-0221-x. [DOI] [PubMed] [Google Scholar]
- 49.Rhee SG, Woo HA, Bae SH, Park S. Sestrin 2 Is Not a Reductase for Cysteine Sulfinic Acid of Peroxiredoxins. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2008.2360. In Press. [DOI] [PubMed] [Google Scholar]
- 50.Hattori F, Oikawa S. Peroxiredoxins in the central nervous system. Subcell Biochem. 2007;44:357–74. doi: 10.1007/978-1-4020-6051-9_17. [DOI] [PubMed] [Google Scholar]
- 51.Leveille F, Soriano FX, Papadia S, Hardingham GE. Excitotoxic insults lead to peroxiredoxin hyperoxidation. Oxidative Medicine and Cellular Longevity. 2009 doi: 10.4161/oxim.2.2.8300. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 53.Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, Endres M. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008;210:531–42. doi: 10.1016/j.expneurol.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 54.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 55.Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci USA. 2003;100:4281–6. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]