Abstract
The NMDA subtype of ionotropic glutamate receptors plays a Jekyll and Hyde role in the mammalian central nervous system. In pathological scenarios such as ischemia, Ca2+ influx through the NMDA receptor is a key mediator of cell death. However, physiological levels of NMDA receptor activity can promote neuronal survival and resistance to trauma, and play important roles in synaptic plasticity and transmission. This dichotomy may explain the poor tolerance and efficacy of NMDA receptor antagonists in clinical trials for excitotoxic trauma. There is a growing understanding of the signalling events that mediate the opposing effects of NMDA receptor activity, and the factors that determine whether an episode of NMDA receptor activity will promote survival or death. This knowledge may lead to therapeutic strategies that enable the selective blockade of pro-death signalling cassettes, while sparing physiological signalling to survival and plasticity.
1. Introduction
NMDARs (N-methyl-D-aspartate (NMDA) receptors) are cationotropic receptors gated by the neurotransmitter glutamate. They play a central role in synaptic transmission and in mediating synaptic plasticity, learning and memory. NMDARs are also present in other cell types such as oligodendrocytes (Karadottir and others 2005), osteoclasts (Szczesniak and others 2005) and lymphocytes (Tuneva and others 2003) where their function is not well understood. Structurally they are heterotetramers, typically containing two glycine-binding NR1 subunits and two glutamate-binding NR2 subunits. The NR1 subunit is essential for a functional NMDAR, and has several splice variants that can influence the properties of the channel. In addition, NMDAR variety is provided by the four different NR2 subunits (NR2A-D) as well as two NR3 genes (NR3A and NR3B) which can take the place of NR2. The most widely expressed NMDARs contain the obligate subunit NR1 plus either NR2B or NR2A or a mixture of the two. NR2B (and NR2D) is expressed at high levels in early developmental stages which decline post-natally, while NR2A and NR2C expression levels increase (Kohr 2006). In adults, NR2A is ubiquitously expressed in the brain, NR2B is mostly restricted to the forebrain, NR2C to the cerebellum and NR2D is expressed in small numbers of cells in selected brain regions. However, NR2A and NR2B exist concurrently in the same brain region and participate in the formation of a heterotrimeric NR1/NR2A/NR2B functional NMDARs (Waxman and Lynch 2005).
The NMDAR channel pore is blocked in a voltage-dependent manner by Mg2+. Release of glutamate, the main excitatory neurotransmitter in the mammalian CNS (central nervous system), causes Na+ influx through AMPA receptors in the postsynaptic cell, resulting in partial membrane depolarisation sufficient to lift the Mg2+ block. The activated NMDAR is permeable to Na+ but, crucially, also to Ca2+. This Ca2+ mediates most of the physiological effects of NMDAR activity.
At the synapse, the NMDAR is linked to a large multi-protein complex via the cytoplasmic C-termini of NR1 and NR2 subunits (Collins and others 2006). This complex facilitates localisation of the receptor in specific areas, such as the postsynaptic density- PSD, and facilitates connection to a variety of downstream signalling molecules through which many NMDAR activity downstream effects are mediated (Waxman and Lynch 2005). The extreme C-termini of NR2 subunits link to a group of proteins called MAGUKs (Membrane-associated Guanylate Kinase) which include PSD-95, SAP-102 (synapse-associated protein) and PSD-93. These proteins contain several PDZ protein interaction domains through which other proteins are connected. This highly organised structure facilitates signal coupling, by bringing together cytoplasmic signal-transducing enzymes and cell-surface receptors. Moreover, it can bring Ca2+ dependent signalling molecules into close proximity with the site of Ca2+ entry at NMDAR. For example, nNOS, in interacting with PSD-95, is brought close to the NMDAR and can thus be efficiently activated by NMDAR-mediated Ca2+ influx (Aarts and others 2002).
NMDARs are essential mediators of synaptic plasticity and also mediate aspects of development and synaptic transmission (Aamodt and Constantine-Paton 1999; Bliss and Collingridge 1993). However, when excessively activated, NMDARs cause cell death in many neuropathological scenarios (Lipton and Rosenberg 1994). During an ischemic episode, extracellular glutamate builds up due to synaptic release and impaired/reversed uptake mechanisms (Camacho and Massieu 2006; Rossi and others 2000) resulting in overactivation of NMDARs (Choi 1988). The destructive effects of excessive NMDAR activity are in contrast to the recent findings that survival of several neuronal types is dependent on physiological synaptic NMDAR activity (Hardingham and Bading 2003; Ikonomidou and Turski 2002a). Thus, responses of neurons to glutamate or NMDA follow a bell-shaped curve: both too much and too little NMDAR activity is potentially harmful (Lipton and Nakanishi 1999).
2. Failed clinical trials for stroke with NMDA receptor antagonists
Despite an overwhelming body of evidence from animal studies implicating NMDAR activity in neuronal loss following ischemia, the many clinical trials of different NMDAR antagonists for stroke have failed due to poor tolerance and efficacy (Ikonomidou and Turski 2002a; Muir 2006). Aptiganel, one of the final generation of antagonists, is a case-in-point. Clinical trials were suspended due to lack of efficacy and emerging data that high-dose Aptiganel patients exhibited worse neurological improvement and higher mortality compared to placebo (Albers and others 2001). Lack of efficacy was observed despite using doses that caused side-effects such as hypertension, as well as CNS-adverse events such as sedation, confusion, headache and psychosis (Dyker and others 1999). The important role of the NMDAR in normal CNS functioning may mean that for any antagonist the maximal tolerated dose is lower than the minimum therapeutically effective dose. Moreover, the growing body of evidence that physiological synaptic NMDAR activity exerts a neuroprotective effect has led to suggestions that it may play a role in promoting recovery and preventing delayed neuronal loss in the penumbra (Albers and others 2001; Ikonomidou and Turski 2002a). Indeed, preconditioning type events are likely to be initiated in the marginal area in the penumbra, and since preconditioning is NMDAR dependent (Grabb and Choi 1999), blockade may be undesirable in this region. Thus, global NMDAR antagonists may block NMDAR-activated pro-survival signals triggered in response to an ischemic challenge, and actually delay recovery in some areas. This dichotomy of NMDAR signalling (Hardingham and Bading 2003) means that any anti-excitotoxic strategy that interferes with NMDAR signalling should be assessed to determine its effects on NMDAR pro-survival signalling. This will require a thorough understanding of the nature of both survival and death pathways triggered by the NMDAR. In this review we will focus on survival and death signalling through the NMDA receptor, exploring newly emerged concepts and the molecules involved.
3. Pro-survival signalling from the NMDAR
In contrast to the adverse effects of excessive NMDAR activity, physiological levels of synaptic NMDAR activity are essential for neuronal survival, since activity blockade was shown to have deleterious effects. Blockade of NMDAR activity in vivo caused a decrease in the number of healthy cells, increased density of pyknotic cells and severe deterioration of the dentate gyrus morphology in first week postnatal rat pups (Gould and others 1994). Elimination of NMDA receptor activity in vivo causes widespread apoptosis and enhances trauma-induced injury in developing neurons (Adams and others 2004; Gould and others 1994; Ikonomidou and others 1999; Monti and Contestabile 2000; Pohl and others 1999). In the adult CNS, NMDAR blockade exacerbates neuronal loss when applied after traumatic brain injury and during ongoing neurodegeneration (Ikonomidou and others 2000), and prevents the survival of newborn neurons in the adult dentate gyrus (Tashiro and others 2006).
NMDAR signalling to survival has been recapitulated in vitro in neuronal cultures (Hardingham and Bading 2003; Hetman and Kharebava 2006), allowing scientists to study the underlying signalling events. Network disinhibition using the GABAa receptor antagonist bicuculline enabled selective studies of synaptic NMDAR activity signalling (Hardingham and others 2001b), as opposed to using the unphysiological bath application of glutamate which would activate synaptic and extrasynaptic NMDARs. Bicuculline blockade of the tonic inhibition mediated by GABAergic interneurons promotes action potential bursting which are associated with synaptic NMDAR-dependent Ca2+ transients (Hardingham and others 2002).
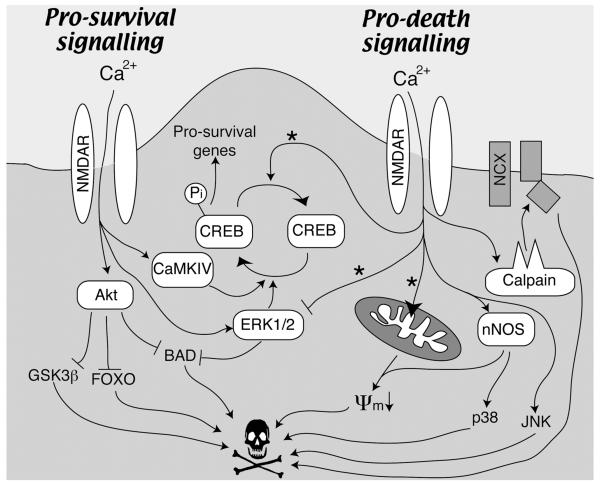
Pro-survival signalling from the NMDA receptor can involve posttranslational modifications of proteins or indeed de novo gene expression. Different signals may have varying importance under different circumstances (Papadia and others 2005). We outline below examples of pro-survival pathways triggered by synaptic NMDAR activity (see also Fig. 1), and the pro-survival events that they mediate.
Fig. 1. Pro-survival and-death signaling from the NMDA receptor (see text for details).
Note that pro-death signaling generally requires more intense activation of NMDARs than pro-survival signalling. Also, the pathways marked with a * are favoured by extrasynaptic NMDAR activation (Hardingham and others 2002; Ivanov and others 2006). ψm denotes mitochondrial membrane potential.
3a. The PI3K-Akt pathway
The PI3K (phosphoinositide-3-kinase) - Akt kinase cascade is a key signalling pathway responsible for pro-survival effects of NMDAR activity (Lafon-Cazal and others 2002; Papadia and others 2005). It is strongly activated by NMDAR in many neuronal types, but not in all (Hetman and Kharebava 2006). Briefly, in response to extracellular stimuli, PI3K, which can be activated in a Ca2+-dependent manner by Ca2+/calmodulin (Joyal and others 1997), catalyses the phosphorylation of the lipid PIP2 (phosphatidylinositol 4,5-biphosphate) to PIP3 (phosphatidylinositol 3,4,5-triphosphate) in the membrane. The kinase PDK1 (phosphoinositide-dependent protein kinase) (Alessi and others 1997) as well as its substrate Akt/PKB (Protein Kinase B) are recruited to the membrane via their interactions with PIP3 through their pleckstrin homology (PH) domains. PDK1, along with PDK2 (which is possibly rictor-mTOR- (Sarbassov and others 2005)) then phosphorylates and activates Akt. Akt promotes cell survival and growth via phosphorylation and activation or inactivation of its many targets: Akt phosphorylates and inactivates GSK3β (Glycogen synthase kinase-3 beta; (Cross and others 1995)), an event that also contributes to NMDAR signalling to neuroprotection (Soriano and others 2006). In the context of neurotrophic factor and growth factor signalling Akt triggers phosphorylation of the FOXO (forkhead box O) subfamily of forkhead transcription factors, promoting translocation from the nucleus to the cytoplasm (Brunet and others 1999). FOXOs control the expression of pro-death genes such as Fas ligand (Brunet and others 1999) and Bim (Gilley and others 2003), and these are down-regulated by Akt activation. NMDAR signalling to Akt-dependent FOXO export has also been observed in the context of NMDAR signalling (Soriano and others 2006). Akt also inactivates the pro-apoptotic Bcl-2 family member BAD (Bcl2/Bcl-XL-antagonist causing cell death) by phosphorylation, thus stopping its interaction with and blockade of the pro-survival Bcl-2 family members Bcl-2 and Bcl-XL (Downward 1999). The JNK/p38 activator ASK1 can also be inhibited by Akt phosphorylation (Kim and others 2001) and p53 transcriptional activity is suppressed by Akt, leading to reduction of Bax expression and of neuronal death (Yamaguchi and others 2001).
3b. CREB (cAMP response element-binding protein)
A key mediator of activity-dependent gene expression is the transcription factor CREB, which binds to the cAMP response element (CRE) (Lonze and Ginty 2002). CRE-dependent gene expression is strongly induced by synaptic NMDAR activity (Hardingham and others 2002). Ca2+ influx through the synaptic NMDAR activates the Ras/ERK pathway in the cytoplasm and the nuclear Ca2+/calmodulin dependent protein kinases, principally CaMKIV. CaMKIV mediates fast CREB phosphorylation at Ser133, whereas the ERK1/2 pathway promotes CREB phosphorylation in a slower, more long lasting manner (Hardingham and others 2001a; Wu and others 2001). Ser133 phosphorylation of CREB is necessary to recruit CBP (CREB-binding protein), a transcriptional cofactor, to CREB. The transactivation potential of CBP is itself positively regulated by NMDAR activity (Hardingham and others 1999) by a mechanism involving its phosphorylation on Ser301 by CaMKIV (Impey and others 2002). Gene expression mediated by CREB can also be mediated by another family of CREB co-activators, the TORCs (Transducers of Regulated CREB activity, (Kovacs and others 2007; Screaton and others 2004) and references therein). TORC2 translocates to the nucleus as a result of the synergistic action of Ca2+ and cAMP signalling, through calcineurin-mediated TORC2 dephosphorylation and cAMP-mediated inhibition of the TORC2 kinase SIK2, respectively (Screaton and others 2004). In hippocampal neurons TORC1, which is expressed more abundantly than TORC2, senses the coincidence of Ca2+ and cAMP signalling and converges this dual signalling to target gene expression by translocating to the nucleus and potently coactivating CREB (Kovacs and others 2007).
The potential of CREB family-regulated gene products to promote neuronal survival was first demonstrated in the context of neurotrophin signalling (Bonni and others 1999; Riccio and others 1999) and exogenous overexpression (Walton and others 1999). In addition, studies of mice where CREB and/or CREB family members have been deleted also point to a pro-survival role for CREB: CREB null mice exhibit considerable death of dorsal root ganglion neurons in vivo, and sympathetic neurons in vitro (Lonze and others 2002). Mice lacking CREB (and that also lack CREM-another CREB family member) in the CNS during development show extensive neuronal apoptosis (Mantamadiotis and others 2002). Postnatal disruption of the CREB gene (in CREM null mice) results in progressive neurodegeneration in the hippocampus and dorsolateral striatum (Mantamadiotis and others 2002).
CREB-dependent gene expression is causally linked to the long-lasting phase of activity-dependent neuroprotection against apoptotic insults (Papadia and others 2005). This long-lasting phase is dependent on nuclear Ca2+/calmodulin signalling (Papadia and others 2005), consistent with the known requirement for nuclear Ca2+ in CREB activation via CaMKIV (Deisseroth and others 1998; Hardingham and others 2001b; Hardingham and others 1997; Limback-Stokin and others 2004). Activity-dependent CRE activation in developing CNS neurons also protects against excitotoxic trauma (Lee and others 2005) which would otherwise result in rapid cell death resembling necrosis. Expression of the inhibitory CREB family member, ICER increases in neurons in response to apoptotic and excitotoxic insults and can promote neuronal death (Jaworski and others 2003). Thus, CREB activation by NMDAR signalling may be important in antagonizing the CREB-inhibiting effects of ICER accumulation.
The importance of NMDAR signalling to CREB in promoting survival in vivo is not as well established. Activation of CREB in response to transient ischemic episodes is proposed to contribute to the establishment of ischemic tolerance or preconditioning and may well be mediated by NMDAR activation (Mabuchi and others 2001). Interestingly, resistance to hypoxic/ischemic death in different neuronal populations correlates positively with an ability to sustain active (phosphorylated) CREB (Walton and Dragunow 2000): in neurons destined to die CREB is only transiently phosphorylated (Walton and Dragunow 2000).
The identity of the CRE-regulated gene(s) responsible for long lasting protection against apoptosis is beginning to be understood. Genome-wide profiling studies have identified a large number of CREB target genes, although only a subset of these are induced by CREB-activating stimuli (Impey and others 2004; Mayr and Montminy 2001; Zhang and others 2005). Furthermore, the identity of the genes within the subset is cell-type specific and may be stimulus-specific, so is hard to predict in any particular scenario.
A recent study identified two genes that contribute to synaptic NMDAR-dependent neuroprotection (Zhang and others 2007): Btg2, a potentially anti-apoptotic CREB target gene, and Bcl6, a transcriptional repressor implicated in suppression of p53, also potentially a CREB target. Both Btg2 and Bcl6 genes confer neuroprotection in the face of apoptotic stimuli like trophic deprivation and staurosporine treatment, and siRNA-mediated knockdown demonstrated that their induction was also necessary for synaptic NMDAR-mediated neuroprotection.
Another CRE-regulated candidate is the pro-survival neurotrophin BDNF (brain-derived neurotrophic factor), which is upregulated by NDMAR activity (Hardingham and others 2002) and is known to promote neuronal survival (Shieh and others 1998; Tao and others 1998) (Hetman and Kharebava 2006). NMDAR blockade in vivo reduces BDNF mRNA expression and in vitro supplementation of neurons with BDNF can rescue them from NMDAR antagonist-caused neuronal death (Hansen and others 2004). Upregulation of other known CREB targets involved in metabolism and respiration (e.g. Cytochrome c), responses to oxidative stress (e.g. Sod2-superoxide dismutase 2) or in inhibiting apoptosis (e.g. Bcl-2) (Riccio and others 1999) are additional potential ways in which CREB can promote survival and resistance to trauma. It is possible that different CRE-regulated genes are responsible for neuroprotection against different types of insult (e.g. apoptotic-like, excitotoxic/necrotic-like, oxidative stress).
4. NMDAR-mediated cell death
Pathological activation of NMDARs is a major cause of neuronal death following acute excitotoxic trauma such as brain ischaemia, hypoxia and mechanical trauma (Arundine and Tymianski 2004). Chronic neurodegenerative diseases may also be associated with excessive NMDAR activation (Lipton 2006; Lipton and Rosenberg 1994). Neuronal cultures respond to high levels of NMDAR activity, induced by bath application of glutamate or NMDA, by undergoing delayed Ca2+ deregulation that precedes and predicts necrotic cell death (Hartley and others 1993) and references therein). Several mechanisms are implicated in cell death triggered by Ca2+ influx through the NMDAR (see below and also Fig. 1).
Mitochondrial dysfunction caused by excessive Ca2+ uptake by the mitochondria through the potential-driven uniporter (Stout and others 1998) is one mechanism. The mitochondrial membrane gets depolarised due to this uptake, which inhibits ATP production, and can even cause depletion of cytosolic ATP due to reversal of the mitochondrial ATPase. This loss of ATP further limits the ability of the neuron to regulate intracellular Ca2+ levels. Furthermore, mitochondrial Ca2+ uptake can promote ROS production (Nicholls 2004) and also release of cytochrome c, necessary for the formation of the apoptosome and subsequent activation of caspases, leading to apoptosis.
In addition to causing mitochondrial dysfunction, toxic levels of NMDAR activation cause Ca2+ efflux to be impaired (Bano and others 2005; Pottorf and others 2006). In neurons Ca2+ exit from the cell is achieved through the plasma membrane Ca2+ ATPase pump (PMCA) and Na+/Ca2+ exchangers (NCXs). Calpains, Ca2+-dependent proteases, are activated by the excessive NMDAR-mediated Ca2+ influx and cleave a major isoform of the plasma membrane Na+/Ca2+ exchanger (NCX3) impairing its function in cerebellar granule neurons (CGNs) (Bano and others 2005). Inhibition of calpains or overexpression of a functional NCX isoform prevented Ca2+ overload while siRNA knockdown of NCX3 exacerbated it (Bano and others 2005). The PMCA, which would utilise the energy from ATP hydrolysis to transport Ca2+ across the plasma membrane, is inactivated by excitotoxic insults via mechanisms attributed to both caspases (Schwab and others 2002) and calpains (Pottorf and others 2006).
NMDAR activity-regulated overactivation of the Ca2+-dependent nNOS (neuronal nitric oxide synthase) also has toxic downstream responses, including mitochondrial dysfunction, p38 mitogen-activated protein kinase signalling and TRPM (transient receptor potential melastatin) channel activation. nNOS is bound by its N-terminal PDZ domain to the post-synaptic density protein PSD95, which brings it in the proximity of the synaptic NMDAR that is also bound to PSD95 via the C-terminal tail of its NR2 subunit. nNOS activation leads to Nitric Oxide (NO) production, which in excess can be toxic both on its own and when combined with other reactive oxygen species (ROS) such as superoxide to form ONOO- (peroxynitrite). Both NO and ONOO− can damage cellular components, inhibit mitochondrial respiratory chain enzymes and promote mitochondrial depolarisation (Arundine and Tymianski 2004). Another important target of NMDAR signalling to NO production in excitotoxicty is the cation channel TRPM7 (Aarts and others 2003). NMDAR-dependent Ca2+ influx triggers both NO production via nNOS activation and superoxide production via mitochondrial Ca2+ uptake, which combine to form ONOO−, an activator of TRPM7. Since TRPM7 itself passes Ca2+, this results in a positive feedback loop.
Stress-activated protein kinases (SAPKs) are another class of signalling molecules that mediate NMDAR-dependent cell death. In CGNs, NMDAR-dependent excitotoxic death relies upon p38 activation, leading to caspase-independent cell death (Cao and others 2004). In cortical neurons another SAPK family, the c-Jun N-terminal kinases (JNKs) mediated NMDAR-dependent cell death in cortical neurons in vitro and in vivo (Borsello and others 2003). New components of the excitotoxic cell death pathway are emerging; Rho, a member of the Rho-family of GTPases, was recently found to link glutamate-induced Ca2+ elevation to p38α-dependent excitotoxic neuronal death (Semenova and others 2007).
5. What determines if an episode of NMDAR activity is neuroprotective or excitotoxic?
As outlined above, there is a multitude of ways in which NMDAR-dependent Ca2+influx can convey the signal downstream to either confer neuroprotection or to trigger programmes of cell death. A key issue, potentially relevant for designing clinically tolerated drugs against neurodegenerative conditions caused by excitotoxicity, is how the distinction between what pathway will be followed is made and what factors affect this. A number of possibilities are presented here.
5a. Stimulus intensity
The magnitude of activation, be it intensity or duration, is very important in determining the nature of the response to an episode of NMDAR activity. The classical bell-shaped curve model of the neuronal response to NMDA or glutamate contends that intermediate, physiological NMDAR activity levels are necessary for neuroprotection whereas too low or too high pathological NMDAR activity promotes cell death. This implies that the effectors of the pro-survival Ca2+ signalling have a higher affinity (or considerably lower requirements for Ca2+) than the Ca2+ effectors of death. Therefore the Ca2+ concentration threshold for activating pro-survival signalling by PI3K, ERK1/2 and CaMKIV must be lower than that necessary to trigger toxic levels of calpain activation, mitochondrial Ca2+ uptake or NO production. The key Ca2+ -dependent components of the survival pathways are in the main activated not by Ca2+ directly, but by Ca2+/Calmodulin. Calmodulin is a ubiquitous Ca2+ binding protein which changes conformation when it binds to Ca. Ca2+/Calmodulin then activates a number of downstream signals, including CaMKIV, PI3K and upstream activators of ERK1/2. As a physiological sensor of elevated Ca2+ levels, calmodulin is designed to be activated by relatively modest increases in Ca2+. In contrast, central mediators of NMDAR-dependent cell death, calpains, are not Ca2+/Calmodulin dependent but are activated by Ca2+ directly, and require higher levels to be fully induced (Tompa and others 1996). The same can be said of the mitochondrial uniporter, which senses Ca2+ directly and is fully activated only by high concentrations (Nicholls 2004). Consistent with this, when increasing doses of NMDA are applied to neurons, only the higher, toxic doses evoke sustained loss of mitochondrial membrane potential and increases in mitochondrial Ca2+ (Soriano and others 2006). Another Ca2+-dependent promoter of neuronal death, nNOS, is Ca2+/Calmodulin-dependent and indeed plays important roles in non-pathological signalling processes such as synaptic plasticity. However, nNOS is also regulated by phosphorylation, and a recent study showed that, in contrast to non toxic stimuli, high levels of glutamate fail to trigger an inhibitory phosphorylation event that ensures that nNOS activation is transient (Rameau and others 2007). Thus, nNOS becomes excessively active and contributes to excitotoxic cell death (Rameau and others 2007).
5b. Location of the receptor
Aside from stimulus intensity, the location of the NMDAR may also profoundly affect the signals that emanate from the NMDAR. Developing neurons have sizeable pools of NMDARs at extrasynaptic, as well as synaptic locations, which signal very differently. Ca2+ influx dependent on intense synaptic NMDAR activation is well tolerated by cells whereas activation of extrasynaptic NMDARs, either on their own or in the presence of synaptic NMDAR activation, causes a loss of mitochondrial membrane potential and cell death (Hardingham and others 2002).
Differential synaptic vs extrasynaptic NMDAR effects also extend to other signal pathways. While synaptic NMDAR activity strongly induces CREB-dependent gene expression, extrasynaptic NMDARs are coupled to a CREB shut-off pathway that causes CREB dephosphorylation of its activator site (serine 133)(Hardingham and others 2002). The developmentally regulated mechanism of this is discussed elsewhere (Hardingham and Bading 2002; Sala and others 2000). The extrasynaptic NMDAR activity-dependent dephosphorylation of CREB is generally dominant over CREB-activating signals (Hardingham and others 2002), possibly by activating a CREB phosphatase such as PP1 (protein phosphatase 1). In neurons exposed to low protecting doses of NMDA, synaptic NMDAR signalling is able to dominate because synaptic NMDARs are preferentially activated due to the NMDA causing a dramatic increase in action potential firing (Soriano and others 2006). This enhanced firing mediates the NMDA-induced pro-survival signalling to Akt, ERK1/2 and CREB. In contrast, higher, toxic doses of NMDA strongly suppress firing rates and hence do not favour synaptic NMDAR activation (Soriano and others 2006). In addition to signalling to CREB, it has also been shown that there is opposing regulation of the ERK1/2 pathway by synaptic and extrasynaptic NMDARs in hippocampal neurons: Synaptic NMDARs activate the ERK pathway whereas extrasynaptic NMDARs evoke ERK inactivation (Ivanov and others 2006).
A recent study involving genome-wide expression analysis has extended our understanding of synaptic vs. extrasynaptic signalling (Zhang and others 2007). While synaptic NMDARs activated a number of pro-survival genes (including the aforementioned Btg2 and Bcl6), extrasynaptic NMDARs failed to do this, and in fact activated expression of a gene Clca1 which kills neurons. It will be of interest to know whether Clca1 is activated in vivo during excitotoxic trauma, and whether it represents a potential therapeutic target.
Chronic exposure of neurons to glutamate and activation of extrasynaptic NMDARs is unlikely to occur under normal physiological conditions. However, it may occur under pathological conditions such as brain injury, or during hypoxic/ischemic insults where glutamate transporters operate in reverse (Rossi and others 2000) thereby pumping glutamate out of the cells into the extracellular medium, building up in the restricted extracellular space. Indeed, exposing hippocampal neurons to oxygen-glucose-free conditions causes a NMDAR-dependent decay of CREB phosphorylation (Hardingham and others 2002). This is consistent with the observation that CREB dephosphorylation has been observed in in vivo stroke conditions (Tanaka and others 1999; Walton and Dragunow 2000). Given the role of CREB in controlling several pro-survival genes, this shut-off may contribute to the slowly progressing apoptotic death of neurons (in response to excitotoxic trauma) that are exposed to toxic levels of glutamate that are insufficient to cause immediate necrotic cell death (Dirnagl and others 1999; Hossmann 1994; Lee and others 1999). The dominant action of the shut-off signal means that any CREB activating ligand present (such as a neurotrophin) would be rendered impotent.
The molecular basis for the apparent differences in synaptic/extrasynaptic NMDAR signalling could be due to differences in the composition of the NMDAR signalling complexes as opposed to the location of the receptors per se, though these potential differences await study. Molecules involved in the CREB shut-off mechanism or in the coupling to mitochondrial Ca2+ uptake may directly interact with extrasynaptic NMDARs. They may interact less well with synaptic NMDARs, due to shielding by the post-synaptic density or differences in receptor subunit composition. While extrasynaptic NMDARs are preferentially enriched with NR2B-containing NMDARs, there is little evidence that differences in subunit composition are dramatic enough to explain the effects observed. However, a recent study has contended that NR2B-containing NMDARs tend to promote neuronal death, irrespective of location (synaptic or extrasynaptic) while NR2A-containing NMDARs promote survival (Liu and others 2007). The investigation of subunit-specific differences in NMDAR signalling is hampered by the lack of a NR2A-specific antagonist that is sufficiently selective to discriminate in the physiological scenario of trans-synaptic stimulation (Frizelle and others 2006; Neyton and Paoletti 2006). The antagonist NVP-AAM077 has some selectivity for NR2A-containing NMDARs but the difference in the equilibrium constants is not sufficient to discriminate between NR2A-containing or NR2B-containing NMDA receptors being trans-synaptically activated (Frizelle and others 2006).
6. Clinical implications and concluding remarks
The pro-survival role of synaptic NMDARs under physiological conditions and especially during CNS development, points to the potential dangers of NMDAR antagonism during this period in humans (Olney and others 2002). Several paediatric/obstetric anaesthetics and anticonvulsants reduce NMDAR activity directly or indirectly; in addition, in utero exposure to NMDAR-antagonistic recreational drugs such as phencyclidine, ketamine and ethanol pose very real risks (Olney and others 2002).
Moreover, the capability of NMDAR signalling to promote both survival and cell death under different circumstances, highlights the problem of designing anti-excitotoxic therapeutic drugs without causing undesirable adverse effects. As outlined earlier, trials of NMDAR antagonists for treating excitotoxic trauma such as stroke have failed in the past due to poor tolerance and efficiency (Ikonomidou and Turski 2002b), likely caused by inhibition of physiological NMDAR-mediated processes such as pro-survival signalling and plasticity. It is becoming more widely accepted that the way forward is to block the excitotoxic consequences of NMDAR activation while preserving pro-survival signals, and indeed synaptic plasticity and cognitive functioning. Thus, specifically targeting downstream proteins and pathways involved in excitotoxicity seems to be a more promising approach. Such strategies are already being developed, such as the use of cell-permeable peptides to disrupt the interaction of NR2B with PSD-95 and so uncouple NMDAR activation from nNOS activation (Aarts and others 2002). Other promising approaches include the specific targeting of the pro-death JNK signalling pathway, again with a cell-permeable peptide (Borsello and others 2003).
The precise pathway, or pathways that mediate NMDAR-dependent cell death in ischemia may depend on the neuronal subtype, the severity and duration of the episode, and the position of the neuron within the lesion (infarct core or penumbra). Thus, it may be that targeting JNK signalling or NO production may ameliorate neuronal loss in some scenarios, but that blocking calpain or TRPM7 activation is more effective in others. Thus, a cocktail of anti-death signalling drugs may be needed for an optimal effect. Moreover, their use, where appropriate, in conjunction with the thrombolytic drug TPA (tissue plasminogen activator) may aid in rapid drug delivery to the site of insult. In any case, the selective targeting of multiple pro-death pathways downstream of the NMDARs may represent an effective and better-tolerated therapeutic strategy than simple NMDAR blockade.
References
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–44. [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115(7):863–77. doi: 10.1016/s0092-8674(03)01017-1. and others. [DOI] [PubMed] [Google Scholar]
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298(5594):846–50. doi: 10.1126/science.1072873. and others. [DOI] [PubMed] [Google Scholar]
- Adams SM, de Rivero Vaccari JC, Corriveau RA. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. J Neurosci. 2004;24(42):9441–50. doi: 10.1523/JNEUROSCI.3290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. Jama. 2001;286(21):2673–82. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–9. doi: 10.1016/s0960-9822(06)00122-9. and others. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61(6):657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120(2):275–85. doi: 10.1016/j.cell.2004.11.049. and others. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9(9):1180–6. doi: 10.1038/nm911. and others. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. and others. [DOI] [PubMed] [Google Scholar]
- Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37(1):11–8. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cao J, Semenova MM, Solovyan VT, Han J, Coffey ET, Courtney MJ. Distinct requirements for p38alpha and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol Chem. 2004;279(34):35903–13. doi: 10.1074/jbc.M402353200. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11(10):465–9. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. and others. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392(6672):198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in Neuroscience. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999;1(2):E33–5. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. Safety and tolerability study of aptiganel hydrochloride in patients with an acute ischemic stroke. Stroke. 1999;30(10):2038–42. doi: 10.1161/01.str.30.10.2038. [DOI] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJA. Equilibrium constants for NVP-AAM077 acting at recombinant NR1/NR2A and NR1/NR2B NMDA receptors: implications for studies of synaptic transmission. Mol Pharmacol. 2006;70(3):1022–32. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162(4):613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, McEwen BS. Blockade of NMDA receptors increases cell death and birth in the developing rat dentate gyrus. Journal of Comparitive Neurology. 1994;340:551–565. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. Journal of Neuroscience. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16(2):440–53. doi: 10.1016/j.nbd.2004.03.013. and others. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001a;4(6):565–6. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001b;4(3):261–7. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600(1-2):148–53. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26(2):81–9. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22(4):789–98. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385(6613):260–5. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Kurth MC, Bjerkness L, Weiss JH, Choi DW. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J Neurosci. 1993;13(5):1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6(8):787–99. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Hossmann K-A. Viability thresholds and the penumbra of focal ischemia. Annals of Neurology. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. and others. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Stefovska V, Turski L. Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci U S A. 2000;97(23):12885–90. doi: 10.1073/pnas.220412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? The Lancet Neurology. 2002a;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002b;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Impey S, Fong AL, Wang Y, Cardinaux JR, Fass DM, Obrietan K. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34(2):235–44. doi: 10.1016/s0896-6273(02)00654-2. and others. [DOI] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119(7):1041–54. doi: 10.1016/j.cell.2004.10.032. and others. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the ERK activity in cultured rat hippocampal neurons. J Physiol. 2006;572(3):789–798. doi: 10.1113/jphysiol.2006.105510. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Mioduszewska B, Sanchez-Capelo A, Figiel I, Habas A, Gozdz A. Inducible cAMP early repressor, an endogenous antagonist of cAMP responsive element-binding protein, evokes neuronal apoptosis in vitro. J Neurosci. 2003;23(11):4519–26. doi: 10.1523/JNEUROSCI.23-11-04519.2003. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JL, Burks DJ, Pons S, Matter WF, Vlahos CJ, White MF. Calmodulin activates phosphatidylinositol 3-kinase. J Biol Chem. 1997;272(45):28183–6. doi: 10.1074/jbc.272.45.28183. and others. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21(3):893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326(2):439–46. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104(11):4700–5. doi: 10.1073/pnas.0607524104. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Cazal M, Perez V, Bockaert J, Marin P. Akt mediates the anti-apoptotic effect of NMDA but not that induced by potassium depolarization in cultured cerebellar granule cells. Eur J Neurosci. 2002;16(4):575–83. doi: 10.1046/j.1460-9568.2002.02124.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-Dependent Neuroprotection and cAMP Response Element-Binding Protein (CREB): Kinase Coupling, Stimulus Intensity, and Temporal Regulation of CREB Phosphorylation at Serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci. 2004;24(48):10858–67. doi: 10.1523/JNEUROSCI.1022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5(2):160–70. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Nakanishi N. Shakespeare in Love - with NMDA receptors? Nature Medicine. 1999;5(3):270–271. doi: 10.1038/6481. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330(9):613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27(11):2846–57. doi: 10.1523/JNEUROSCI.0116-07.2007. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. Journal of Neuroscience. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Villalba AM, et al. Disruption of CREB function in brain leads to neurodegeneration. Nature Genetics. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Monti B, Contestabile A. Blockade of the NMDA receptor increases developmental apoptotic elimination of granule neurons and activates caspases in the rat cerebellum. Eur J Neurosci. 2000;12(9):3117–23. doi: 10.1046/j.1460-9568.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6(1):53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26(5):1331–3. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4(2):149–77. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–98. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25(17):4279–87. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl D, Ishmaru MJ, Bittigau P, Stadhaus D, Hubner C, Olney JW, et al. NMDA antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proceedings of the National Academy of Sciences USA. 1999;96:2508–2513. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottorf WJ, 2nd, Johanns TM, Derrington SM, Strehler EE, Enyedi A, Thayer SA. Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem. 2006;98(5):1646–56. doi: 10.1111/j.1471-4159.2006.04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, et al. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27(13):3445–55. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286(5448):2358–61. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. Journal of Neuroscience. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, et al. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 2002;9(8):818–31. doi: 10.1038/sj.cdd.4401042. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Semenova MM, Maki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, et al. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci. 2007;10(4):436–43. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20(4):727–40. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26(17):4509–18. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1(5):366–73. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone. 2005;37(1):63–73. doi: 10.1016/j.bone.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nagata E, Suzuki S, Dembo T, Nogawa S, Fukuuchi Y. Immunohistochemical analysis of cyclic AMP response element binding protein phosphorylation in focal cerebral ischemia in rats. Brain Research. 1999;818:520–526. doi: 10.1016/s0006-8993(98)01263-3. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–26. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tompa P, Baki A, Schad E, Friedrich P. The calpain cascade. Mu-calpain activates mcalpain. J Biol Chem. 1996;271(52):33161–4. doi: 10.1074/jbc.271.52.33161. [DOI] [PubMed] [Google Scholar]
- Tuneva EO, Bychkova ON, Boldyrev AA. Effect of NMDA on production of reactive oxygen species by human lymphocytes. Bull Exp Biol Med. 2003;136(2):159–61. doi: 10.1023/a:1026366907084. [DOI] [PubMed] [Google Scholar]
- Walton M, Woodgate AM, Muravlev A, Xu R, During MJ, Dragunow M. CREB phosphorylation promotes nerve cell survival. J Neurochem. 1999;73(5):1836–42. [PubMed] [Google Scholar]
- Walton MR, Dragunow M. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23(2):48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11(1):37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98(5):2808–13. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, et al. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J Biol Chem. 2001;276(7):5256–64. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Steijaert MN, Lau D, Schutz G, Delucinge-Vivier C, Descombes P, et al. Decoding NMDA Receptor Signaling: Identification of Genomic Programs Specifying Neuronal Survival and Death. Neuron. 2007;53(4):549–62. doi: 10.1016/j.neuron.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102(12):4459–64. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]