Abstract
The pathological activation of proteases within the pancreatic acinar cell is critical to initiating pancreatitis. Stimulation of acinar cells with supraphysiological concentrations of the CCK analog caerulein (CER) leads to protease activation and pancreatitis. Agents that sensitize the acinar cell to the effects of CCK might contribute to disease. The effects of physiological ligands that increase acinar cell cAMP [secretin, VIP, and pituitary adenylate cyclase activating peptide (PACAP)] on CER-induced responses were examined in isolated rat pancreatic acini. Each ligand sensitized the acinar cell to zymogen activation by physiological concentrations of CER (0.1 nM). VIP and PACAP but not secretin also enhanced activation by supraphysiological concentrations of CER (0.1 μM). A cell-permeable cAMP analog also sensitized the acinar cell to CER-induced activation. The cAMP antagonist Rp-8-Br-cAMP inhibited these sensitizing effects. These findings suggest that ligands that increase acinar cell cAMP levels can sensitize the acinar cell to the effects of CCK-induced zymogen activation.
Keywords: caerulein, chymotrypsin, secretin, trypsin
the premature activation of digestive zymogens within the pancreatic acinar cell contributes to the initiation of pancreatitis. In the rodent pancreas, ligands such as CCK or the muscarinic agonist carbachol can cause zymogen activation in isolated pancreatic acini. These ligands activate specific G protein-linked receptors that stimulate phospholipase C and increase cytosolic Ca2+. Several reports (7, 9) have observed that zymogen activation within the acinar cell requires an increase in cytosolic Ca2+. CCK-induced activation depends on the presence of extracellular Ca2+ and an increase in cytosolic Ca2+ (9). Removal of extracellular Ca2+ or chelation of intracellular Ca2+ blocks caerulein (a CCK homolog)-induced trypsin activation (7, 9). Whether an increase in intracellular Ca2+ alone is sufficient to cause activation is controversial. Increasing cytosolic Ca2+ by thapsigargin was found to cause zymogen activation in one study but not in another (7, 9). Thus additional signaling pathways may act independently or with Ca2+ to cause zymogen activation.
The pancreatic acinar cell has receptors for pathways that are coupled to increasing both cytosolic Ca2+ and cAMP. Stimulation of acinar cell CCK or muscarinic receptors is linked to increased cytosolic Ca2+. Secretin, pituitary adenylate cyclase activating peptide (PACAP), and VIP stimulate receptors that are primarily coupled to cAMP generation. However, some receptors can generate both Ca2+ and cAMP signals. For example, acinar cells stimulated by high concentrations of CCK but not by muscarinic agonists cause prominent elevations in both cytosolic Ca2+ and cAMP (5). Moreover, although physiological concentrations of CCK do not cause a measurable increase in cAMP, they do activate cAMP-dependent protein kinase (5). Similarly, secretin has been reported in some but not all studies to not only increase cellular cAMP, but to also stimulate phospholipase C activity causing an increase in cytosolic Ca2+ in the acinar cell (10, 12). Because ligands that increase both acinar cell Ca2+ and cAMP are released in response to many small intestinal stimuli, including a meal, examining their interactions is physiologically relevant.
Activation of acinar cell receptors that are linked to cAMP generation has been reported to sensitize the acinar cell to CCK-induced enzyme secretion, but the effect of these stimuli on zymogen activation has not been reported. In the present study, the effects of ligands that stimulate acinar cell cAMP generation were examined alone and with caerulein. We report that agents that increase acinar cell cAMP have little effect on zymogen activation alone but sensitize the cell to caerulein-induced activation.
MATERIALS AND METHODS
Preparation of isolated pancreatic acini
Acini were isolated as described in Ref. 4. Briefly, fasted male Sprague-Dawley rats, ~150 g (Charles River Laboratories, Wilmington, MA), were killed by CO2 using a protocol approved by the Yale University and the Veterans Administration Animal Care and Use Committees. The pancreas was removed and placed into 6 ml acinar media [in mM: 40 Tris (pH 7.4), 95 NaCl, 4.7 KCl, 0.6 MgCl2, 1.3 CaCl2, 1 NaH2PO4, 10 glucose, and 2 glutamine, with 0.1% BSA, and 1 × MEM amino acids (GIBCO-BRL, San Jose, CA)] containing 50 U/ml collagenase Type-4 (Worthington, Freehold, NJ). The collagenase media was injected into the pancreas and after 5 min, the pancreas was minced and placed into a 50-ml flask with 12 ml of collagenase-media for 60 min at 37°C with shaking (120 rpm). The digest was filtered through a 300–400 μm mesh (Sefar American, Depew, NY). Isolated acini were distributed among the 24 wells (0.5 ml suspension/well) of a 24-well Falcon tissue culture plate (Becton Dickinson, Franklin Lakes, NJ). All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Acinar experimental protocol
Tissue culture plates containing acini were incubated for 60 min at 37°C under constant O2 with shaking (80 rpm). After the media were exchanged, acini were incubated for an additional 60 min. At this time, acini were treated without changing media. Treatments included caerulein (0.1 nM, 100 nM), secretin (0.1–100 nM), VIP (1,100 nM), PACAP (1,100 nM), 8-Br-cAMP (1–1,000 μM), and Rp-8-Br-cAMP (100 μM; Calbiochem, La Jolla, CA). Acini were incubated for 60 min (unless otherwise noted), at which time samples (media and cells) were collected, placed in 1.5-ml microcentrifuge tubes (USA Scientific, Waltham, MA), and stored at −80°C.
Enzymatic activity assays
Samples were thawed on ice, homogenized in the 1.5-ml microcentrifuge tubes with a tight-fitting pestle, and subjected to centrifugation at 1,000 g for 1 min. From the resulting postnuclear supernatant, 100 μl were added to wells of a 24-well tissue culture plate containing 350 μl of trypsin assay buffer (50 mM Tris, pH 8.1, 150 mM NaCl, 1 mM CaCl2, 0.01% BSA). Lastly, 50 μl of 400 μM enzyme substrate (trypsin #MQR3135-v, chymotrypsin #MAA3114-v; Peptides International, Louisville, KY) diluted in trypsin assay buffer (40 μM final) were added to each well. The plate was read using a fluorometric microtiter plate reader (HTS 7000; Perkin-Elmer Analytical Instruments, Shelton, CT) using an excitation wavelength of 380 nm and an emission of 440 nm for 11 min. The slope of the line represented enzyme activity and was normalized to total amylase activity and expressed as relative fluorescence units per second per microgram total amylase.
Amylase assay
Amylase activity in acinar homogenates was determined using a commercial kit (Phaebadas kit; Pharmacia Diagnostic, Rochester, NY).
cAMP assay
cAMP content in acinar homogenates was determined using a commercial ELISA assay (Amersham Biosciences, Piscataway, NJ). Briefly, acini were preincubated with 1 mM IBMX for 15 min to inhibit endogenous cAMP-phosphodiesterase activities, and then they were treated with specified agents for 60 min. cAMP content was calculated using a standard curve and normalized to amylase content.
Statistical analysis
The data represent the means ± SE of at least three individual experiments unless otherwise noted, with each experiment being performed in at least duplicate. A Student’s t-test analysis was used to determine statistical significance.
RESULTS
The effects of the CCK analog caerulein on stimulation of trypsin and chymotrypsin activity in isolated pancreatic acini were examined (Fig. 1). A supraphysiological concentration (100 nM) of caerulein caused a time-dependent increase in both trypsin and chymotrypsin activities. In vivo, supraphysiological concentrations of caerulein cause zymogen activation and acute pancreatitis. This supraphysiological concentration of caerulein is referred to as high-dose treatment. Stimulation with low-dose (0.1 nM) caerulein for 60 min generated no increase in either enzyme activity over background (not shown). The relative increase (compared with background) in chymotrypsin activity after high-dose caerulein treatment was greater than that observed for trypsin after 30 or 60 min (Fig. 1). Although we did observe a significant increase in trypsin activities with high-dose caerulein, these values were of a smaller magnitude than reported by others. The explanation for this difference is unclear. However, we found that measurement of chymotrypsin activity was both a more reproducible and sensitive marker of zymogen activation than trypsin, particularly after 60 min of treatment. This observation may be related to the processes of trypsin inhibition and degradation that have little effect on chymotrypsin. For these reasons, the enzymatic activities generated after 60 min of treatment were used to examine zymogen activation.
Fig. 1.
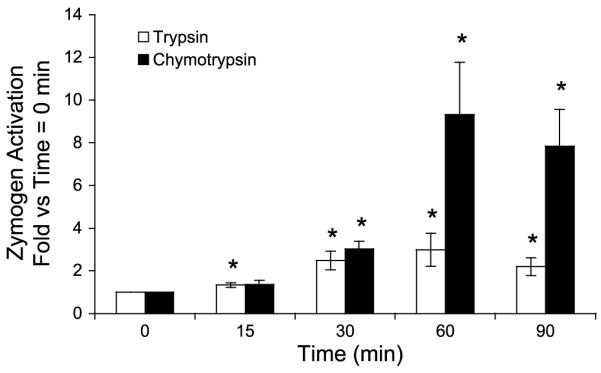
Caerulein generates a time-dependent increase in enzyme activities in isolated pancreatic acini. After high-dose caerulein (100 nM) treatment, enzyme activities were assayed in homogenates as described in materials and methods. Values are means ± SE from 6 separate experiments. *P < 0.05 compared with untreated control.
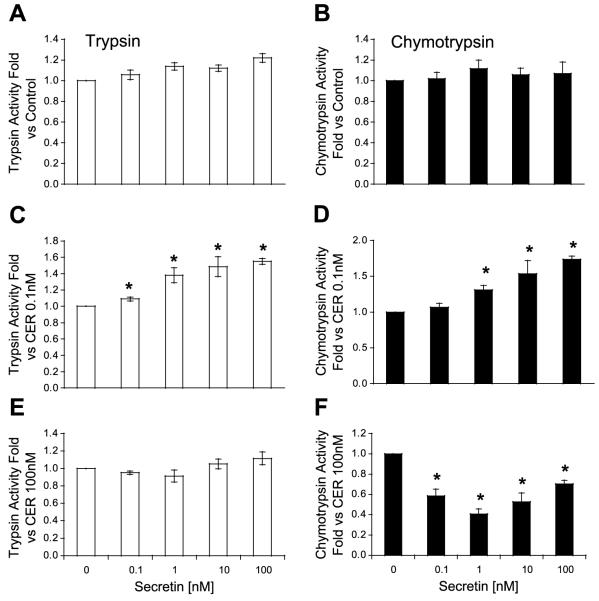
The effects of secretin on zymogen activation were next examined. As shown in Fig. 2, secretin alone had little effect on enzyme activities. However, secretin enhanced the effects of a low-dose caerulein on activation in a concentration-dependent manner. Thus secretin (10 nM) increased trypsin and chymotrypsin activities by ~60% over caerulein alone. Secretin did not enhance the effects of high-dose caerulein on trypsin or chymotrypsin activities. Indeed, secretin significantly reduced the effects of high-dose caerulein on chymotrypsin activity. Preliminary studies from our laboratory suggest that this inhibitory effect of secretin on high-dose caerulein may be the result of interactions between these receptors. Because secretin can stimulate cAMP-dependent and independent mechanisms in the acinar cell, the effects of other agents that increase cAMP were examined.
Fig. 2.
Secretin sensitizes the acinar cell to caerulein (CER)-induced zymogen activation. Secretin has little to no effect on basal (A), trypsin (B), or chymotrypsin activities (C and D). Secretin sensitizes the acinar cell to activation induced by low-dose CER (0.1 nM; E and F). Secretin does not sensitize to high-dose CER (100 nM). *P < 0.05 compared with no secretin.
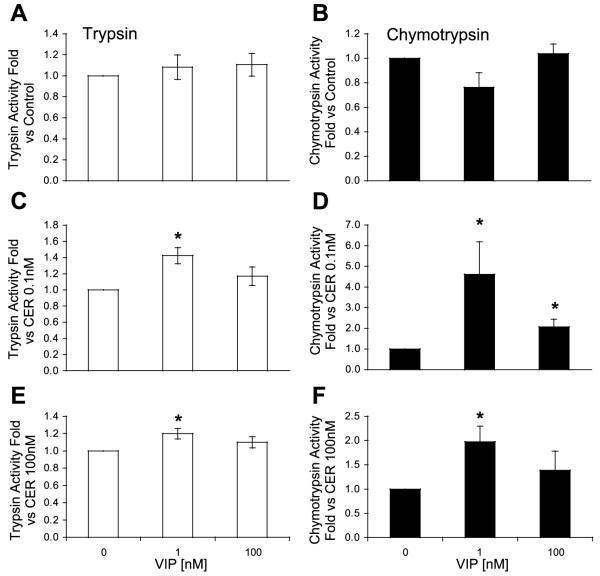
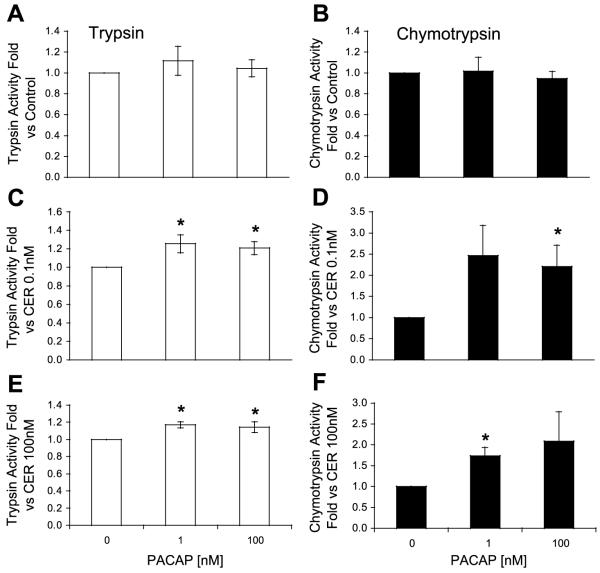
VIP and PACAP are known to activate receptors on the acinar cell that are linked to cAMP generation. The effects of these ligands on zymogen activation were similar. Alone, the ligands had little effect on trypsin and chymotrypsin activities (Figs. 3 and 4). However, both VIP and PACAP caused sensitization to low-dose caerulein (Figs. 3 and 4). In contrast to secretin, these agents also sensitized the acinar cell to high-dose caerulein. Furthermore, VIP and PACAP were more potent than secretin in sensitizing the acinar cell to caerulein.
Fig. 3.
Vasoactive intestinal peptide (VIP) sensitizes the acinar cell to CER-induced trypsinogen (A, C, and E) and chymotrypsinogen (B, D, and F) activation. VIP does not affect trypsin or chymotrypsinogen activation (A and B). VIP sensitizes the acinar cell to activation induced by low-dose (0.1 nM; C and D) and high-dose CER (100 nM; E and F). *P < 0.05 compared with no VIP.
Fig. 4.
Pituitary adenylate cyclase activating peptide (PACAP) sensitizes the acinar cell to CER-induced trypsinogen (A, C, and E) and chymotrypsinogen (B, D, and F) activation. PACAP does not affect trypsin or chymotrypsinogen activation (A and B). PACAP sensitizes the acinar cell to activation induced by low-dose (0.1 nM; C and D) and high-dose CER (100 nM; E and F). *P < 0.05 compared with no PACAP.
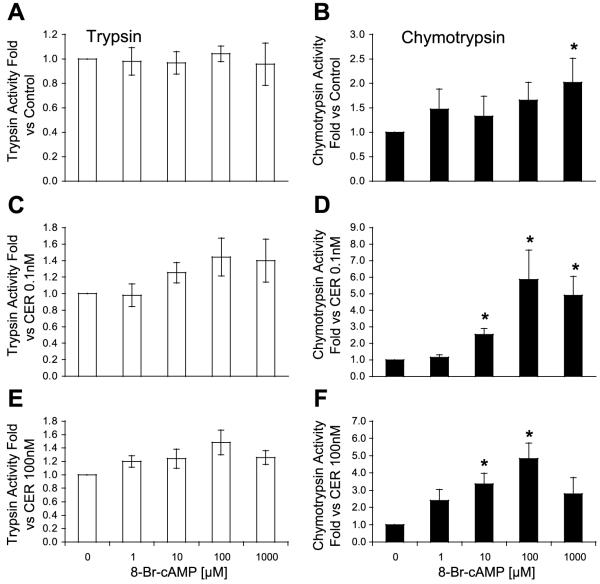
To confirm that these ligands were acting through a cAMP-dependent pathway, the effect of elevating cellular cAMP by a receptor-independent mechanism was examined. As shown in Fig. 5, addition of the cell-permeable analog of cAMP, 8-Br-cAMP, caused a small increase in chymotrypsin activity only at the highest concentration (1,000 μM). However, 8-Br-cAMP caused a concentration-dependent enhancement of zymogen activation when combined with either low- or high-dose caerulein (Fig. 5, C-F). The maximal level of sensitization was observed with 100 μM 8-Br-cAMP; higher concentrations caused less sensitization. Similarly, high concentrations of VIP also caused less sensitization than observed with lower concentrations (Fig. 3). The mechanism of the reduced sensitization at high concentrations of VIP and 8-Br-cAMP is unclear. 8-Br-cAMP (100 μM) also increased both trypsin and chymotrypsin activities in response to 30 min of 100 nM caerulein (not shown). These findings suggest that elevations in cellular cAMP can enhance the effects of low- and high-dose caerulein on zymogen activation. With respect to high-dose caerulein, the effects of 8-Br-cAMP were similar to the sensitizing effects of VIP and PACAP but different from those observed with secretin. Three additional studies were performed to demonstrate the link between cAMP and zymogen activation.
Fig. 5.
Directly increasing cytosolic cAMP with a permeable cAMP analog enhances CER-induced trypsinogen (A, C, and E) and chymotrypsinogen (B, D, and F) activation. Concentration-dependent effects of 8-Br-cAMP: alone (A and B) or with low-dose (0.1 nM; C and D) and high-dose CER (100 nM; E and F) on trypsin and chymotrypsin activities. *P < 0.05 compared with no 8-Br-cAMP.
The intracellular cAMP response to ligands was next examined. As shown in Fig. 6 and previously reported in Ref. 5, high-dose caerulein increased cAMP over low-dose caerulein or control values. However, the increase in cAMP levels caused by high-dose caerulein was substantially less than that generated by secretin, PACAP, or VIP. Furthermore, the combination of cAMP agonists with caerulein did not increase cAMP levels above those generated by the cAMP agonists alone.
Fig. 6.
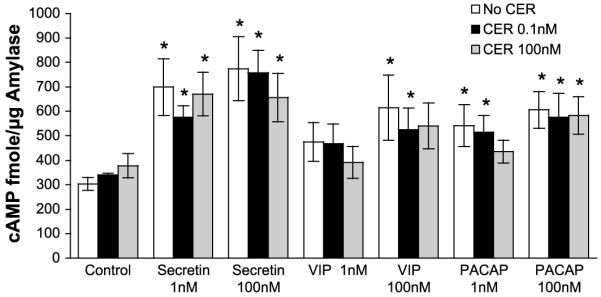
Agonists increase acinar cell cAMP levels. After treatments, cAMP content was assayed in homogenates as described in materials and methods. The cAMP content was calculated from a standard curve and normalized to amylase activity. *P < 0.05 (peptides are compared with unstimulated controls, and peptides with CER are compared with CER alone).
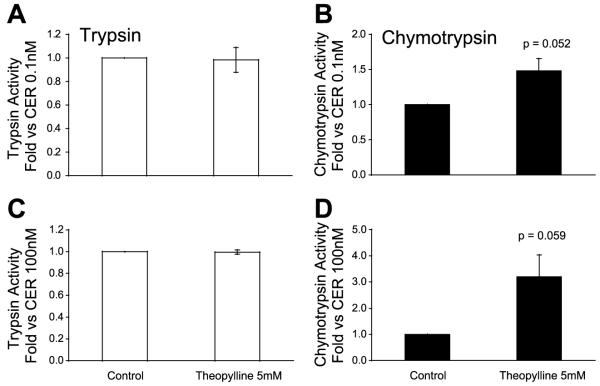
To enhance the endogenous cAMP response to caerulein, acinar cells were pretreated with the inhibitor of cAMP-phosphodiesterase, theophylline. As shown in Fig. 7, theophylline alone did not cause activation, but it tended to sensitize the acinar cells to low and high doses of caerulein.
Fig. 7.
Inhibition of phosphodiesterase augments CER-induced activation. Trypsin and chymotrypsin activities were assayed after 30 min of pretreatment with 5 mM theophylline and 60 min of additional incubation with vehicle or CER: low-dose CER (0.1 nM; A and B), high-dose CER (100 nM; C and D).
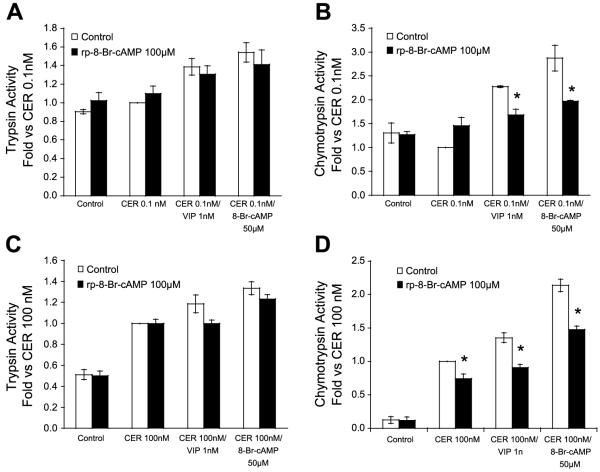
To confirm the role of cAMP in activation and sensitization, acini were pretreated (60 min) with Rp-8-Br-cAMP, a cell-permeable cAMP inhibitor. Such treatments reduced sensitization induced by both VIP and the 8-Br-cAMP to both low- and high-dose caerulein (Fig. 8). Moreover, it also reduced chymotrypsin activation induced by high-dose caerulein alone. These findings demonstrate the importance of cAMP in both the sensitization to caerulein and the activation caused by high-dose caerulein.
Fig. 8.
Inhibition of cAMP reduces chymotrypsinogen activation. Isolated acini were pretreated with the cAMP antagonist 8Br-Rp-cAMP (100 μM) or vehicle for 60 min before stimulation. After pretreatment, acini were treated as shown for 60 min and assayed for trypsin (A and C) and chymotrypsin activities (B and D). *P < 0.05 compared with paired treatment condition without 8Br-Rp-cAMP.
DISCUSSION
The pathological activation of pancreatic digestive zymogens within the pancreatic acinar cell is a central feature of acute pancreatitis. Stimulation of isolated acinar cells with supraphysiological concentrations of CCK or the muscarinic agonist carbachol results in zymogen activation. Supraphysiological concentrations of CCK in vivo also cause zymogen activation within the acinar cell and pancreatitis. Sensitization of the acinar cells to the pathological effects of these ligands may be relevant to the pathogenesis of pancreatitis. The present study examined the sensitizing effect of agents that increase cAMP in the acinar cell. We find that secretin, PACAP, and VIP sensitize the acinar cell to caerulein-induced zymogen activation. PACAP and VIP caused sensitization to physiological and supraphysiological concentrations of caerulein. Secretin was less potent than PACAP and VIP in sensitizing to physiological concentrations of caerulein and did not cause activation in response to supraphysiological concentrations of caerulein. The explanation for the greater potency of PACAP and VIP compared with secretin remain unclear but did not appear to be the result of differences in the levels of cAMP generation.
To demonstrate that the sensitizing effects of these agents were linked to cAMP generation, additional studies were performed. The first demonstrated sensitization to caerulein by a cell-permeable cAMP analog (8-Br-cAMP) that bypassed the secretin, PACAP, and VIP receptors. The CCKAI1 receptor predominately acts through calcium signaling, but it is also known to increase cAMP levels, particularly at supraphysiological concentrations (5). We confirmed that high-dose caerulein increased cellular cAMP but to much lower levels than generated by cAMP agonists alone or with caerulein. To enhance the effects of caerulein on cAMP generation, cAMP-phosphodiesterase was inhibited by theophylline. Such inhibition caused an increase in zymogen activation by caerulein. Finally, an inhibitor of cAMP binding to its receptors, Rp-8-Br-cAMP, reduced sensitization by both VIP and 8-Br-cAMP. The cAMP inhibitor also reduced activation caused by high-dose caerulein. These observations support the conclusion that sensitization by the secretin-related ligands to caerulein is related, at least in part, to cAMP generation. Furthermore, increased cellular cAMP appears to contribute to the effects of high-dose caerulein on activation.
The mechanisms of cAMP-related sensitization remain unclear. cAMP can signal through two major pathways: cAMP-dependent protein kinases (PKA) and the recently described Epac mechanism (8). Signaling in the latter pathway is initiated by cAMP activation of the guanine nucleotide exchange factors (GEF) Epac-1 or Epac-2. These GEFs regulate the small GTPases, Rap1 and Rap2. Although the PKA and Epac pathways have a number of potential targets, they may converge on two pathways that are linked to zymogen activation: calcium and phosphatidylinositol 3 (PI3)-kinase. PKA has been found to modulate calcium-release through the inositol 1,4,5-trisphosphate (IP3) receptor. However, the effects of PKA on IP3 receptors are complex and may vary with the receptor subtype (1, 2). Recent studies have also demonstrated that the Epac pathway may modulate levels of intracellular calcium by regulating movement through the calcium-dependent calcium-release channel (3). Studies are underway in our laboratory to determine the contribution of the PKA and Epac pathways to cAMP-dependent sensitization in acinar cells. cAMP may be causing sensitization to caerulein through a calcium-dependent or independent mechanism. The effects of cAMP agonists on peak calcium responses and other characteristics of the cytosolic calcium response to caerulein need to be examined. PKA and Epac have been found to modulate the activity of PI3-kinase in pancreatic islet cells (6). Because PI3-kinase has been reported to regulate trypsin activation in response to caerulein in isolated pancreatic acini, it too represents a potential target of the cAMP response (11). These observations suggest that the mechanism of cAMP sensitization to caerulein may be complex.
In summary, these studies demonstrate that increasing cAMP levels in the pancreatic acinar cell can sensitize the cell to caerulein-induced zymogen activation. Notably, this sensitization is observed with physiologically relevant concentrations of caerulein. Whether the sensitizing action of cAMP is through PKA or the newly described Epac pathway is unknown. Additionally, the effects of cAMP on calcium signaling need to be determined. In a clinical context, these studies provide an example of sensitization. Thus combinations of physiological agonists, such as CCK and secretin, with specific exogenous agents or conditions might lead to pancreatitis. The findings of this study suggest that agents that increase cAMP levels in the pancreatic acinar cell may predispose the cell to pathological zymogen activation and contribute to the development of acute pancreatitis. This hypothesis needs to be examined using in vivo models of disease.
Acknowledgments
We thank E. Thrower and C. Shugrue for critical review of this manuscript.
DISCLOSURES
This work was supported by and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-54021 (to F. Gorelick) and the Training Program in Gastroenterology Research Fellowship Grant KD-07017 (to S. Karne).
REFERENCES
- 1.Dyer JL, Mobasheri H, Lea EJ, Dawson AP, Michelangeli F. Differential effect of PKA on the Ca2+ release kinetics of the type I and III InsP3 receptors. Biochem Biophys Res Commun. 2003;302:121–126. doi: 10.1016/s0006-291x(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci DR, Groblewski GE, Sneyd J, Yule DI. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J Biol Chem. 2000;275:33704–33711. doi: 10.1074/jbc.M004278200. [DOI] [PubMed] [Google Scholar]
- 3.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Z, Karne S, Kolodecik T, Gorelik FS. Alcohols enhance caerulein-induced zymogen activation in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2002;282:G501–G507. doi: 10.1152/ajpgi.00388.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marino CR, Leach SD, Schaefer JF, Miller LJ, Gorelick FS. Characterization of cAMP-dependent protein kinase activation by CCK in rat pancreas. FEBS Lett. 1993;316:48–52. doi: 10.1016/0014-5793(93)81734-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 7.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehmann H, Prakash B, Wolf E, Rueppel A, De Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 9.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 1999;276:G835–G842. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 10.Singh J, Lennard R, Salido GM, Wisdom D, Render CL, Pozo MJ, Pariente JA, Camello PJ. Interaction between secretin and cholecystokinin-octapeptide in the exocrine rat pancreas in vivo and in vitro. Exp Physiol. 1992;77:191–204. doi: 10.1113/expphysiol.1992.sp003573. [DOI] [PubMed] [Google Scholar]
- 11.Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–1395. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trimble ER, Bruzzone R, Biden TJ, Meehan CJ, Andreu D, Merrifield RB. Secretin stimulates cyclic AMP and inositol trisphosphate production in rat pancreatic acinar tissue by two fully independent mechanisms. Proc Natl Acad Sci USA. 1987;84:3146–3150. doi: 10.1073/pnas.84.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]