Introduction
Acanthamoeba keratitis (AK) may result in incapacitating pain and significant visual and ocular morbidity. A pronounced dichotomy of visual outcomes has been observed with some patients doing very well while others require corneal transplantation or even enucleation. 1–4 While the depth of corneal involvement is an independent risk factor for poorer visual outcome, the duration of untreated disease is not, suggesting that chronicity alone is not the reason for these widely divergent outcomes. The benefits of recent advances in early diagnosis may then be limited, increasing the importance of understanding the factors associated with cases demonstrating increased clinical pathogenicity.
One subset of AK which has historically done poorly consists of patients with combined AK and infectious crystalline keratopathy (ICK). These patients generally require surgical intervention but, nevertheless, experience poor outcomes. 5–8 This is not surprising since both AK and ICK, independently, have very guarded prognoses. Polymicrobial keratitis, specifically combined bacteria and fungus, has been shown to worsen experimental infections, and is clinically thought to create more severe inflammation often resulting in delayed recognition and treatment of all active pathogens.9–11 Acanthamoeba keratitis, especially, has been linked to a number of co-infections including bacterial, fungal and viral pathogens.12–16
In most of these co-infections, however, Acanthamoeba is considered as either an opportunistic pathogen, taking advantage of compromised ocular defenses or as a primary pathogen in the setting of poor general hygiene and gross overgrowth of microbes. Reported almost exclusively in patients on long term corticosteroid therapy, usually months after initiating treatment for AK, ICK is an exception and, in contrast, is characterized as a secondary, opportunistic infection.5–8
In these patients, corticosteroids have been used to reduce aggressive scleritis or keratolysis, but their modulatory role in AK is controversial and it is less clear what effect they have in encouraging the development of ICK. We present the medical management of three cases of combined Acanthamoeba keratitis and infectious crystalline keratopathy treated with current anti-acanthamoebal medications and discuss the clinical presentation and implications of this unusual relationship between two rare corneal infections.
Methods
A retrospective review of all cases of Acanthamoeba keratitis diagnosed at the University of Illinois Eye and Ear Infirmary (IEEI) between June, 2003 and November, 2008 was undertaken to identify patients with an additional diagnosis of bacterial keratitis, and, specifically, for a clinical diagnosis of ICK. Our diagnostic criteria for AK have been described previously.17 Briefly, a patient was Considered to have AK When demonstrating any combination of two or more of the following positive findings: clinical characteristics consistent with AK, confocal microscopic evidence, histologic smear, pathology or culture. Bacterial keratitis was considered present when a positive bacterial culture was obtained at any time during diagnosis or treatment for AK. Bacterial cultures were plated on blood agar, chocolate agar, and eugonic broth and presumed a contaminant if read as few colonies or less or if isolated from broth only. Cultures were seldom performed after the initial diagnostic visit, primarily in cases of poor resolution or increasing infiltration.
Each case was reviewed for demographic information, prior treatment, diagnostic interventions and their post-AK diagnosis treatment course, including risk factors, medications and slit lamp findings.
Results
A total of 111 cases of Acanthamoeba keratitis were identified in the study period. Five patients demonstrated bacterial growth either on cultures obtained at the time of initial evidence of AK or during their subsequent treatment course. Three of the five patients with this microbiologic evidence of bacterial keratitis during active AK manifested clinical signs of ICK. Five other patients demonstrated light growth (broth only (2), rare colony (2) and a single colony (1)) of a presumed contaminant, coagulase negative Staphylococci. None of these patients manifested clinical signs of bacterial keratitis. Two additional patients contracted bacterial keratitis several months after penetrating keratoplasty and clinical resolution of their active AK. These resolved with antibacterials alone without the reintroduction of anti-acanthamoebal drugs, and were, therefore, not considered to be concomitant with the original AK infection.
Patient 1
A 32 year old frequent replacement soft contact lens wearer with a history of recreational water exposure was referred to the Cornea section at the IEEI with a 10 week history of steroid dependent keratitis. He initially developed blurry vision and was given topical antibiotics, reportedly, for a corneal abrasion, without improvement. The addition and continued use of topical corticosteroids after 2 weeks led to a resolution of his symptoms until his supply of steroids was exhausted five weeks later. Severe photophobia, blurred vision, tearing and frank eye pain then responded to a re-introduction of topical corticosteroids 5 times daily.
Visual acuity on presentation was 20/40 in the affected left eye and 20/25 in the normal right eye. Slit lamp examination of his left eye revealed a central irregular geographic ring infiltrate, radial keratoneuritis extending from the 7 o’clock position to the limbus, centralized corneal haze and a corneal epithelial defect. No focal infiltrates were present initially. Confocal microscopy revealed suspicious acanthamoeba-like figures in the form of dense white opacities and bright centered round opacities. 17 A superficial scraping confirmed the presence of Acanthamoeba. Antibacterials were discontinued.
Propamidine isethionate 0.1% and chlorhexidine gluconate 0.02% every 1–2 hours was initiated and prednisolone tapered with improvement in signs and symptoms over the next week. After cultures returned positive for Acanthamoeba and negative for any bacterial or fungal growth, the patient returned to his referring ophthalmologist. He returned to the IEEI pharmacy two weeks later serendipitously reporting a recent worsening of his symptoms despite steady improvement noted by his referring ophthalmologist a few days earlier. An impromptu exam revealed two areas suspicious for infectious crystalline keratopathy (Figure 1 (Top Left)). The patient was re-cultured and empirically started on fortified vancomycin 15 mg/cc and gatifloxacin hourly. These cultures later returned positive for Streptococcus oralis. Symptoms subsided rapidly with the keratitis resolving over the next several weeks. Visual quality was limited by corneal irregularity and an optical penetrating keratoplasty (PKP) was performed resulting in visual acuity of 20/40. No active disease was present on pathology.
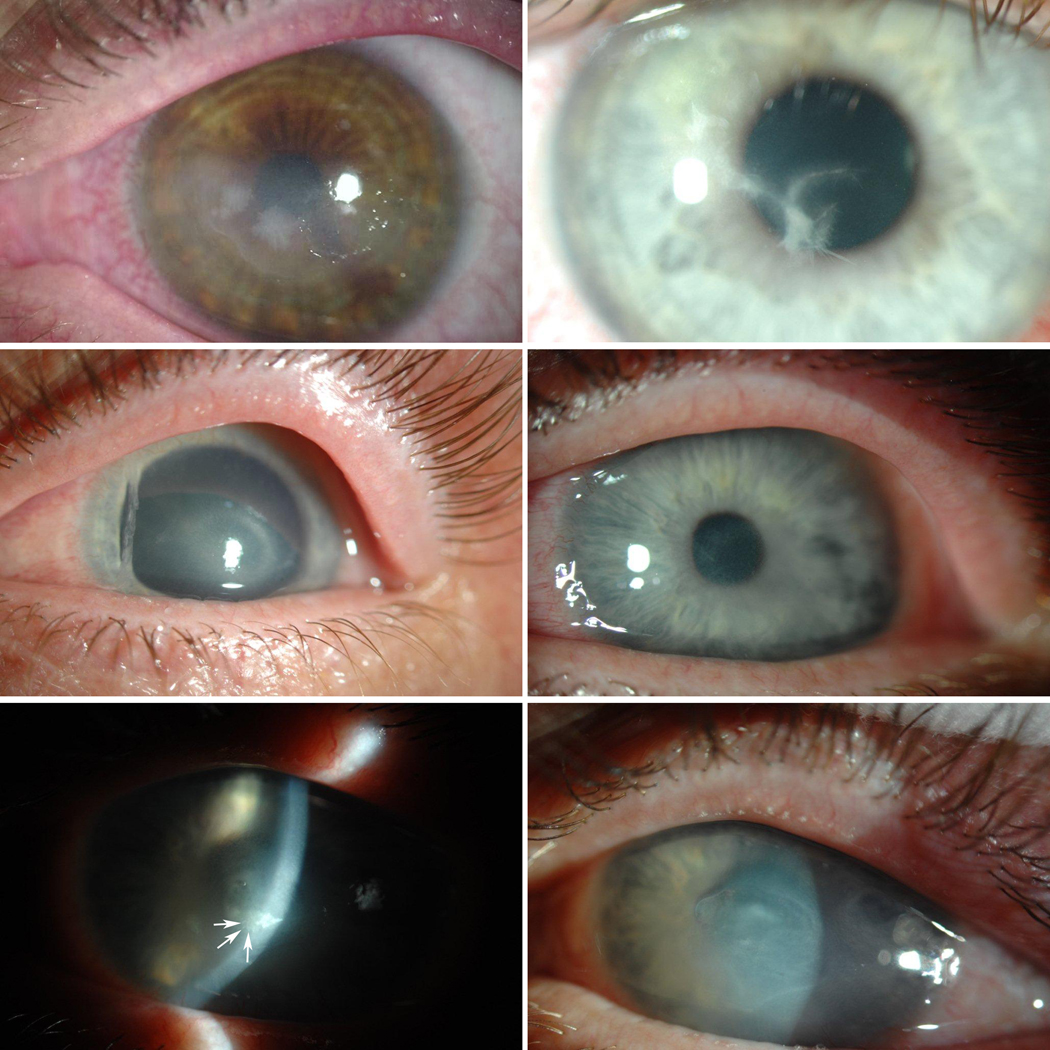
Figure 1.
Presentation of Infectious Crystalline Keratopathy in Acanthamoeba Keratitis. (Top Left) Patient 1, 3 weeks after initiation of anti-acanthamoeba therapy presents with increasing pain and photophobia and two areas of mid-stromal crystalline keratopathy. (Top Right) Patient 3, 1 week after starting anti-acanthamoeba therapy with deep needle- like mid-stromal extensions from the original ulcer. Patient 2: (Middle Left) Immune ring on initial presentation at the IEEI. (Middle Right) Symptomatic and visual improvement 1 week after initiation of anti-acanthamoebal therapy. (Bottom Left) New pain and new deep stromal infiltrate with small finger-like projections (arrows) 3 weeks after initiating treatment. (Bottom Right) Resolution of S. oralis infection with increased corneal haze and tissue loss.
Case 2
A 38 year old soft contact lens wearer presented to the IEEI with a chronic keratitis of the left eye, treated as herpetic keratitis with a waxing and waning course over the prior 4 months. Vision was stable until 2 days prior when he had a significant decrease in vision. Medications at presentation included trifluridine and prednisolone acetate three times daily and oral valacyclovir 500 mg daily. Corrected visual acuity was 20/20 and 20/200 in the normal right and left eye, respectively. Slit lamp exam revealed a superficial pannus in the left eye and an immune disciform-appearing infiltrate of the paracentral inferotemporal cornea (Figure 1 (Middle Left)). Confocal microscopy and Diff-Quik (Difco, Detroit. Michigan) smear was positive for Acanthamoeba.
Treatment was initiated as in case 1 with the exception of the addition of voriconazole 200mg twice daily prompted by the presence of a deep infiltrate. Marked improvement was seen in symptoms over the next three weeks with a peak visual acuity of 20/40 (Figure (Middle Right)). Rapidly after that time, however, the patient noted a severe increase in pain and photophobia accompanying increasing corneal infiltration (Figure (Bottom Left)). One week later, deep stromal crystalline extensions were noted extending from the main infiltrate. The lesion was re-cultured, and vancomycin 15mg/cc every two hours was added with a significant improvement in pain over the next week (Figure (Bottom Left)). Cultures grew S. oralis. All medications were tapered 6 weeks later, resulting in a quiet eye, but with new central scarring limiting best corrected vision to 20/50.
Case 3
A 21 year old overnight silicone hydrogel soft contact lens wearer was referred to the University of Illinois Hospital emergency room for a 3 day history of acute onset pain, redness and photophobia of the right eye with an atypical keratitis unresponsive to 2 days of frequent topical gatifloxacin. After cultures were obtained, fortified cefazolin and gentamicin were initiated hourly. Because of the unusual clinical appearance, the patient was referred to the Cornea service of the IEEI the next day. Visual acuity was 20/40 and 20/20 in the right and left eyes respectively. Slit lamp examination revealed a broad, anterior stromal, serpiginous infiltrate extending from the superotemporal limbus into the visual axis. Small, isolated extensions from the infiltrate, suggestive of atypical radial keratoneuritis, were observed. Confocal microscopy and smear was positive for Acanthamoeba. Propamidine and chlorhexidine were started as noted in case 1. The patient returned 3 days later noting modest improvement in symptoms but with an increasing number and size of deep crystalline intralamellar infiltrates (Figure (Top Right)). Original cultures, performed on the partially treated keratitis, were positive for broadly sensitive methicillin sensitive S. aureus (MSSA). Vancomycin was substituted for cefazolin with significant improvement in symptoms over the next two weeks. Best spectacle corrected vision returned to 20/20 over the following several weeks.
Discussion
We present three cases of primary Acanthamoeba keratitis with a secondary manifestation of infectious crystalline keratopathy. While this unusual juxtaposition of two very rare corneal infections has been previously reported,5–8 our cases are unique in that medical therapy alone was successful for cure with a reasonable visual outcome. Most previous cases have required one or more PKPs for medically unresponsive disease, most without reported resolution (Table).5–8 The availability of more effective anti-acanthamoebal medications combined with earlier recognition, demonstrated to improve visual outcomes in AK,4 may have obviated the need for therapeutic surgical intervention. Further, it has been previously suggested that chronic corticosteroid use, common in the treatment of AK in the 1980’s, in the setting of a compromised ocular surface is a primary factor in developing a secondary ICK.5–8 With the exception of one case of ICK post-PKP, all previous reports have been under active topical and/or systemic corticosteroid therapy when ICK was recognized. Our cases are also unique in that two of these patients were not actively treated with topical corticosteroids at the time of ICK diagnosis; one without any history of corticosteroid exposure and ICK developed as early as 1 week after initial symptoms. These cases indicate that chronicity and corticosteroid use are not obligatory risk factors for developing ICK in Acanthamoeba-infected eyes and suggest the possibility that this association of two very rare infections and their associated pathogens may extend beyond simple opportunism.
Table.
Previously reported and current cases of concomitant Acanthamoeba Keratitis and Infectious Crystalline Keratopathy.
| Contact Lens Use |
HSV Therapy |
Previous steroid therapy |
ICK after Sx Onset |
Presentation of ICK after Initiation of AK Tx |
Anti- parasitic Therapy |
Continue d Steroid therapy |
Surgical therapy during for AK |
Culture | Result | |
|---|---|---|---|---|---|---|---|---|---|---|
| Davis 19876 | Yes | Yes | Yes | 3 months | ~ 1 month | Propamidine Paromycin | Yes | PKP × 2 | None | Active |
| No | Yes | Yes | 12 months | >4 months | None | Yes | PKP × 2 | S. viridans | Active | |
| Cohen 19875 | Yes | No | Yes | 8 months | 6 months | Propamidine Miconazole Neomycin Ketoconazole | Yes | PKP | S. viridans | 20/40 |
| Mathers 19878 | Yes | Yes | Yes | 5 months | 1 month (post-PKP) | Neomycin Propamidine | No | PKP × 4 | S. viridans | Active |
| Loffler, 19987 | Yes | Yes | No | Unk | Unk | Propamidine | Yes | PKP | None | 20/40 |
| Current | Yes | No | Yes | 13 weeks | 3 weeks | Propamidine Chlorhexidine | Yes | None* | S. oralis | 20/40 *Post-PKP |
| Yes | Yes | Yes | 5 months | 3 weeks | Propamidine Chlorhexidine Voriconazole | No | None | S. oralis | 20/50 | |
| Yes | No | No | 1 week | 1 week | Propamidine Chlorhexidine | No | None | MSSA | 20/20 |
Legend: AK- Acanthamoeba Keratitis, ICK- Infectious Crystalline Keratopathy, HSV- Herpes simplex keratitis, Sx- Symptoms, Tx – treatment, Active – Final visual acuity and/or resolution unavailable at time of last report, PKP- Penetrating Keratoplasty, Unk – unknown, MSSA – Methicillin Sensitive Staphylococcus aureus, S.- Streptococcus.
Only five patients in our series of 111 AK cases had established microbiologic evidence of a bacterial keratitis during their active amoebic infection. ICK, a rare keratitis, constituted 3 of 5 of these cases. Interestingly, initial cultures were only positive in patient 3 (MSSA), presenting on topical antibacterials, with the earliest presentation of ICK. Although many different organisms have been associated with ICK, almost all of the cases previously described in AK-associated ICK have involved the S. viridans group which was isolated in the form of Strep. oralis in patients 1 and 2, only after the appearance of clinical signs of ICK. Two additional patients developed bacterial keratitis months after successful penetrating keratoplasty for recalcitrant AK, one of which also developed ICK.18 However, the liberal, empiric use of modern, broad spectrum antibacterials, community culture practices, restriction of subsequent cultures to atypical AK progression and the reduced yield characteristic of partially-treated organisms, makes it probable that the number of coinfections is higher than could be practically detected in our cohort.
Infectious crystalline keratopathy is primarily associated with slow growing cocci like S. viridans, but may also involve other more aggressive bacteria as well as Mycobacteria sp. and Candida sp. It is characterized by intralamellar crystalline spread, thought to be secondary to a polysaccharide-rich biofilm secreted by the responsible pathogen and is most commonly associated with post-keratoplasty infections potentiated by chronic corticosteroid use and recent ocular surgery. Initial presentations of ICK are notably minimally inflammatory with very slow progression even with steroid treatment. Unfortunately, the suppressive effect expected from chlorhexidine which has been shown to have good activity against isolates of S. oralis (Delaney CM, et al. IOVS 2005;46: E-Abstract 4881.) and S. aureus,19 the etiologic agents isolated in our cases, was not effectively prophylactic even at the high doses used to treat AK.
Effective prophylaxis and treatment of ICK in AK is heavily dependent on the origin of the inoculum. Corneal clarity would not have prevented an observation of ICK at the time of AK diagnosis and our patients demonstrated progressive, symptomatic and objective improvement with AK treatment alone. Prior to the development of ICK, the epithelium had completely healed in patient 1, no epithelial defect was observed in patient 2 prior to the onset of ICK and only a small peripheral epithelial defect was noted in patient 3, spatially removed from the main area of ICK. Finally, the onset of ICK was rapid, atypically deep stromal in origin and associated with an acute increase in pain and inflammation in patients under frequent observation. Although it is possible that S. oralis was a successive pathogen, introduction of a new, opportunistic stromal keratitis in the face of hourly, effective antimicrobial administration with an intact epithelium seems less likely.
This raises the possibility that S. oralis may have originally been present as an acanthamoebal endosymbiont, released only after effective lysis of the host amoeba, consistent with the rapid, deep stromal origin seen in our patients. While Acanthamoebae normally utilize bacteria as prey, certain amoeba resistant bacteria (ARB), e.g. legionella, pseudomonas, mycobacterium, and parachlamydiae, among others, are able to resist digestion and, instead, enter a stable endosymbiotic relationship with the host amoeba.20,21 Internalized, endosymbionts are sheltered from both disinfection with antimicrobials, e.g. antibiotics or chlorhexidine, and from detection, since they cannot be isolated unless released from the host.22, 23 Regardless of the length of AK infection, all previous reports cite the onset of ICK only after initiation of AK therapy (Table) supporting the possibility that treatment plays some role in the development of ICK and that more effective AK treatment might lead to an earlier post-treatment presentation as was seen in our patients.
In fact, acanthamoebae have been hypothesized to serve as a “Trojan Horse” allowing pathogens safe harbor in other sites of human infection.24 Gram positive bacteria have not, however, been well characterized as ARB in nature,20,21,25 but, when exposed, methicillin resistant S. aureus can be easily incorporated.26 Further, recent evidence indicates that the distribution of endosymbionts found in corneal acanthamoeba isolates is more reflective of pathogens found in the human environment and are detectable in greater than 50% of IEEI (unpublished data) and others’ clinical keratitis isolates. (Lovieno, et al. OMIG 2008; Abstract 19) This is well above the 25% noted in previous reports of corneal and environmental sources 25 Their contribution in our cases remains hypothetical because despite observation of endosymbionts in the Acanthamoeba isolate in Patient 1, we were unable to type the endosymbionts, probably due to the age of the acanthamoeba isolate.
Regardless of the origin, early, aggressive treatment of ICK at first presentation achieved a rapid reduction in pain and inflammation without the need for therapeutic keratoplasty in these cases. Constituting nearly 3% of our AK cases, patients with concomitant ICK presented at an advanced stage of disease and, despite initial improvement, subsequently experienced sudden severe pain and inflammation accompanying definitive signs of crystalline keratopathy. Significant stromal loss and scarring less characteristic of simple AK developed in these geographic areas of co-infection suggesting an increased pathogenicity when combined with ICK.4 While breakdown of the biofilm associated with ICK may increase inflammation in later disease, the timing was atypical in our patients. Coinfection itself has been shown in laboratory studies to increase both bacterial and amoebic viability and pathogenicity in coculture.21,26–28 Despite a previous hypothesis of a modulatory effect,29 evidence suggests that some endosymbiotic relationships confer increased pathogenicity.25,30 Clinical precedent exists in Onchocerciasis in which the presence of its endosymbiont Wolbachia sp. produces more severe corneal disease,31–33 the aggressive treatment of which reduces neutrophil recruitment, the infection’s primary mechanism for corneal scarring.34,35 Although topical vancomycin was used in our cases, the organisms isolated were also broadly sensitive in vitro to both 4th generation fluoroquinolones and cefazolin.
Clinically, a high index of suspicion should be maintained for ICK in the setting of Acanthamoeba keratitis especially in recalcitrant or worsening clinical disease after an initial response to appropriate therapy. Successful medical management of these infections is possible with early recognition of polymicrobial disease and aggressive treatment without therapeutic surgical intervention. Anti-acanthamoebal drugs have a broad spectrum of anti-microbial activity, but alone are not sufficiently prophylactic against the rise of ICK, suggesting that the use of more effective, specific anti-bacterial drugs may be required in certain patients. Importantly, neither chronicity, corticosteroid use nor an epithelial defect is a requisite for the emergence of ICK in AK. Consequently, this nexus of two rare corneal infections should prompt consideration of the complex interactions of acanthamoebal coinfection and, possibly, endosymbiosis not only in the divergent outcomes seen in Acanthamoeba keratitis but, more broadly, in the pathogenesis of other contact lens-related keratitides and other human disease.
ACKNOWLEDGMENTS/DISCLOSURE
Funding / Support (including none): NIH 15689, Prevent Blindness America, Midwest Eye-Banks, UIC Campus Research Board, AOF AAO William C. Ezell Fellowship, Karl Cless Foundation
Financial Disclosures (including none): Dr. Tu reports receiving honoraria of <$10,000 for speaking at the invitation of Alcon and Allergan and an equity interest in Genentech of <$10,000. Dr. Joslin is supported by the National Eye Institute (NIH 15689). Dr. Feder reports book royalties and provision of expert legal testimony. Dr. Jain reports receiving <$10,000 as a member of the Alcon Dry Eye Advisory Board and research funding by the Warren and Clara Cole Foundation. Dr Shoff has received support from the National Eye Institute (NIH EY 09073).
Contributions to Authors in each of these areas: Contributions of Authors: Design of the study (ET, CJ, LN); Analysis and Interpretation (ET, CJ, RF), Writing the Article; (ET, LN); Critical Revision of the Article (ET,CJ, LN, RF,SJ, MS); Final Approval (ET, CJ, LN, RF, SJ, MS); Data Collection (ET, LN); Provision of Materials (ET, RF, SJ); Obtaining Funding (ET, CJ); Literature Search (ET, CJ); Administrative/Technical Support (ET, CJ, MS)
Statement about Conformity with Author Information: This research was approved by the Institutional Review Board of the University of Illinois at Chicago
Other Acknowledgments: None
Biographies
Elmer Y. Tu, MD, is currently an Associate Professor of Clinical Ophthalmology at the University of Illinois at Chicago serving as director of the Cornea and External Disease Service. Dr Tu completed his residency at the University of Wisconsin-Madison and cornea fellowship at the Bascom Palmer Eye Institute in Miami, Florida. Dr Tu was previously director of the Cornea and External Disease Service and Residency Program Director at the University of Texas Health Science Center, San Antonio, Texas.

Charlotte E. Joslin, OD, is currently an Assistant Professor at the University of Illinois at Chicago, Department of Ophthalmology and Visual Sciences. Dr Joslin received her undergraduate degree from Illinois Wesleyan University and professional degree from The Ohio State University, and completed her residency training at the VA Chicago Healthcare, West Side and Hines Divisions. Dr Joslin is currently a NEI K23 awardee and is pursuing her PhD in epidemiology at the University of Illinois at Chicago School of Public Health.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, Wright P. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984–1992. Eye. 1993;7(Pt 6):719–725. doi: 10.1038/eye.1993.168. [DOI] [PubMed] [Google Scholar]
- 2.Mathers WD, Sutphin JE, Folberg R, Meier PA, Wenzel RP, Elgin RG. Outbreak of keratitis presumed to be caused by Acanthamoeba. Am J Ophthalmol. 1996;121:129–142. doi: 10.1016/s0002-9394(14)70577-x. [DOI] [PubMed] [Google Scholar]
- 3.Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86:536–542. doi: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu EY, Joslin CE, Sugar J, Shoff ME, Booton GC. Prognostic Factors Affecting Visual Outcome in Acanthamoeba Keratitis. Ophthalmology. 2008;115:1998–2003. doi: 10.1016/j.ophtha.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen EJ, Parlato CJ, Arentsen JJ, et al. Medical and surgical treatment of Acanthamoeba keratitis. Am J Ophthalmol. 1987;103:615–625. doi: 10.1016/s0002-9394(14)74320-x. [DOI] [PubMed] [Google Scholar]
- 6.Davis RM, Schroeder RP, Rowsey JJ, Jensen HG, Tripathi RC. Acanthamoeba keratitis and infectious crystalline keratopathy. Arch Ophthalmol. 1987;105:1524–1527. doi: 10.1001/archopht.1987.01060110070034. [DOI] [PubMed] [Google Scholar]
- 7.Loffler KU, Witschel H. Whitish crystalloid corneal deposits. Infectious crystalline keratopathy in amoebic keratitis. Ophthalmologe. 1998;95:576–577. doi: 10.1007/s003470050319. [DOI] [PubMed] [Google Scholar]
- 8.Mathers W, Stevens G, Jr, Rodrigues M, et al. Immunopathology and electron microscopy of Acanthamoeba keratitis. Am J Ophthalmol. 1987;103:626–635. doi: 10.1016/s0002-9394(14)74321-1. [DOI] [PubMed] [Google Scholar]
- 9.Jones DB. Polymicrobial keratitis. Trans Am Ophthalmol Soc. 1981;79:153–167. [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan M, Sangawe JL, Mahajan VM. Pathogenesis of experimentally produced corneal ulcers in rabbits. Ann Ophthalmol. 1984;16:246–248. 250–252. [PubMed] [Google Scholar]
- 11.Pate JC, Jones DB, Wilhelmus KR. Prevalence and spectrum of bacterial coinfection during fungal keratitis. Br J Ophthalmol. 2006;90:289–292. doi: 10.1136/bjo.2005.081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dini LA, Cockinos C, Frean JA, Niszl IA, Markus MB. Unusual case of Acanthamoeba polyphaga and Pseudomonas aeruginosa keratitis in a contact lens wearer from Gauteng, South Africa. J Clin Microbiol. 2000;38:826–829. doi: 10.1128/jcm.38.2.826-829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajdatsy AD, Kosmin A, Barrett GD. Coexistent adenoviral keratoconjunctivitis and Acanthamoeba keratitis. Clin Experiment Ophthalmol. 2000;28:434–436. doi: 10.1046/j.1442-9071.2000.00352.x. [DOI] [PubMed] [Google Scholar]
- 14.Mathers WD, Goldberg MA, Sutphin JE, Ditkoff JW, Folberg R. Coexistent Acanthamoeba keratitis and herpetic keratitis. Arch Ophthalmol. 1997;115:714–718. doi: 10.1001/archopht.1997.01100150716002. [DOI] [PubMed] [Google Scholar]
- 15.Rumelt S, Cohen I, Rehany U. Spontaneous corneal graft ulcerative perforation due to mixed Acanthamoeba and herpes simplex keratitis: a clinicopathologic study. Cornea. 2000;19:240–242. doi: 10.1097/00003226-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Slade DS, Johnson JT, Tabin G. Acanthamoeba and fungal keratitis in a woman with a history of Intacs corneal implants. Eye Contact Lens. 2008;34:185–187. doi: 10.1097/ICL.0b013e3181506ad1. [DOI] [PubMed] [Google Scholar]
- 17.Tu EY, Joslin CE, Sugar J, Booton GC, Shoff ME, Fuerst PA. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea. 2008;27:764–772. doi: 10.1097/ICO.0b013e31816f27bf. [DOI] [PubMed] [Google Scholar]
- 18.Munir WM, El Mallah MK, Janda WM, Tu EY. Gemella haemolysans infectious crystalline keratopathy. Cornea. 2008;27:258. doi: 10.1097/ICO.0b013e31815b8535. author reply 258. [DOI] [PubMed] [Google Scholar]
- 19.Bu P, Riske PS, Zaya NE, Carey R, Bouchard CS. A comparison of topical chlorhexidine, ciprofloxacin, and fortified tobramycin/cefazolin in rabbit models of Staphylococcus and Pseudomonas keratitis. J Ocul Pharmacol Ther. 2007;23:213–220. doi: 10.1089/jop.2006.81. [DOI] [PubMed] [Google Scholar]
- 20.Fritsche TR, Horn M, Seyedirashti S, Gautom RK, Schleifer KH, Wagner M. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl Environ Microbiol. 1999;65:206–212. doi: 10.1128/aem.65.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snelling WJ, Moore JE, McKenna JP, Lecky DM, Dooley JS. Bacterial-protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect. 2006;8:578–587. doi: 10.1016/j.micinf.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Garcia MT, Jones S, Pelaz C, Millar RD, Abu Kwaik Y. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ Microbiol. 2007;9:1267–1277. doi: 10.1111/j.1462-2920.2007.01245.x. [DOI] [PubMed] [Google Scholar]
- 24.Thomas V, Herrera-Rimann K, Blanc DS, Greub G. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol. 2006;72:2428–2438. doi: 10.1128/AEM.72.4.2428-2438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritsche TR, Gautom RK, Seyedirashti S, Bergeron DL, Lindquist TD. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol. 1993;31:1122–1126. doi: 10.1128/jcm.31.5.1122-1126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huws SA, Smith AW, Enright MC, Wood PJ, Brown MR. Amoebae promote persistence of epidemic strains of MRSA. Environ Microbiol. 2006;8:1130–1133. doi: 10.1111/j.1462-2920.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 27.Alsam S, Jeong SR, Sissons J, Dudley R, Kim KS, Khan NA. Escherichia coli interactions with Acanthamoeba: a symbiosis with environmental and clinical implications. J Med Microbiol. 2006;55:689–694. doi: 10.1099/jmm.0.46497-0. [DOI] [PubMed] [Google Scholar]
- 28.Ly TM, Muller HE. Ingested Listeria monocytogenes survive and multiply in protozoa. J Med Microbiol. 1990;33:51–54. doi: 10.1099/00222615-33-1-51. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch D, Gray TB, Cursons R, Parr D. Acanthamoeba keratitis in New Zealand, including two cases with in vivo resistance to polyhexamethylene biguanide. Aust N Z J Ophthalmol. 1998;26:231–236. doi: 10.1111/j.1442-9071.1998.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 30.Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol. 2005;71:20–28. doi: 10.1128/AEM.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluxen G, Horauf A. Ocular onchocerciasis: a key role for Wolbachia. Ophthalmologe. 2007;104:860–865. doi: 10.1007/s00347-007-1594-x. [DOI] [PubMed] [Google Scholar]
- 32.Pearlman E, Gillette-Ferguson I. Onchocerca volvulus, Wolbachia and river blindness. Chem Immunol Allergy. 2007;92:254–265. doi: 10.1159/000099276. [DOI] [PubMed] [Google Scholar]
- 33.Saint Andre A, Blackwell NM, Hall LR, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 34.Hall LR, Pearlman E. Pathogenesis of onchocercal keratitis (River blindness) Clin Microbiol Rev. 1999;12:445–453. doi: 10.1128/cmr.12.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brattig NW, Buttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]