Abstract
Background
The Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial (BARI 2D) in 2,368 patients with stable ischemic heart disease assigned pre-randomization to percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) strata reported similar 5-year all cause mortality rates with insulin sensitization (IS) vs. insulin provision (IP) therapy and with a strategy of prompt initial coronary revascularization and intensive medical therapy (REV) or intensive medical therapy alone with REV reserved for clinical indication(s) (IMT). In this report, we examine the predefined secondary endpoints of cardiac death and myocardial infarction (MI).
Methods and Results
Outcome data were analyzed by intent to treat; the Kaplan Meier method was used to assess 5-year event rates. Nominal p-values are presented. During an average 5.3 year follow-up, there were 316 deaths (43% were attributed to cardiac causes) and 279 first MI events. Five-year cardiac mortality did not differ between REV (5.9%) and IMT (5.7%, p=0.38) or between IS (5.7%) and IP therapy (6%, p=0.76). In the CABG stratum (n=763), MI events were significantly less frequent in REV vs. IMT (10.0% vs 17.6%; p=0.003) and the composite endpoint of all cause death or MI (21.1% vs. 29.2%; p=0.010) and cardiac death or MI (p=0.03) were also less frequent; Reduction in MI (p=0.001) and cardiac death/MI (p=0.002) was significant only in the IS group.
Conclusions
In many patients with type 2 diabetes and stable ischemic coronary disease in whom angina symptoms are controlled similar to those enrolled in the PCI stratum, IMT alone should be the first-line strategy. In patients with more extensive coronary disease, similar to those enrolled in the CABG stratum, prompt CABG, in the absence of contraindications, IMT and an IS strategy appears to be a preferred therapeutic strategy to reduce the incidence of MI.
Keywords: Diabetes mellitus, Myocardial Infarction, Prognosis, Angioplasty, Bypass
INTRODUCTION
Patients with type 2 diabetes mellitus and coronary disease are at increased risk for death and myocardial infarction (MI) compared to nondiabetic patients (1–4). Interventions to reduce cardiovascular risk include intensive medical therapy to control atherosclerotic risk factors, prompt coronary revascularization, and optimizing glucose homeostasis through insulin sensitization or insulin provision therapies (5,6). In selected diabetic patients with stable ischemic coronary disease, revascularization with percutaneous coronary intervention has not been shown to reduce the risk of all-cause death as compared to IMT in the Bypass Angioplasty Revascularization Investigation in type 2 Diabetes (BARI 2D) trial after an average 5.3 years of follow-up (7). Similar results were reported by the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial after a median 4.6 year follow-up (8,9). A history of diabetes was present in 34% of patients enrolled in the COURAGE trial and the study was not designed to test an initial strategy of coronary artery bypass grafting.
The BARI 2D trial required that physicians determine which REV strategy was preferred for individual patients considered for the study and after the preferred type of REV was selected (either PCI or CABG), then patients were randomized to REV or IMT (10–12). The CABG population was different from the PCI population, having different anatomic characteristics and prognosis (7,11,12). The impact of an IS strategy compared to an IP strategy on cardiovascular outcomes was also tested using a 2×2 factorial design (6). The BARI 2D trial reported no significant differences in all-cause mortality, the primary endpoint of the trial, between the four main treatment strategies (7). However, nonfatal myocardial infarction (MI) events were significantly reduced in REV patients that were in the CABG stratum.
Cardiac mortality, myocardial infarction, and the composite of all cause mortality/myocardial infarction were prespecified secondary endpoints in BARI 2D (10). Comparison of treatment strategies designed to reduce cardiovascular morbidity and mortality require that cardiac endpoints be specifically analyzed as well as all cause mortality. The aim of this report is to examine whether an initial treatment strategy of REV vs. IMT significantly influenced the secondary outcomes of cardiac death, myocardial infarction, the composite endpoint of all-cause mortality/nonfatal MI, or the endpoint of cardiac death/MI. The impact of myocardial infarct events on subsequent cardiac mortality was also examined.
METHODS
Study Population and Treatment Strategies
A detailed description of the BARI 2D study design, protocol, clinical patient characteristics, and consort diagram have been published previously (5–7,10–13). Briefly, the BARI 2D is a large international trial testing two major strategies in a 2 × 2 factorial design: a.) an early vs. only if necessary revascularization strategy (REV vs. IMT); and b.) glycemic control strategy (IS vs. IP therapy to a target HbA1c of <7%). The trial randomized 2,368 patients with angiographically defined coronary artery disease (CAD), between January 1, 2001 and March 31, 2005 (7,13). The choice of REV (PCI or CABG) was determined after coronary angiography by the treating physician, and randomization was stratified by the type of intended revascularization procedure. Patients selected for CABG had more extensive disease, total occlusions, proximal left coronary disease, and greater myocardial jeopardy score than those selected by their physician for PCI (11). After randomization, all patients were treated according to current guidelines for lipid and blood pressure management, smoking cessation, physical activity, and weight loss (6,7,10). Medication usage and achievement of risk factor targeted therapeutic goals were measured at prespecified intervals during follow-up and treatment optimized (7).The baseline characteristics of the study population were similar across the 4 treatment strategies (7,12,13). Their average age was 62.4 yrs and 29.6% were women. The average duration of type 2 diabetes was 10.4 years. Patients were excluded if they required immediate revascularization or had revascularization within the prior 12 months, had left main coronary disease, class III or IV heart failure, a creatinine >2mg/dL, a glycated HbA1c >13%, or significant hepatic dysfunction. The protocol was approved by the institutional review board at the University of Pittsburgh and at each participating site. All patients provided written informed consent. The trial was supported by the National Institutes of Health, with additional support from industry.
Outcome Evaluation
Myocardial Infarction
Reported myocardial ischemic events requiring hospitalizationwere classified at a Core Electrocardiography and Myocardial Infarction Classification Laboratory in St Louis (7,10). The adjudication was based on source documents such as the emergency room records, admission history and physical, electrocardiograms and biomarkers associated with the admission, discharge summary, and records of revascularization procedures when available. The BARI 2D criteria for MI were modified from the Universal MI definition criteria by requiring that an abnormal biomarker profile exceed at least twice the upper limits of normal for the local laboratory (14). When cardiac troponin and CKMB were simultaneously acquired, cardiac troponin took precedence over CKMB in establishing the diagnosis. MI was confirmed if abnormal cardiac biomarkers occurred and there was evidence of angina or angina equivalent symptoms, ECG or imaging evidence of new myocardial ischemia, or autopsy evidence of recent MI.
All ECGs were interpreted at the Core Laboratory using the Minnesota code with an adaptation of the Novacode for serial ECG comparison (15,16). A Q-wave MI required the development of new pathologic Q waves (15) or the new occurrence of a left bundle branch block in addition to abnormal biomarkers. Non Q-wave MI required the above MI criteria without new pathologic Q waves. Fatal MI’s were defined as death occurring within 30 days after the event with a causal relationship to the death.
Cardiac biomarkers were not routinely collected within 96 hours of the index coronary revascularization procedure in patients assigned to the revascularization strategy. When biomarkers were collected, a 3 fold elevation in CKMB following a PCI procedure and a 10 fold increase in CKMB following CABG were used as the cut-points to define periprocedural myocardial necrosis. The Universal MI definition subcategorizes MI into spontaneous (type 1) or PCI or CABG related (types 4 or 5) events (14). In this report, PCI or CABG related MI’s are defined as occurring in the periprocedure phase or within 30 days of the procedure.
Cardiac Mortality
Cause of death was classified by an independent Mortality and Morbidity Classification Committee as cardiac (direct or contributory), noncardiac but related to atherosclerotic disease, noncardiac medical cause (e.g. cancer, pulmonary disease) trauma, suicide, accident, other, or unknown (Table S-1). Cardiac death was defined as death within 1 hour to 30 days after a documented or probable MI, death from intractable congestive heart failure or cardiogenic shock, or other documented cardiac cause. Sudden cardiac death was defined as occurring instantaneously or within 60 minutes after the onset of cardiac symptoms. Cardiac death was considered contributory if cardiac dysfunction contributed to the death but it was unclear if it was the direct cause of death. Classification was based on the report from the clinical center’s principal investigator; death certificate; surgical and catheterization laboratory reports within the 30 days; ECG’s and biomarker data within 24 hours; patients baseline, procedural, and hospital study data; and if available, a coroner’s report. Each case was reviewed by two classification committee members. Disagreements were resolved by consensus of the full committee.
Statistical Analysis
As of November 30, 2008, the vital status was known for 2,283 patients (96%). The mean follow-up interval was 5.3 years (range, 3.4–7.8). The data were analyzed by intention to treat, and Kaplan Meier analyses were used to assess 5-year cumulative event rates for all cause death, cardiac death, MI, and the composite end-point of cardiac death or myocardial infarction. MI rates include fatal and nonfatal events. The MI endpoint was censored at the last clinic follow-up visit, and the mortality endpoint was censored at the last patient contact. Noncardiac deaths were censored at time of death in the analysis of cardiac mortality. Kaplan-Meier estimates of event rate distributions were compared using the log-rank test. Cox regression analyses with a time-dependent variable to indicate presence of a follow-up MI were used to determine the hazard ratio (HR) between the randomization arms for death or cardiac death in the setting of an MI. An interaction term was added to the models to test the equality between the HR after the occurrence of an MI and the HR when no MI was suffered. If no MI event occurred, deaths were censored at the last known MI free time. Cox regression was also used to estimate the HR for REV vs. IMT in each of the glycemic treatment arms.
A p-value of 0.05 was used to determine statistical significance. Nominal p-values are presented. The tables also indicate significance after Bonferroni adjustment for multiple testing. Analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA.) and figures were created using R 2.8.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
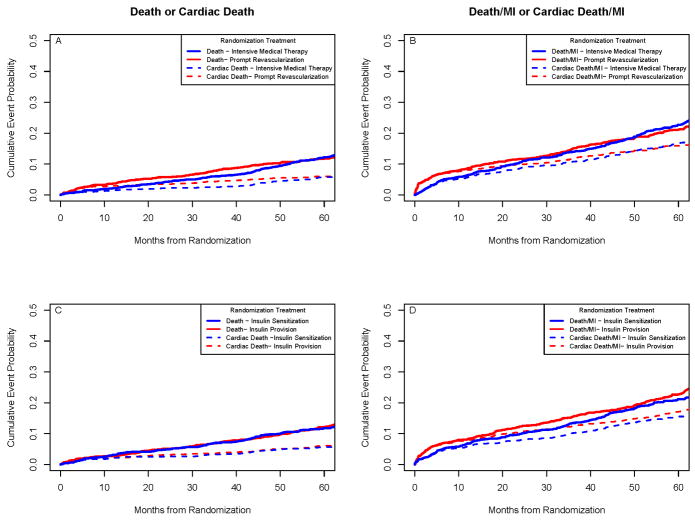
The 5-year Kaplan-Meier all-cause mortality rates did not differ significantly between REV (11.7%) and IMT (12.2%, p=0.97) or between IS (11.8%) and IP strategies (12.1%, p=0.89) as previously reported (7) (Table 1). Of the 316 deaths in BARI 2D, 136 (43%) were attributed to cardiac causes and 94 of these (69.1%) were sudden deaths. The 5-year cardiac mortality did not differ significantly between REV (5.9%) and IMT (5.7%, p=0.38) or between IS (5.7%) and IP therapy (6%, p=0.76). Similar results were observed for the endpoint of death or MI, and for the endpoint of cardiac death or MI (Table 1) (Figure 1).
Table 1.
Five Year Kaplan-Meier Estimates for Death and the Composite Endpoints of Death or Myocardial Infarction, Cardiac Death or Myocardial Infarction, According to Initial Treatment Strategy, and According to PCI and CABG Stratum
| Death |
MI |
Composite |
|||||
|---|---|---|---|---|---|---|---|
| 5 year KM estimate(n) | All Cause Death | Cardiac Death† | Sudden Cardiac Death† | MI†† | Death or MI | Cardiac Death or MI | |
| All Patients | 2368 | ||||||
| Prompt Coronary Revascularization | 1176 | 11.7% (155) | 5.9% (72) | 4.0% (48) | 11.5% (128) | 21.1% (247) | 15.9% (178) |
| Intensive Medical Therapy | 1192 | 12.2% (161) | 5.7% (64) | 4.2% (46) | 14.3% (151) | 22.7% (266) | 16.7% (179) |
| Nominal P-Value | 0.97 | 0.38 | 0.72 | 0.27 | 0.61 | 0.78 | |
| PCI Stratum | 1605 | ||||||
| Prompt Coronary Revascularization | 798 | 10.8% (102) | 5.0% (44) | 3.8% (34) | 12.3% (95) | 21.1% (173) | 16.0% (126) |
| Intensive Medical Therapy | 807 | 10.2% (96) | 4.2% (33) | 3.4% (26) | 12.6% (88) | 19.6% (157) | 14.2% (101) |
| Nominal P-Value | 0.48 | 0.16 | 0.25 | 0.42 | 0.19 | 0.045 | |
| CABG Stratum | 763 | ||||||
| Prompt Coronary Revascularization | 378 | 13.6% (53) | 8.0% (28) | 4.3% (14) | 10.0% (33) | 21.1% (74) | 15.8% (52) |
| Intensive Medical Therapy | 385 | 16.4% (65) | 9.0% (31) | 6.0% (20) | 17.6% (63) | 29.2% (109) | 21.9% (78) |
| Nominal P-Value | 0.33 | 0.79 | 0.35 | 0.003** | 0.010* | 0.03* | |
| Insulin Provision Therapy | 1185 | 12.1% (160) | 6.0% (70) | 4.2% (47) | 13.6% (149) | 22.7% (265) | 17.1% (189) |
| Insulin Sensitization Therapy | 1183 | 11.8% (156) | 5.7% (66) | 4.0% (47) | 12.2% (130) | 21.2% (248) | 15.6% (168) |
| Nominal P-value | 0.89 | 0.76 | 0.97 | 0.21 | 0.29 | 0.21 | |
Cause of Death determined by independent Mortality and Morbidity committee;
Includes documented MI’s from the Core ECG lab and/or Fatal MI’s as determined by death classification
significant at nominal level
significant after Bonferroni adjustment for multiple tests done in the table
Figure 1.
Rates of All-Cause Death or Cardiac Death and of Death/MI or Cardiac Death/MI. There were no significant differences in the rates of death or cardiac death between the revascularization group and the medical-therapy group (Top Left Panel) or in the rates of the composite endpoint of all-cause death/MI or cardiac death/MI (Top Right Panel). Similar results were seen between the insulin-sensitization group and the insulin-provision group (Bottom Panels).
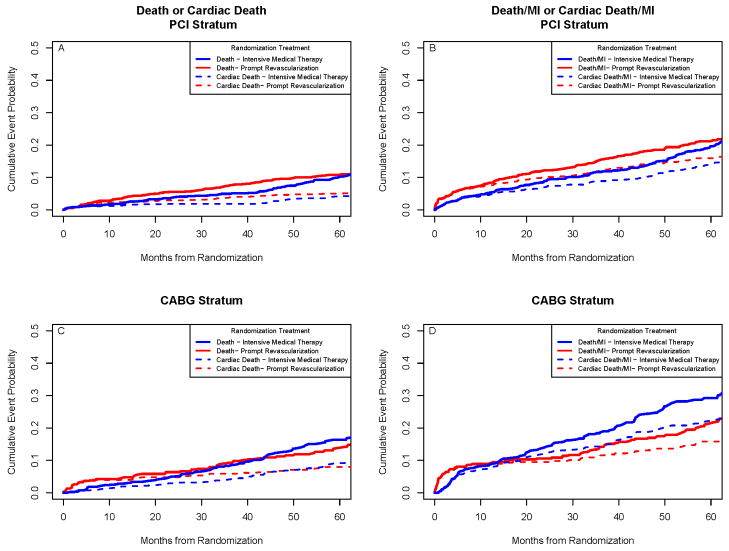
Examining the 1,605 patients in the PCI stratum, there were no significant differences in the rates of cardiac death or MI between the treatment groups. Examining the 763 patients in the CABG stratum, MI events were significantly less frequent in the REV group compared to IMT (10% vs. 17.6%; p=0.003). The rate for the composite endpoint of all cause death or MI was also significantly less in the REV CABG stratum group (21.1% vs. 29.2%; p=0.010) and the composite endpoint of cardiac death or MI was also less frequent (p=0.03) (Figure 2). When procedure-related MI events were not counted, the difference in the composite endpoint of cardiac death or MI was 13.7% vs. 21.4% (p<0.01) (Table S-2). The hazard ratio of cardiac death for REV/IMT in the CABG stratum was significantly different (p=0.004) in the first 6 months after randomization (4.48; Kaplan-Meier rates 3.4%/0.8%) compared to >6 months to the end of follow-up (0.55; 4.1%/7.5%). The data suggest that once the risk of CABG surgery is accounted for, there is a reduction in the subsequent hazard of cardiac death. This difference was not seen in the PCI stratum. The hazard ratio for cardiac death for REV/IMT in the PCI stratum was not significantly different (p =0.70) in the first 6 months after randomization (1.58; Kaplan-Meier rates 1.8%/1.1%) compared to > 6 months to the end of follow-up (1.30; 4.8%/3.0%).
Figure 2.
Rates of All-Cause Death or Cardiac Death and of Death/MI or Cardiac Death/MI, According to PCI and CABG Strata. There were no significant differences in the rates of all-cause death or cardiac death between the revascularization group and the medical-therapy group among patients who were selected for the percutaneous coronary intervention (PCI) stratum (Top Left Panel) or in the rates of the composite endpoint of all-cause death/MI or cardiac death/MI (Top Right Panel). Among patients who were selected for the coronary artery bypass strategy (CABG) stratum, the rates for all-cause or cardiac mortality were not significantly different (Lower Left Panel), but the rates for all-cause death free of MI (p=0.009) or cardiac death free of MI (p=0.03) were significantly better than in the medical-therapy group (Lower Right Panel).
Myocardial Infarction
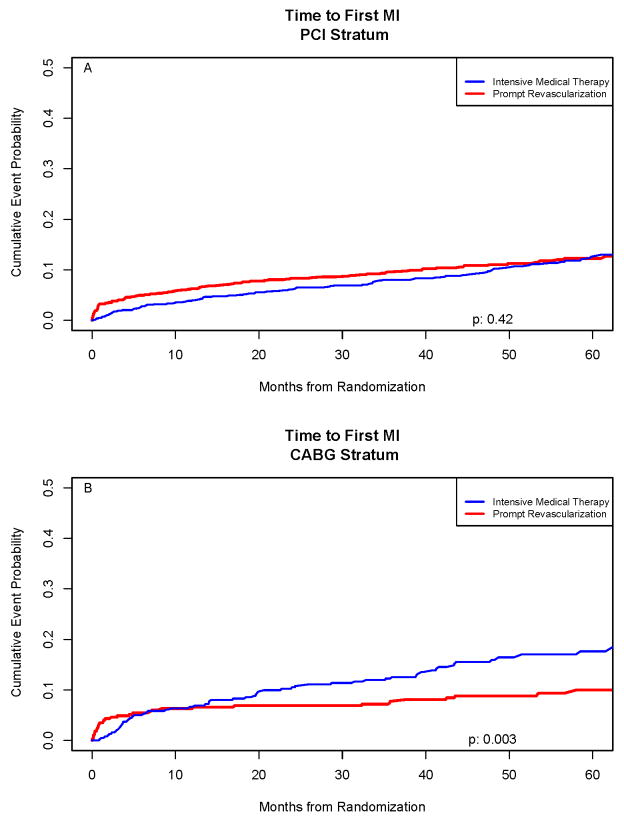
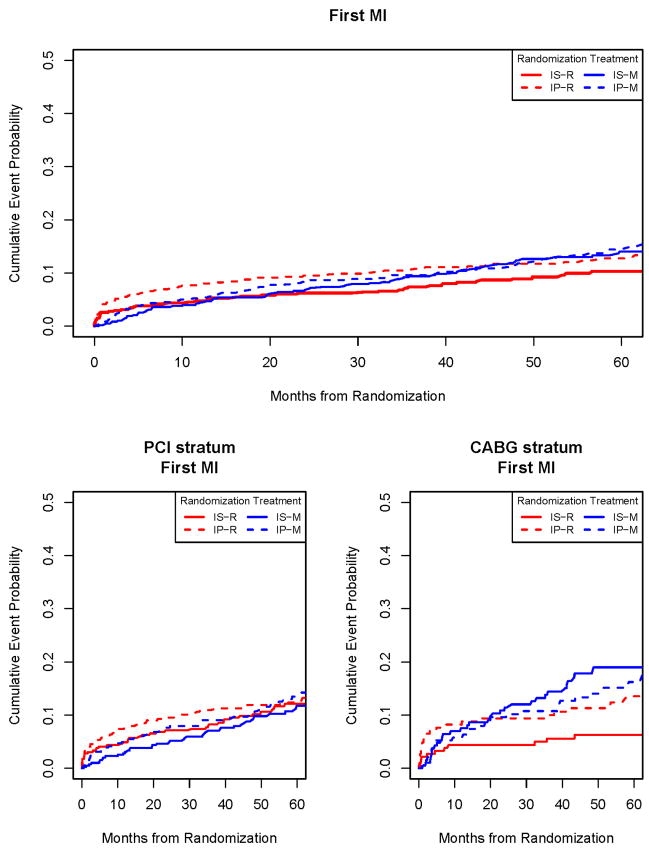
Of the 2,368 BARI 2D patients, 279 were diagnosed with a first post randomization MI (Figure 3). In the PCI stratum, 5-year MI rates were 12.3% and 12.6%; p=0.42 for PCI vs. IMT. The rates were 9.4% vs. 11.4% (p=0.69) when procedure related MI events were not counted (Table S-2). In the CABG stratum, 5-year MI rates were 10% vs. 17.6% (p=0.003) for CABG vs. IMT; the rates were 5.9% and 14.8% (p<0.001) when procedure related MI events were not counted. There were no overall differences in the rates of MI between IS and IP therapies (Table 1).
Figure 3.
Time to First Myocardial Infarction (MI) in the PCI Stratum (Top Panel) and in the CABG Stratum (lower Panel). The incremental risk of myocardial infarction was continuous over time in the Intensive Medical Therapy Groups. In the CABG stratum, the difference in myocardial infarction rates between initial REV with CABG compared to an initial Intensive Medical Therapy strategy was significant (p=0.003).
The hazard ratio of MI for REV/IMT in the CABG stratum was significantly different (p=0.003) in the first 6 months after randomization (1.13; Kaplan-Meier rates 5.5%/5.0%) compared to >6 months to the end of follow-up (0.29; 3.9%/12.5%). A trend was seen in the HR of MI in the PCI stratum (p=0.03); (1.81, 5.0%/2.8%) in the first 6 months after randomization and (0.90, 7.7%/8.8%) > 6 months to end of follow-up.
At 5 years, 452/1192 (KM estimate, 42.1%) of patients in the IMT group (315/807 (KM estimate, 43.3%) in the PCI stratum and 137/385 (KM estimate, 39.7%) in the CABG stratum) had crossed over and undergone clinically indicated revascularization. Of the 452 patients in the IMT group that crossed-over to REV, the procedure was precipitated by an MI event in 50 patients.
Impact of MI Events on Cardiac Mortality
In the overall BARI 2D cohort, the risk of death during the entire follow-up was 5.2 times higher following an MI compared to those with no MI. Thirty six (12.9%) of the 279 first MI events were fatal. Among the remaining 243 non-fatal first MI events, 169 (69.5%) and 23 (9.4%) were type I symptomatic and silent MI events; 51 (21.0%) were procedure related MI events. The 3-year cardiac mortality rates were 16.1% (HR 8.2; p=<0.001) and 10.7% (HR 4.8; p=0.03) for type 1 symptomatic and silent MI events, and 9.6% (HR 3.4; p=0.008) for procedure related MI events. The silent Q-wave MI events were detected during protocol ECG follow-up. The 3-year cardiac mortality rate for patients without a myocardial infarction during follow-up was 2.4%.
The 30-day cardiac mortality rate after an MI was 12.5% for patients initially assigned to REV versus 14.1% initially assigned to IMT. When these events were analyzed by the intended method of revascularization, the 30-day cardiac death rates were 9.5% and 11.6% for PCI vs. IMT and 21.2% and 17.5% for CABG vs. IMT. The difference in the 30-day cardiac mortality after MI was marginally greater after CABG (p = 0.09). There were no significant long-term differences in the risk of all-cause death after an MI between the patients in the REV and IMT groups (Table 2). However, for cardiac death, the relative risk for REV versus IMT was 0.58 (p=0.048) after an MI compared to RR=1.95 (p=0.006) for patients who did not suffer an MI, and these relative risks differed significantly from each other (p <0.001). In the PCI stratum, the risk of death and cardiac death after an MI was lower among those assigned to REV compared with those assigned to IMT; in contrast, in the CABG stratum, there was no significant treatment difference for cardiac mortality rates after MI.
Table 2.
Hazard Ratio of Death or Cardiac Death by Post Randomization MI Status
| Number of Deaths† | HR of Death† (95% CI) | Nominal p-value for equality between HR | Number of Cardiac Deaths† | HR of Cardiac Death* (95% CI) | Nominal p-value for equality between HR | |||
|---|---|---|---|---|---|---|---|---|
| Rev | IMT | Rev/IMT | Rev | IMT | Rev/IMT | |||
| All Patients(n = 2368) | ||||||||
| MI | 36 | 46 | 0.82 (0.53–1.27) | 0.22 | 22 | 36 | 0.58* (0.34–0.99) | <0.001** |
| No MI | 101 | 92 | 1.13 (0.86–1.50) | 49 | 26 | 1.95* (1.21–3.14) | ||
| PCI Stratum(n = 1605) | ||||||||
| MI | 24 | 27 | 0.65 (0.37–1.13) | 0.033* | 13 | 20 | 0.44* (0.22–0.90) | <0.001** |
| No MI | 67 | 53 | 1.33 (0.93–1.91) | 31 | 13 | 2.49* (1.30–4.75) | ||
| CABG Stratum (n = 763) | ||||||||
| MI | 12 | 19 | 1.3 (0.63–2.71) | 0.35 | 9 | 16 | 0.95 (0.41–2.17) | 0.48 |
| No MI | 34 | 39 | 0.86 (0.55–1.37) | 18 | 13 | 1.41 (0.69–2.87) | ||
| IP | IS | IP/IS | IP | IS | IP/IS | |||
| All Patients (n = 2368) | ||||||||
| MI | 44 | 38 | 0.87 (0.57–1.35) | 0.54 | 30 | 28 | 0.79 (0.47–1.32) | 0.34 |
| No MI | 97 | 96 | 1.03 (0.77–1.36) | 39 | 36 | 1.10 (0.70–1.73) | ||
censored at last known MI follow-up
significant at nominal level
significant after Bonferroni adjustment for multiple tests done in the table
CABG = coronary artery bypass grafting; IP/IS = insulin provision/insulin sensitization; MI = myocardial infarction; PCI = percutaneous coronary intervention; Rev/Med = revascularization/medicine
Patients in the CABG stratum had more atherosclerotic lesions than in the PCI stratum (mean number of lesions > 20% (5.6 vs. 4.3)). Analysis of 15 baseline angiographic descriptors analyzed by the core angiographic laboratory identified a greater number of atherosclerotic lesions (p<0.001) and greater myocardial jeopardy score (p<0.001) as predictors of MI in the IMT group. In the REV-PCI strata, number of atherosclerotic lesions at baseline was a significant predictor of MI (p<0.001) whereas no significant angiographic descriptor differences were found in the REV-CABG stratum.
Interaction of the Four Treatment Strategies on Cardiac Death and MI Endpoints
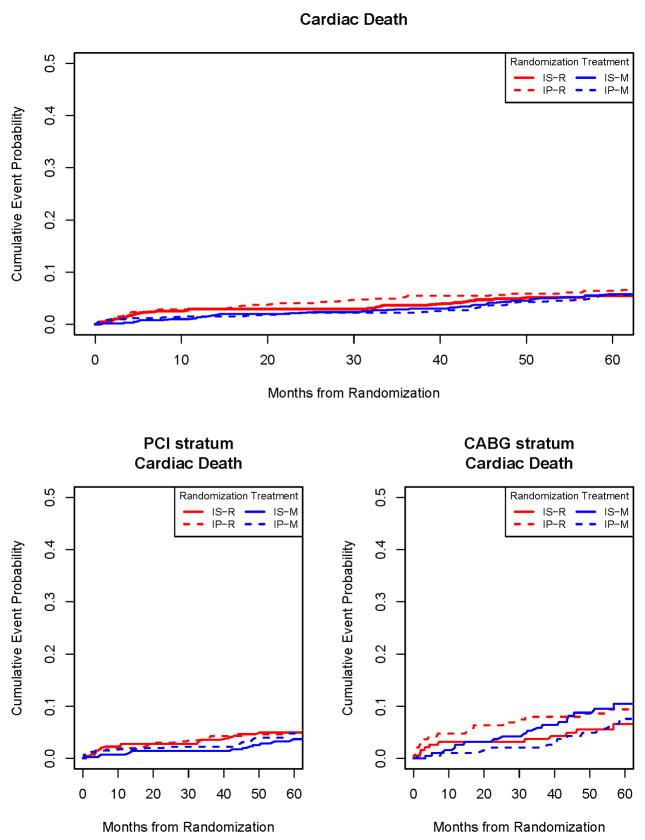
The hazard ratios of REV/IMT for MI, cardiac death, and the composite of cardiac death/MI were not significantly different in the total population. This was also true in the PCI stratum. However, when patients in the CABG stratum were examined, the hazard ratio of MI for REV/IMT was significantly different (p=0.001) in the IS group (0.32; Kaplan-Meier rates 6.3%/19%) whereas the difference was not significant in the IP group (Table 3; Figures 4,5). The p-value for the overall four-way comparison among the mutually exclusive treatment groups for first myocardial infarction in the CABG stratum was p=0.007. The p-value for the difference in the revascularization group with insulin sensitization as compared to insulin provision therapy was p=0.046. The hazard ratio of cardiac death was 0.62 for REV/IMT (p=0.21) in the IS group and 1.4 in the IP group (p=0.39).
Table 3.
Hazard Ratio (HR) of Rev/IMT for MI, Cardiac Death, and Cardiac Death or MI within Glycemic Randomization Arm
| MI |
Cardiac Death |
Cardiac Death/MI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 Year KM Estimate | HR Rev/IMT (95% CI) | Nominal p-value for HR | 5 Year KM Estimate | HR Rev/IMT (95% CI) | Nominal p-value for HR | 5 Year KM Estimate | HR Rev/IMT (95% CI) | Nominal p-value for HR | |
| All Patients (n = 2368) | |||||||||
| Insulin Sensitization | |||||||||
| Rev(n= 584) | 10.3% (56) | 0.78 (0.55–1.10) | 0.16 | 5.5% (34) | 1.11 (0.68–1.80) | 0.68 | 14.2% (79) | 0.92 (0.68–1.24) | 0.57 |
| IMT(n=599) | 14.1% (74) | 5.8% (32) | 16.9% (89) | ||||||
| Insulin Provision | |||||||||
| Rev(n= 592) | 12.7% (72) | 0.97 (0.70–1.34) | 0.85 | 6.4% (38) | 1.21 (0.76–1.94) | 0.42 | 17.6% (99) | 1.15 (0.86–1.52) | 0.35 |
| IMT(n=593) | 14.4% (77) | 5.7% (32) | 16.5% (90) | ||||||
| PCI stratum (n=1605) | |||||||||
| Insulin Sensitization | |||||||||
| Rev(n=396) | 12.1% (45) | 1.18 (0.77–1.80) | 0.45 | 5.0% (23) | 1.74 (0.89–3.38) | 0.10 | 15.7% (61) | 1.39 (0.95–2.03) | 0.09 |
| IMT(n=408) | 11.7% (41) | 3.7% (14) | 13.3% (47) | ||||||
| Insulin Provision | |||||||||
| Rev(n= 402) | 12.4% (50) | 1.08 (0.73–1.62) | 0.69 | 5.0% (21) | 1.12 (0.60–2.07) | 0.73 | 16.3% (65) | 1.23 (0.86–1.76) | 0.26 |
| IMT(n=399) | 13.5% (47) | 4.8% (19) | 15.2% (54) | ||||||
| CABG stratum (n =763) | |||||||||
| Insulin Sensitization | |||||||||
| Rev(n=188) | 6.3% (11) | 0.32 (0.16–0.64) | 0.001** | 6.6% (11) | 0.62 (0.29–1.32) | 0.21 | 11.0% (18) | 0.41 (0.24–0.72) | 0.002** |
| IMT(n=191) | 19.0% (33) | 10.5% (18) | 24.6%(42) | ||||||
| Insulin Provision | |||||||||
| Rev(n= 190) | 13.5% (22) | 0.79 (0.45–1.37) | 0.40 | 9.4% (17) | 1.37 (0.67–2.83) | 0.39 | 20.5% (34) | 1.03 (0.64–1.64) | 0.91 |
| IMT(n=194) | 16.2% (30) | 7.6% (13) | 19.3% (36) | ||||||
CABG = coronary artery bypass grafting; MI = myocardial infarction; PCI = percutaneous coronary intervention; Rev/Med = revascularization/medicine;
Kaplan-Meier values are expressed as percentage (n). CI indicates confidence interal.
significant after Bonferroni adjustment for multiple tests done in the table
Figure 4.
Time to cardiac death according to the 4 initial treatment strategies (top panel) and in the PCI stratum (lower left panel) or CABG stratum (lower right panel). IS–R indicates revascularization IS group; IP–R, revascularization IP group; IS–M, IMT IS group; and IP–M, IMT IP group.
Figure 5.
Time to First Myocardial Infarction (MI) (Top Panel) according to the initial treatment strategy and in the PCI stratum (lower left panel) and CABG Stratum (lower right Panel). A marked reduction in MI incidence is seen in the IS-R treatment group. The relative risk of MI for REV/IMT was significantly different (p=0.001) in the IS group whereas the difference was not significant in the IP group. IS–R indicates revascularization IS group; IP–R, revascularization IP group; IS–M, IMT IS group; and IP–M, IMT IP group.
DISCUSSION
The BARI 2D trial reported no significant differences in the primary endpoint of all-cause mortality or in the principal secondary end point of all-cause death/MI/stroke between patients with type 2 diabetes undergoing REV and those undergoing IMT or between strategies of IS and IP. However, in the CABG stratum, a significant reduction in major cardiovascular events (all-cause mortality/MI/stroke) was seen with CABG compared to IMT, largely due to a reduction in MI in patients within the IS strategy (7). In our original report, MI rates included only nonfatal MI events; in this paper, the MI rates reported include both fatal and nonfatal MI events
In BARI 2D, 43% of the total mortality was attributed to cardiac causes over the average 5.3 year follow-up. Cardiovascular deaths accounted for 37–53% of all-cause mortality in the Action to Control Cardiovascular Risk in Diabetes Study Group (ACCORD), Veterans Affair Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) T2D, and Randomized Intervention Treatment of Angina (RITA-2) trials (17–19, 20). BARI 2D differs from the other diabetes trials in several respects, in particular, by the protocol requirement that all patients needed to have angiographic documented coronary disease suitable for coronary revascularization (32% severe enough to be considered for CABG). The cardiac mortality in BARI 2D was much lower than originally anticipated considering the fact that all patients had documented coronary disease, and that other reports of type 2 diabetes estimate cardiac deaths to be 60–75% of all cause death (1–4). We believe that the lower cardiac death rates in BARI 2D are a consequence of IMT that included frequent visits with aggressive implementation of IMT in all patients regardless of choice of REV procedure or randomization to IMT or REV. In BARI 2D at 3-year follow-up as compared to baseline, the average reduction in LDL cholesterol was −16 mg/dL, triglycerides −20mg/dL, systolic blood pressure −6mm Hg, and HbA1c −0.45%. The percent of patients that smoked in the prior year was reduced from 22% to 11% after randomization with smoking cessation counseling.
REV as compared to IMT did not affect all-cause death rates (7) but comparison of treatment effects on prevention of subsequent cardiovascular events such as cardiac death or MI (fatal and nonfatal) are diluted by noncardiac causes of death such as suicide, trauma, sepsis, etc. that would not be affected by the therapy received, and thus confound the interpretation of potential therapeutic benefit. Therefore, the BARI 2D protocol pre-specified that the secondary endpoints of cardiac death, myocardial infarction, and all cause death or MI be examined to determine if there were significant treatment effects. In this report, we present nominal p-values. This should be taken into account when interpreting the results. A number of tests have retained significance after Bonferroni adjustment for multiple comparisons; this is noted in the tables. An important aspect of the BARI 2D trial design was that the REV arm was stratified according to the intended type of revascularization procedure (5). Thus, as expected, CABG patients as compared to those selected for PCI, on average, had more three vessel disease (52% vs. 20%; p<0.001), more total occlusions (61% vs. 32%; p<0.001), more proximal left anterior descending stenosis ≥ 50% (19% vs. 10%; p<0.001) as well as a greater number of nonobstructive and obstructive atherosclerotic, and class C lesions (p<0.001), and as shown in this report, an increased cardiac death rate. We did not observe a treatment benefit, in terms of prevention of all cause death, cardiac death, MI, or the composite endpoint of cardiac death or MI in the PCI stratum. Our data from patients with stable ischemic coronary disease are consistent with the findings from the COURAGE, RITA-2 and other randomized trials showing no survival benefit with prompt PCI compared to state-of-the-art initial IMT with REV reserved for worsening symptoms or clinical indications after ~5 years of follow-up (8,20,21).
This was not the case in the patients with more severe and extensive coronary disease in the CABG stratum. As compared to IMT patients in the CABG stratum, there were significantly fewer MI events during follow-up in the CABG group and a significant reduction in the composite endpoint of all-cause death or MI (driven by the MI endpoint) and the cardiac death or MI endpoint in which both components contributed to the reduction in event rates. MI events were associated with a significant increased risk of subsequent cardiac death regardless of MI type, and regardless of initial treatment or REV stratum as compared to patients without an MI during follow-up. One possible explanation as to why patients in the CABG stratum had fewer MI’s during follow-up, as compared to the IMT group and in contrast to patients in the PCI stratum may be that patients in the CABG stratum had more severe and extensive coronary artery disease at baseline (12). The initial selection process of patients for the CABG stratum resulted in a population of patients with a much greater atherosclerotic burden and more lesions than in the PCI strata, and the rates of subsequent MI and cardiac death in the IMT group were greater in the CABG stratum than in the PCI stratum. Increased number of atherosclerotic lesions at baseline was associated with increased MI events during follow-up. The more comprehensive revascularization required for patients in the CABG stratum resulted in more vessels bypassed (average of 3.0±1.0 compared to 1.5± 0.8 for PCI treated patients) (7) thus offering greater protection against plaque rupture (if it occurred proximal to a patent graft insertion) compared to the IMT patients in the CABG stratum. Furthermore, the difference in MI rates was dramatically affected by the type of initially assigned glycemic strategy. IP as compared to IS in the CABG strata was associated with a higher rate of MI whereas initial IS and CABG therapy significantly reduced MI rates as compared to the IS and IMT group. A reduction in MI rates with IS therapy has been reported previously in observational studies (22,23). It is not clear why IS was specifically beneficial in those receiving CABG but not in those receiving PCI, a question that remains to be determined. One factor that was entertained in the design and implementation of the BARI 2D trial was the development of insulin-sensitizing drugs in part because they appeared to retard vasculopathy and the evolution of atherosclerotic plaques vulnerable to rupture (24–28). One mechanism implicated in conferring such benefits was the impact of these drugs in attenuating the increased expression of plasminogen activator inhibitor type-1 (PAI-1) (28) in blood and vessel walls associated with insulin resistance thereby potentially enhancing endogenous thrombolysis and facilitating formation of biologically stable atherosclerotic plaques as opposed to plaques vulnerable to rupture (29). In view of these observations, results in the present study that show a significant reduction of MI in those receiving CABG are particularly cogent, and consistent with laboratory observations demonstrating vascular protective effects of insulin sensitizers in the context of insulin resistance. Although we did not observe a significant difference in cardiac death rates in patients initially assigned to IMT or to CABG, prevention of MI events is an important goal of REV therapy to reduce morbidity, and potentially reduce long term cardiac death. The Coronary Drug project enrolled 8,341 men <65 years old with a prior MI and reported a significant 17% reduction in MI events after 5 years of follow-up without a mortality difference between treatments (30). It was only 9 years after termination of the trial that a significant mortality difference was observed with treatment (niacin) as compared to placebo. Differential treatment trends in outcome that become statistically significant only after a more prolonged follow-up are well known and have been reported with trials in type 2 diabetes as well as trials comparing REV to medical therapy (31–33) The average duration of follow-up in the patients that had a nonfatal MI in BARI 2D was 3.2 years, a relatively limited amount of time for nonfatal MI events to increase mortality risk that could significantly impact longer-term treatment differences in cardiac death rates. In the BARI 2D CABG stratum, REV was associated with a small nonsignificant reduction in total mortality and cardiac mortality. The data support the notion that evaluation of treatment strategies in chronic disease states such as diabetes and associated coronary disease may require more than 5 years to determine the impact of non-fatal events known to reduce long-term survival, such as MI.
In the PCI stratum, we did not observe a significant difference in the rate of MI or cardiac mortality compared to IMT although the relative risk of later cardiac death after sustaining a nonfatal MI was significantly less in patients in the PCI group compared to IMT alone. This difference was not seen in the CABG stratum even though MI events were reduced. Insertion of bypass conduits distal to significant obstructive disease in the native circulation can result in competitive flow and may result in proximal occlusion of the bypassed vessel (34). If graft occlusion later developed in this type of graft-dependent patient with more extensive and diffuse disease at baseline, the resultant MI may be larger and of greater consequence over time if the area of myocardium at risk were substantial.
The increased 30 day cardiac mortality rates seen after MI in the CABG as compared to the PCI group is explained, in part, by the fact that the selection process for the intended revascularization at baseline resulted in the CABG strata being at greater prognostic risk to start with as demonstrated by increased 5.3 year cardiac mortality rates in the CABG-IMT group (9%) as compared to the PCI-IMT group (4.2%). Other explanations that might explain this observation include the fact that the upper reference limit to define periprocedure-related MI after CABG was 10 times as compared to 3 times the upper limits of normal after PCI, thus restricting the diagnosis to larger MI events in the CABG as compared to the PCI group where a lower biomarker threshold was used.
We did not observe any significant treatment differences overall between IS and IP therapy in prevention of death, cardiac death, death or MI, and cardiac death or MI. However, because BARI 2D was a 2×2 factorial design, we were able to examine interactions between the 4 treatment strategies. In the CABG stratum, the relative risk of MI for REV/IMT was significant (p=0.001) in the IS group but not in the IP group and the interaction term was significant. The explanation for this observation requires additional research. The glycemic strategies used in BARI 2D tested IS vs. IP therapy, and within each strategy, various doses and combinations of drugs were used. Since, the benefit in terms of MI reduction in the CABG stratum was only seen in patients initially assigned to the IS therapy, and IS therapy is cardioprotective against late MI events in patients with more severe and extensive coronary disease referred for CABG, this should be considered as the initial therapeutic approach in this type of patient when there is no clinical contraindication(s).
Limitations
The BARI 2D trial design did not require collection of cardiac biomarkers immediately following the index revascularization procedure. Abnormal cardiac troponin or CK-MB elevations are common after a PCI or CABG procedure and the greater the magnitude of biomarker release, the greater the increase in mortality (35–39). Most patients do not have a marked increase in these biomarkers, and therefore, the impact of these smaller procedural MI events on the treatment outcome mortality comparisons should be relatively small over the follow-up period in this study. In BARI 2D, total and cardiac death rates were similar between treatment strategies after 5.3 years of follow-up, although there was a nonsignificant reduction in total and cardiac mortality in the CABG stratum and MI events were significantly reduced as compared to IMT alone.
The low cardiac mortality in patients with type 2 diabetes and anatomically defined coronary disease in BARI 2D, and other recently published trials in which coronary anatomy definition was not a prerequisite to enrollment, is less than had been expected based on natural history studies in this population where the majority of deaths are cardiovascular (1–4, 40). The reasons for this finding might be the beneficial results of IMT, or the duration of follow-up in the natural history studies, which allows a longer exposure time for cardiovascular events to occur and is longer than the average 5.3 year follow-up in BARI 2D. Fewer cardiac deaths in BARI 2D would result in less power to detect differences between REV and IMT and a longer duration of follow-up might be required to demonstrate longer term treatment differences. BARI 2D patients had their coronary anatomy defined prior to randomization, and a preselection entry bias that excluded patients with even more severe and extensive CAD than those enrolled in BARI 2D cannot be excluded. The number of patients lost to follow-up was small (4%), and not significantly different between treatment strategies.
The BARI 2D has several design advantages; it allowed clinician to assess patient revascularization needs and choose PCI or CABG accordingly, thus, simulating real practice scenarios and challenges. By the nature of this choice, it permitted recruitment of a wider spectrum of patients with coronary disease, allowing for a broader application of our results to patients with type 2 diabetes and coronary artery disease. In the glycemic trial strategy, clinicians were allowed to maximize IS and IP therapy drugs, as in clinical practice, if the HbA1c could not be satisfactorily lowered with drugs in the randomly assigned therapy group alone.
CONCLUSIONS
Our data show that for many patients with type 2 diabetes mellitus and stable but less severe and extensive CAD, intensive medical therapy is an excellent first-line strategy, and does not require immediate PCI to prevent cardiac death or myocardial infarction Revascularization can be applied later if drug therapy does not adequately control symptoms without incurring an increased risk of MI or cardiac death by waiting until symptoms worsen. This is consistent with recently conducted trials in patients with stable CAD (8,17). However, prompt revascularization in the absence of clinical containdications, IMT and an IS strategy is appropriate in type 2 patients with diabetes with more severe and extensive CAD similar to those enrolled in BARI 2D to prevent MI. Longer term follow-up will determine if the reduction in MI rates seen with CABG as compared to IMT translate into a treatment reduction in cardiac mortality.
BARI 2D was not designed to address whether PCI may produce similar outcome results to CABG in patients with more extensive disease, a question that is being addressed in the ongoing Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) trial (41).
Commentary.
In this report from the BARI 2D trial, we comment on the secondary endpoints of cardiac death and myocardial infarction. Cardiac death accounted for 43% of all-cause mortality during an average 5.3 year follow-up, emphasizing the critical importance of intensive medical therapy to reduce death rates from cardiovascular disease in patients with type 2 diabetes and obstructive coronary artery disease. The low cardiac mortality rate is even more impressive when one considers that ACCORD and ADVANCE reported 50–53% of deaths were cardiovascular and only 32–35% of the population entered had a prior history of known CAD.
The BARI 2D trial enrolled 2,368 patients with stable ischemic heart disease assigned pre-randomization to PCI or CABG strata and we found similar 5-year all cause and cardiac mortality rates with insulin sensitization (IS) vs. insulin provision (IP) therapy and with a strategy of prompt initial coronary revascularization and intensive medical therapy (REV) or intensive medical therapy alone with REV reserved for clinical indication(s) (IMT).
We did not find any difference in the PCI stratum as compared to IMT in all-cause death, cardiac death, or myocardial infarction. Thus, for many patients with type 2 diabetes and stable but less severe and extensive CAD, IMT is an excellent first-line strategy, and does not require immediate prophylactic PCI to prevent cardiac death or MI. In contrast, prompt CABG with an IMT and IS strategy is superior to a strategy of “watchful waiting” and IMT alone in patients with more severe and extensive CAD to prevent non-procedural MI’s that are associated with an increased cardiac mortality risk.
Supplementary Material
Acknowledgments
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) is funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804) with significant supplemental funding from GlaxoSmithKline. A full listing of all sponsors can be found in in the Supplementary Appendix, at NEJM.org. (N Engl J Med 2009;360:2503-15). As an NIH funded trial, we are required to abide by the NIH PubMed Central Policy that we retain the right to provide a copy of the final manuscript to the NIH upon acceptance for publication by your journal, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication. From the Department of Medicine, Division of Cardiology, Saint Louis University School of Medicine, St. Louis, Missouri.
We thank Burton E. Sobel, M.D., Maria M. Brooks, Ph.D. and Sheryl F. Kelsey, Ph.D., for their support of this study.
FUNDING SOURCE
BARI 2D receives significant supplemental funding provided by GlaxoSmithKline, Collegeville, PA, Lantheus Medical Imaging, Inc. (formerly Bristol-Myers Squibb Medical Imaging, Inc.), North Billerica, MA, Astellas Pharma US, Inc., Deerfield, IL, Merck & Co., Inc., Whitehouse Station, NJ, Abbott Laboratories, Inc., Abbott Park, IL, and Pfizer, Inc, New York, NY. Generous support is given by Abbott Laboratories Ltd., MediSense Products, Mississauga, Canada, Bayer Diagnostics, Tarrytown, NY, Becton, Dickinson and Company, Franklin Lakes, NJ, J. R. Carlson Labs, Arlington Hts., IL, Centocor, Inc., Malvern, PA, Eli Lilly and Company, Indianapolis, IN, LipoScience, Inc., Raleigh, NC, Merck Sante, Lyon, France, Novartis Pharmaceuticals Corporation, East Hanover, NJ, and Novo Nordisk, Inc. Princeton, NJ. A full listing of sponsors can be found in the Supplementary Appendix at http://www.NEJM.org (N Engl J Med 2009; 360:2503-2515).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Chaitman reports receiving consulting/advisory board fees from Eli Lilly and lecture fees from Gilead. No other potential conflict of interest relevant to this article was reported.
Clinical Trial Registration: ClinicalTrials.gov number, NCT00006305
BIBLIOGRAPHY
- 1.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30:834–839. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR on behalf of the United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci. 2001;101:671–679. [PubMed] [Google Scholar]
- 3.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Association of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 4.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In: Aubert RE, Ballard DJ, Barrett-Connor E, et al., editors. Diabetes in America. 2. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1995. pp. 233–57. (NIH publication no. 95-1468.) [Google Scholar]
- 5.Barsness GW, Gersh BJ, Brooks MM, Frye RL. Rationale for the revascularization arm of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97(Suppl 12A):31G–40G. doi: 10.1016/j.amjcard.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97(Suppl 12A):20G–30G. doi: 10.1016/j.amjcard.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Frye RL, August P, Brooks M, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TLZ, Molitch ME, Nesto RW, Sako EY, Sobel BE the BARI IID Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris C, Chaitman BR, Shaw L, Gosselin G, Nawaz Sh, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GBJ, Weintraub WS for the COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 9.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk W, Knudtson M, Dada M, Casperson P, Harris CL, Spertus JA, Shaw L, Chaitman BR, Mancini GBJ, Berman DS, Weintraub WS on behalf of the COURAGE trial coinvestigators and study coordinators. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial: Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151:1173–9. doi: 10.1016/j.ahj.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97(Suppl 12A):9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Kim LS, King SB, Kent K. Factors related to the selection of surgical versus percutaneous revascularization in diabetic patients with multivessel coronary artery disease in the BARI 2D trial. J Am Coll Cardiol Intv. 2009;2:384–92. doi: 10.1016/j.jcin.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz L, Kip KE, Alderman E, Lu J, Bates ER, Srinivas V, Bach RG, Mighton LD, Feit F, King S, Frye RL and the BARI 2D Study Group. Baseline coronary angiographic findings in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial (BARI 2D) Am J Cardiol. 2009;103:632–638. doi: 10.1016/j.amjcard.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Brooks MM, Barsness G, Chaitman BR, Chung SC, Faxon D, Feit F, Frye R, Genuth S, Green J, Hlatky M, Kelsey S, Kennedy F, Krone R, Nesto R, Orchard T, O’Rourke R, Rihal C, Tardif JC. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the BARI 2D Trial. Am Heart J. 2008;156:528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, White HD on behalft of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 15.Chaitman BR, Zhou SH, Tamesis B, Rosen A, Terry AB, Zumbehl KM, Stocke K, Takase B, Gussak I, Rautaharju PM. Methodology of serial ECG classification using an adaptation of the NOVACODE for Q wave myocardial infarction in the Bypass Angioplasty Revascularization Investigation (BARI) J Electrocardiol. 1996;29:265–77. doi: 10.1016/s0022-0736(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 16.Rautaharju PM, Calhoun HP, Chaitman BR. NOVACODE serial ECG classification system for clinical trials and epidemiologic studies. J Electrocardiol. 1992;24 (Suppl):179–187. doi: 10.1016/s0022-0736(10)80041-x. [DOI] [PubMed] [Google Scholar]
- 17.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 19.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson RA, Pocock SJ, Clayton TC, Knight R, Fox KAA, Julian DG, Chamberlain DA for the Second Randomized Intervention Treatment of Angina (RITA-2) Trial participants. Seven-year outcome in the RITA-2 trial. Coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42:1161–1170. doi: 10.1016/s0735-1097(03)00951-3. [DOI] [PubMed] [Google Scholar]
- 21.Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373:911–918. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzucchi SE, Masoudi FA, Wang Y, Kosiborod M, Foody JM, Setaro JF, Havranek EP, Krumholz HM. Insulin-sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction. Insights from the National Heart Care Project. Diabetes Care. 2005;28:1680–1689. doi: 10.2337/diacare.28.7.1680. [DOI] [PubMed] [Google Scholar]
- 23.Sauer WH, Cappola AR, Berlin JA, Kimmel SE. Insulin sensitizing pharmacotherapy for prevention of myocardial infarction in patients with diabetes mellitus. Am J Cardiol. 2006;97:651–654. doi: 10.1016/j.amjcard.2005.09.106. [DOI] [PubMed] [Google Scholar]
- 24.Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, Yoshida K. Impact of insulin resistance on neointimal tissue proliferation after coronary stent implantation: Intravascular ultlrasound studies. J Diabetes Complicat. 2002;16:50–55. doi: 10.1016/s1056-8727(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 25.Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–1820. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 26.Koshiyama H, Shimono D, Kuwamura N, Minamikawa J, Nakamura Y. Rapid communication: Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3452–3456. doi: 10.1210/jcem.86.7.7810. [DOI] [PubMed] [Google Scholar]
- 27.Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1979–1989. doi: 10.1161/01.ATV.0000091250.53231.4D. [DOI] [PubMed] [Google Scholar]
- 28.Kruszynska Y, Yu JG, Sobel BE, Olefsky JM. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes mellitus and in lean and obese normal subjects. Diabetes. 2000;49:633–639. doi: 10.2337/diabetes.49.4.633. [DOI] [PubMed] [Google Scholar]
- 29.Sobel BE. Increased plasminogen activator inhibitor-1 and vasculopathy: a reconcilable paradox. Circulation. 1999;99:2496–2498. doi: 10.1161/01.cir.99.19.2496. [DOI] [PubMed] [Google Scholar]
- 30.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 31.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 33.Passermani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 34.Aldridge HE, Trimble AS. Progression of proximal coronary artery lesions to total occlusion after aorto-coronary bypass saphenous bypass grafting. J Thorac Cardiovasc Surg. 1971;62:7. [PubMed] [Google Scholar]
- 35.Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, Montinaro A. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention. Results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54:558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, Castelli A, Mussardo M, Montorfano M, Ricciardelli B, Colombo A. Novel approaches for preventing or limiting events (Naples) II trial. J Am Coll Cardiol. 2009;54:2157–2163. doi: 10.1016/j.jacc.2009.07.005. [DOI] [PubMed]
- 37.Gavard JA, Chaitman BR, Sakai S, Stocke K, Danchin N, Erhardt L, Gallo R, Chi E, Jessel A, Theroux P for the GUARd During Ischemia Against Necrosis (GUARDIAN) investigators. Prognostic significance of elevated CK-MB after coronary bypass surgery and after an acute coronary syndrome: results from the GUARDIAN trial. J Thorac Cardiovasc Surg. 2003;126:807–813. doi: 10.1016/s0022-5223(03)00735-9. [DOI] [PubMed] [Google Scholar]
- 38.Klatte K, Chaitman BR, Theroux P, Gavard JA, Stocke K, Boyce S, Bartels C, Keller B, Jessel A for the GUARDIAN Investigators. Increased mortality after coronary artery bypass grafting is associated with increased levels of postoperative CK-MB release – results from the GUARDIAN trial. J Am Coll Cardiol. 2001;38:1070–7. doi: 10.1016/s0735-1097(01)01481-4. [DOI] [PubMed] [Google Scholar]
- 39.Mohammed AA, Agnihotri AK, van Kimmenade RRJ, Martinez-Rumayor A, Green SM, Quiroz R, Januzzi JL. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation. 2009;120:843–850. doi: 10.1161/CIRCULATIONAHA.108.837278. [DOI] [PubMed] [Google Scholar]
- 40.Hueb W, Gersh BJ, Costa F, Lopes N, Soares PR, Dutra P, Jatene F, Pereira AC, Gois AFT, Oliveira SA, Ramires JAF. Impact of diabetes on five-year outcomes of patients with multivessel coronary artery disease. Ann Thorac Surg. 2007;83:93–99. doi: 10.1016/j.athoracsur.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Farkouh ME, Dangas G, Leon MB, Smith C, Nesto R, Busse JB, Cohen DJ, Mahoney E, Sleeper L, King S, Domanski M, McKinlay S, Fuster V. Design of the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) Trial. Am Heart J. 2008;155:215–223. doi: 10.1016/j.ahj.2007.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.