SUMMARY
Targeted drugs hold great promise for the treatment of malignant tumors; however, there are several challenges for efficient evaluation of these drugs in preclinical and clinical studies. These challenges include identifying the ‘correct’, biologically active concentration and dose schedule, selecting the patients likely to benefit from treatment, monitoring inhibition of the target protein or pathway, and assessing the response of the tumor to therapy. Although anatomic imaging will remain important, molecular imaging provides several new opportunities to make the process of drug development more efficient. Various techniques for molecular imaging that enable noninvasive and quantitative imaging are now available in the preclinical and clinical settings, to aid development and evaluation of new drugs for the treatment of cancer. In this Review, we discuss the integration of molecular imaging into the process of drug development and how molecular imaging can address key questions in the preclinical and clinical evaluation of new targeted drugs. Examples include imaging of the expression and inhibition of drug targets, noninvasive tissue pharmacokinetics, and early assessment of the tumor response.
Keywords: drug development, molecular imaging, MRI, optical imaging, PET
INTRODUCTION
The success of imatinib in the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors (GISTs) has demonstrated that targeted drugs can induce remarkable tumor responses, and even cure patients of cancer.1 Targeted drugs have been used to treat a variety of common human solid tumors, including breast cancer, colorectal cancer, and non-small-cell lung cancer (NSCLC). Diverse integrated signaling pathways are involved in the development and progression of these malignancies, each of which is genetically heterogeneous. Consequently, inhibition of one specific pathway is likely to be efficacious only in small subsets of patients who share a specific histological tumor type. For example, in unselected patients with metastatic breast cancer, the response rate to the anti-HER2 antibody trastuzumab (Herceptin®; Genentech, San Francisco, CA) is less than 10%.2 Low response rates have also been reported for EGFR and mammalian target of rapamycin (mTOR) kinase inhibitors in patients with NSCLC3 and renal cell carcinoma,4 respectively. In the small subgroup of patients that do respond, however, targeted drugs can dramatically improve the survival rate. Clearly, patient stratification to identify potential responders would be of great benefit; for breast cancer, identification of probable responders to trastuzumab therapy2 by use of immunohistochemical HER2 staining facilitated approval of this drug and its widespread use in the treatment of HER2-positive breast cancer.
Immunohistochemical assays of a comparable benefit are unavailable for many cancers when new targeted drugs enter clinical trials. Moreover, sensitivity to new drugs might not be based on overexpression of target proteins. Instead, sensitivity might be based on factors such as specific mutations of the target molecule or the loss of negative regulators of its activity and other regulatory, redundant and/or compensatory tumor responses that are not currently understood. An inability to identify the appropriate patient subsets complicates drug development when ‘go–no go’ decisions are made according to the response rates in relatively small phase II studies. In such studies, poorly defined study populations might lead to an apparent drug failure because of the large fraction of nonresponders that dilute the positive drug effect on subpopulations detected by the study. The inability to properly identify appropriate patient subsets is confounded by the fact that the conventional size criteria used to assess tumor responses might not be applicable for some targeted drugs, since durable responses to targeted drugs might be cytostatic rather than cytotoxic and are thus not associated with tumor shrinkage. As a consequence, standard response criteria, which define a response as a decrease in tumor size, are of limited value in the assessment of the response to this class of drugs, as discussed subsequently.
Although targeted drugs are promising because of the genetic distinctions and consequent biochemical differences among patients with the same histopathologic tumor types, it is unlikely that a single drug will be effective for all patients with a ‘common’ tumor. Rather, it will be necessary to develop multiple targeted drugs, even for patients that share a single histologically defined tumor type. The result will be smaller patient populations per drug, although there will be a greater fraction of responders. The inevitable consequence is a decreased revenue:cost ratio for the industry, unless the cost per drug can be lowered. Key questions during the development of targeted drugs include the following5: Is the target protein expressed in a particular tumor? Does the drug reach the target at a sufficient concentration? Is the function of the target protein modulated? Does modulation of the function of the target protein result in the expected biological effects?
Molecular imaging provides probes and assays that can address expression of the target protein, drug–target protein interactions and drug effects in both preclinical and clinical studies. Molecular imaging can reduce the costs of drug development,6 stratify patient populations and monitor therapeutic efficacy. With regard to the clinical setting, the two key techniques for molecular imaging that will be emphasized in this article are PET and MRI. Owing to space constraints, we have not discussed other molecular imaging modalities, such as single photon emission CT (SPECT), although studies indicate that these modalities may also be used for molecular imaging in patients.7 For preclinical studies, specialized imaging devices that include small-animal PET, SPECT instrumentation, small-animal MRI, and optical imaging devices for fluorescence and bioluminescence, are currently available for studies of rodents and other animal species. In addition to molecular imaging probes, these devices enable pharmacokinetic and pharmacodynamic studies in animal models of cancer and in humans. The physical performance characteristics of these imaging techniques have been reviewed.8–10
Although there is little doubt that small-animal imaging will facilitate the development of targeted drugs and imaging agents, drug targets can be species-specific; for example, carcinoembryonic antigen is not expressed in mice. Conversely, the monoclonal antibody bevacizumab (Avastin®; Genetech, San Francisco, CA), which targets VEGF, binds specifically to the human, but not the mouse, VEGF target protein. In such cases, the results of clinical imaging studies might differ from those obtained from animal models. Molecular imaging is a burgeoning field; we present selected examples to illustrate the applications of molecular imaging, focusing on how molecular imaging can potentially contribute to ‘go–no go’ decisions earlier in the process of drug development.
IMAGING OF TARGET EXPRESSION
Expression of the protein or proteins targeted by a drug can generally be assessed by standard immunohistochemistry techniques. Tissue samples for immunohistochemical analysis, however, are frequently available only from the primary tumor, which might have a different phenotype and drug response than metastatic lesions. By the time patients enter clinical trials, expression of the target protein, or its function, may have changed considerably. For example, Linden et al. studied 47 patients with recurrent breast cancer.11 At the time of diagnosis, all primary tumors were estrogen receptor positive; however, only 23% of the patients achieved an objective response to salvage hormonal therapy. However, the degree of binding of the radiolabeled estrogen analog [18F]fluoroestradiol (FES) to estrogen receptors in metastatic tumors, as measured by PET, was predictive of the tumor response. Specifically, none of the patients lacking focal FES tumor binding responded to therapy, whereas 34% of patients with FES tumor binding responded to salvage hormonal therapy. Thus, FES–PET can be used to identify patient subgroups that, despite expression of estrogen receptors in the primary tumor, are unlikely to benefit from antiestrogen therapy because the metastatic tumors are estrogen receptor negative.
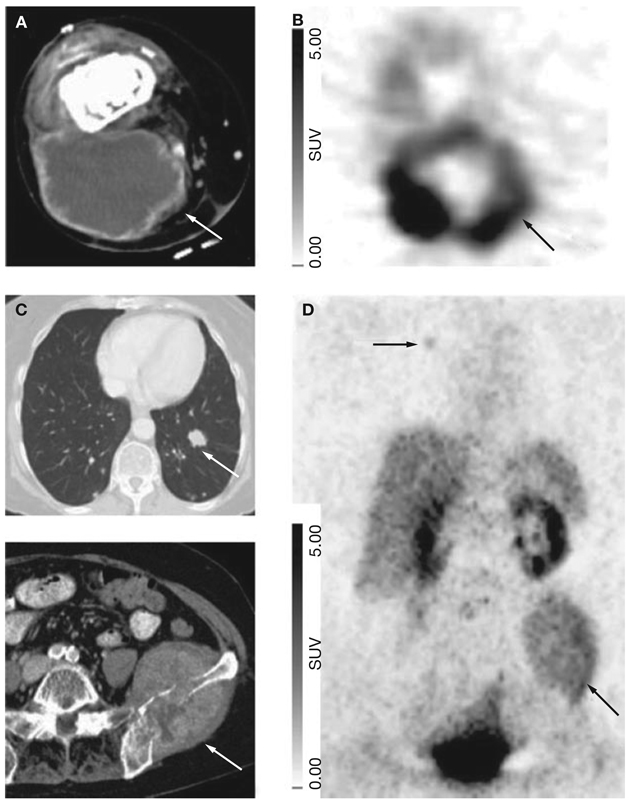
In addition to identifying differences in expression of target proteins among patients, molecular imaging can also show intrapatient heterogeneity of the expression of target proteins. For instance, lesion-to-lesion differences in the expression of αVβ3 integrin in a patient with metastatic soft tissue sarcoma are shown in Figure 1. The marked differences in αVβ3 integrin expression found in this patient demonstrated the potential of molecular imaging for prediction and might explain the heterogeneous responses to different targeted drugs within a single individual.12 Currently, the levels of expression of target proteins are determined by analysis of tumor biopsies. In patients with metastatic disease, analysis of tumor biopsies allows evaluation of only a small part of the total tumor mass. Figure 1 illustrates that the assessment of the expression of protein targets by use of biopsies might, therefore, be misleading, since αVβ3 integrin was highly expressed in the primary tumor but not in the pulmonary metastases.
Figure 1.
Intrapatient heterogeneity in the expression of αVβ3 integrin imaged by PET with the αVβ3 ligand [18F]galacto-RGD. The primary tumor, a soft tissue sarcoma of the thigh (arrow in the CT image in A), demonstrates intense uptake of [18F]galacto-RGD, indicating high expression levels of αVβ3 integrin (arrow in B). Uptake of [18F]galacto-RGD is much less pronounced in a bone metastasis in the pelvis and a right-sided lung metastasis (arrows in D). The bottom image in C shows the pelvic metastasis on CT (arrows). A left-sided lung metastasis, shown on CT (C, top) is negative on the [18F] galacto-RGD PET scan (D). Permission obtained from the Society of Nuclear Medicine © Beer AJ et al. (2005) Biodistribution and pharmacokinetics of the αVβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med 46: 1333–1341. Abbreviations: RGD, arginine–glycine–aspartic acid; SUV, standardized uptake value.
Currently, only a limited number of molecular targets can be imaged in clinical studies because of the restricted number of available probes (Table 1).13–17 Most molecular targets are expressed at nanomolar concentrations in the tumor tissue, which presents a challenge for the development of ligands. Many potential probes not only bind with high affinity to their targets but also demonstrate considerable nonspecific binding and/or unfavorable pharmacokinetic properties for in vivo imaging. Reducing nonspecific binding and optimizing the pharmacokinetic properties have been major challenges in the development of new imaging probes.
Table 1.
Examples of molecular imaging probes for studying the expression of therapeutic targets in patients.
| Therapeutic target | Imaging probe | Reference |
|---|---|---|
| Estrogen receptors | [18F]FES | Mintun et al. (1988)13 |
| Androgen receptors | [18F]FDHT | Larson et al. (2004)14 |
| Somatostatin receptors | [68Ga]octreotide analogs | Hofmann et al. (2001)15 |
| [18F]octreotide analogs | Schottelius et al. (2004)16 | |
| αVβ3 integrins | [18F]RGD-peptides | Haubner et al. (2005)17 |
Abbreviations: FDHT,16β-[18F]fluoro-5α-dihydrotestosterone; FES, [18F]fluoro-17β-estradiol; RGD, arginine–glycine–aspartic acid.
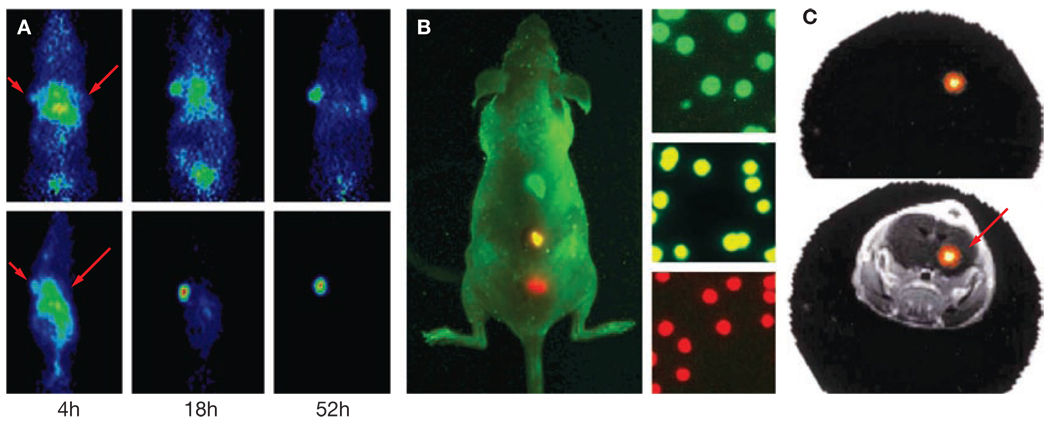
Several new strategies for the development of a variety of molecular imaging probes have been tested successfully in animals. Radiolabeled antibodies have been used to image a variety of targets, but their slow clearance from the bloodstream and tissue results in limited image contrast. Recent advances in antibody engineering18,19 have led to the development of several antibody fragment formats that demonstrate excellent properties for targeted PET imaging in animals (Figure 2A). Translation of modified antibodies for use in the clinic is likely to follow in the near future.
Figure 2.
New approaches for imaging expression of the target protein. (A) Imaging of the expression of carcinoembryonic antigen (CEA) with radiolabeled antibody fragments in mice. Mice were implanted with a CEA-expressing tumor at the left shoulder and CEA-negative tumor at the right shoulder. An [124I]-labeled anti-CEA antibody (top row) accumulates in the CEA-positive tumor (solid arrow), but the background and CEA-negative tumor (open arrow) have relatively low levels of CEA expression. The contrast is much higher for sc-Fv-Fc antibody fragments (bottom row) owing to faster blood clearance. Permission obtained from Nature Publishing Group © Wu AM and Senter PD (2005) Nat Biotechnol 23: 1137–1146. (B) Multiplexed optical imaging using quantum dots. Quantum dots of three different colors were subcutaneously injected into the back of a mouse and imaged simultaneously. The right side of the panel shows microscopic images of the injected quantum-dot-encoded microbeads (diameter 0.5 µm). Permission obtained from Nature Publishing Group © Gao X et al. (2004) Nat Biotechnol 22: 969–976. (C) Optical fluorescence tomography of the expression of cathepsin B in a mouse bearing an orthotopic human glioma xenograft in the right hemisphere of the brain (arrow). The top panel shows the reconstructed optical image (cross-section) obtained after injection of an activatable optical probe that is cleaved by cathepsin B. The bottom panel shows an overlay of the optical image and an MRI scan. Permission obtained from Nature Publishing Group © Ntziachristos V et al. (2002) Nat Med 8: 757–760.
Another research focus is the development of targeted nanoparticles for molecular imaging.20 Nanoparticles can be coated with multiple ligands or probes and the consequent multivalent binding to target molecules could result in dramatically increased uptake and retention of the particles. Nanoparticles can be coated with a large number of such signal-generating molecules, thereby increasing the intensity of the imaging signal. For optical imaging, nanoparticles can be loaded with fluorescent dyes that emit light of different wavelengths, which permits multiplexed imaging of various properties of the tumor tissue in one examination (Figure 2B).21 Optimization of the pharmacokinetics of nanoparticles is, however, still an issue, especially reduction of their accumulation by the reticuloendothelial system in the liver and spleen.
Activatable ‘smart’ probes represent an elegant approach to avoiding the problem of nonspecific binding in fluorescence imaging. These probes emit a signal only after interacting with their target. Activatable imaging probes consist of a fluorochrome coupled to a quenching moiety, such as a peptide.22 For this type of ‘smart’ probe, a fluorescence signal is emitted only after the quenching peptide has been cleaved from the fluorochrome by the activity of a protease. This approach enables the specific imaging of the enzymatic activity of proteases, because the nonspecifically bound imaging probe does not emit a light signal (Figure 2C).
The restricted tissue penetration of light limits the use of optical imaging probes. By use of near-infrared fluorescent reporters, tissue penetration of several centimeters has been achieved in experimental studies, albeit with increasing attenuation with depth.22 Furthermore, image reconstruction algorithms are now being developed to produce tomographic imaging in animal models, with the goal of quantitative assessment of regional concentrations of fluorescent reporters.22 Near-infrared fluorescence imaging might, in the future, have a role in clinical studies of superficial tumors or tumors accessible by endoscopy.22
TISSUE PHARMACOKINETICS
PET imaging has been used to study the pharmacokinetics of several anticancer drugs,23 for example the radiolabeled humanized anti-VEGF antibody HuMV833. Jayson and coauthors used PET imaging with an iodinated drug analog to study the pharmacokinetic properties of this antiangiogenic drug.24 To quantify the drug concentrations in the organ and tumor, labeled and unlabeled antibodies were coadministered during the first treatment cycle. PET was performed at 24 h and 48 h after administration of the drug and probe. The study revealed important differences in drug uptake, clearance and biological activity between primary tumors and metastatic lesions. Some primary tumors exhibited clearance rates that were three times faster than those of metastatic tumors, even within the same patient. Substantial differences in the rates of drug clearance from the tumor were also observed among different patients and tumor types. This heterogeneous pharmacokinetic response might explain the lack of effectiveness of treatment in some patients. The study also revealed discrepancies between the plasma pharmacokinetics of the drug and the rate of drug clearance from tumors, as determined by PET. In patients with multiple lesions, intratumoral drug concentrations varied up to 3.4-fold.24 This variance indicates that plasma pharmacokinetics cannot be used to predict intratumoral drug concentrations.
Examples of radiolabeled drug analogs used for PET imaging include [18F]fluorouracil,25 [18F]paclitaxel,26 [11C]temozolomide27 and [11C]-N-(2-[dimethylamino]ethyl)acridine-4-carboxamide.28 An interesting application of this drug–labeled-drug concept is ‘microdosing’ of drug candidates to study their distribution in the tissues before phase I studies.29 Such ‘phase 0’ studies can be performed at dose levels of the drug that have no significant pharmacologic or toxic effects because of the high sensitivity of PET. Although microdosing studies can provide valuable information about the pharmacokinetic properties of new drugs, there are some limitations of this concept. The parent drug and radiolabeled metabolites cannot be differentiated by PET imaging. By studying temporal changes in the concentrations of tissue radioactivity and performing metabolite analyses of plasma samples, corrections for the effect of metabolites can be made in some cases. These corrections, however, are often complex and require thorough validation.30
Radiolabeled drug analogs targeting specific biological molecules can also be used as imaging agents to study the expression or function of these molecules in patients. This approach requires extensive studies because validation that the uptake of the imaging probe reflects the activity of the studied process in patients is required. Subsequently, more-extensive clinical trials are needed to demonstrate that imaging of this particular process provides clinically useful information. Nunn31 estimated that the development of radiolabeled drug analogs as imaging probes can cost US$100–200 million, an investment that makes sense only if the imaging probe can subsequently be used clinically in large numbers of patients.
Costs are dramatically less if radiolabeled drug analogs are synthesized only to study pharmacokinetics;29 development of imaging-only reagents would require just the less-costly FDA approval of an investigative new drug. Nonetheless, the development of radiolabeling procedures with use of short-lived radioisotopes is a complex, time-consuming and costly process. Under the present conditions of probe discovery and regulatory processes, it is unlikely that the development of molecular imaging probes can keep pace with drug development and be financially feasible. The development of radiolabeled drug analogs is likely to be facilitated in the future by technology platforms such as integrated microfluidics chips32 that enable a more efficient and less expensive process for the preparation and evaluation of radiolabeled probes.
MEASURING TARGET INHIBITION
Noninvasive assessment of the target inhibition is important at various stages of drug development. In phase I studies, assessment of target inhibition can define the biologically active dose of a new drug for further phase II and III studies. Traditionally, the dose of a cancer drug chosen for further investigation has been determined by the maximum tolerated dose (MTD) in phase I studies. Pathway-targeted drugs, however, frequently cause limited acute adverse effects; the biologically active dose might be substantially lower than the MTD. Furthermore, classic cytotoxic agents are administered over a relatively brief period of time, with the intent to eradicate tumor cells. By contrast, many pathway-targeted drugs are developed as oral treatments for long-term use and are designed to inhibit tumor growth. Dosing these drugs at the MTD might result in unnecessary toxicity. Moreover, the effectiveness of some targeted drugs may actually decrease at higher doses, owing to off-target effects on other biologically active molecules.
In phase II studies, new drugs commonly fail to produce the desired responses for two reasons. First, the dose schedule might be inadequate; in other words, the target might not be inhibited. This fact should not, however, lead to ‘no go’ decisions; instead, the dose or schedule of administration must be optimized. Second, drugs might inhibit the target but might not affect tumor growth if the target is not essential. This should lead to ‘no go’ decisions.
If the probes used in imaging bind to the same target as the drug (Figure 3A), inhibition of the target can be monitored by imaging the blocking of probe uptake by the study drug.33,34 Similarly, inhibition of enzymatic activity can also be visualized by imaging probes that are substrates of specific enzymes. This approach has been used to monitor the inhibition of matrix metalloproteinase activity by activatable MRI probes that are cleaved by this enzyme.35
Figure 3.
Monitoring of target inhibition by PET imaging. (A) Blockade of αVβ3 integrin by the cyclic pentapeptide c(RGDfV). Mice were implanted with an αVβ3-positive tumor on the left shoulder. Pretreatment with the αVβ3 ligand c(RGDfV) leads to a dose-dependent decrease in the uptake of the radiolabeled imaging probe [18F]galacto-RGD. Permission obtained from the American Association for Cancer Research © Haubner R et al. (2001) Noninvasive imaging of αVβ3 integrin expression using 18F–labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 61: 1781–1785. (B) Monitoring of Hsp90 inhibition by PET imaging with 68Ga-labeled anti-HER2 antibody fragments. Mice were implanted on the left shoulder with a tumor that overexpressed HER2 (arrows). Treatment with the Hsp90 inhibitor 17-AAG leads, within 24 h, to a marked reduction of the uptake of anti-HER2 antibody fragments, indicating downregulation of HER2 expression. Downregulation of HER2 expression was confirmed by immunoblots of the tumor tissue. Permission obtained from Nature Publishing Group © Smith-Jones PM et al. (2004) Nat Biotechnol 22: 701–706. Abbreviations: 17-AAG, 17-allylaminogeldanamycin; HER2, human epidermal growth factor receptor 2; Hsp90, heat shock protein 90; RGD, arginine–glycine–aspartic acid.
Few ligands have been validated for imaging target inhibition in this way. Also, radiolabeling of drugs frequently does not provide useful imaging probes for monitoring target inhibition because uptake of the drug in the tumor can be dominated by nonspecific binding to the cell membrane or other cellular components. In addition, although the drug concentration does not need to be higher at the target relative to the surrounding tissues, this is a necessary condition for imaging probes. Finally, whether the kinetics of the drug are favorable for imaging and whether analysis of the image is confounded by radiolabeled metabolites must be determined.36
If a probe is unavailable for a known target, imaging of biochemical or biological changes in response to target inhibition can provide a surrogate for imaging the interaction between the drug and the target. Using this approach, Smith-Jones et al. studied levels of expression of HER2 in murine xenograft models of breast cancer in response to treatment with the heat shock protein 90 (Hsp90) inhibitor 17-allylaminogeldanamycin (17-AAG).37 HER2 expression was measured by use of micro-PET with [68Ga]trastuzumab and a [68Ga]trastuzumab F(ab') 2 fragment (because of more rapid blood clearance of this fragment than of trastuzumab). Both imaging probes showed high retention in tumors, which correlated to HER2 expression by immunoblotting. Serial micro-PET imaging to measure the effects of 17-AAG on the levels of HER2 expression in tumors is feasible because 68Ga has a short half-life of only 68 min and the F(ab') fragment is cleared quickly from the circulation. Treatment resulted in a reduction of more than 50% in probe uptake in the tumor 24 h after 17-AAG treatment (Figure 3B). Expression of HER2 measured in excised tumor tissue was reduced by 80% compared with that in vehicle-treated controls. The binding of the probe to the target was specific because 17-AAG treatment did not affect the levels of phosphatidylinositol 3-kinase, an Hsp90-independent protein. The same group demonstrated that the 17-AAG-dependent degradation of HER2 did not result in reduced tumor size or tumorglycolysis, by use of micro-PET analysis of 2-deoxy-2-[18F]fluorodeoxy-D-glucose (FDG) uptake.38
These studies have several potential implications for human trials. First, the [68Ga]trastuzumab F(ab') 2 fragment (or other appropriately engineered antibody fragments) could be used to identify breast cancer patients who overexpress HER2 and are, therefore, more likely to respond to treatment with trastuzumab. Second, an early reduction in [68Ga]trastuzumab F(ab') 2 fragment binding could distinguish effective 17-AAG treatments in vivo. Third, PET imaging might elucidate differences in HER2 expression in distinct metastatic lesions. Fourth, PET imaging might be used to determine noninvasively whether the MTD of the drug can inhibit the therapeutic target. Finally, the dose scheduling could be optimized by monitoring the duration of target inhibition using PET.
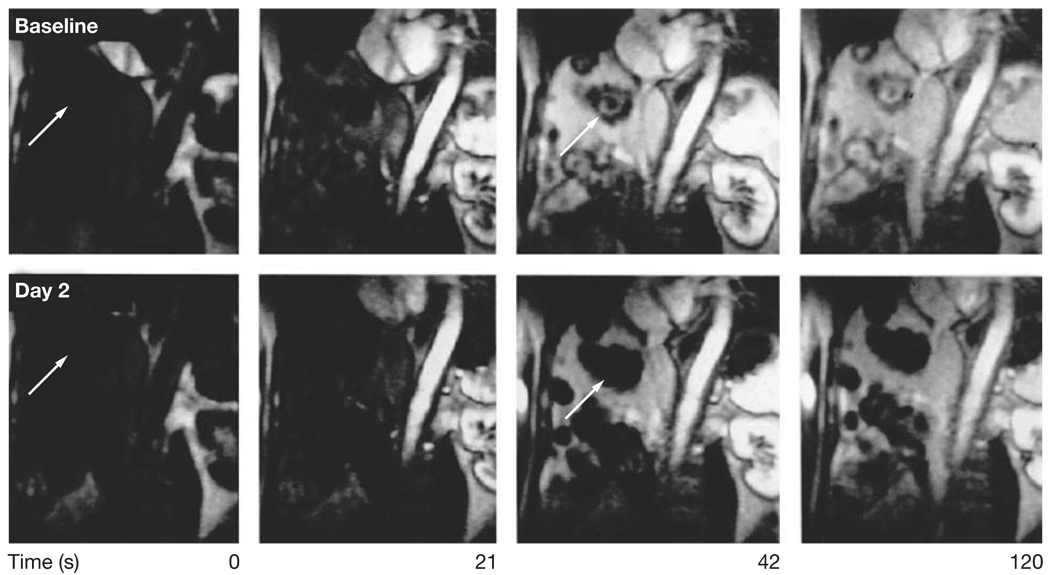
Dynamic contrast-enhanced (DCE-) MRI has also been used to study the effects of inhibitors of VEGF signaling. VEGF markedly increases vascular permeability; monitoring changes in vascular permeability presents an attractive approach to the imaging of target inhibition by anti-VEGF antibodies or VEGF receptor kinase inhibitors.39 DCE-MRI was used to determine the effective dose for the pan-VEGF receptor kinase inhibitor PTK 787/ZK 222584 (PTK/ZK). Treatment with this drug resulted in a dose-dependent decrease of Ktrans, a marker of vascular permeability and perfusion. The effects of the drug on Ktrans plateau at a dose of 1,000 mg/day.40 The MTD of PTK/ZK is 1,500 mg/day and, therefore, the biologically effective dose seems to be significantly lower than the MTD; DCE-MRI was instrumental in determining the appropriate dose (Figure 4).41
Figure 4.
Monitoring of target inhibition by dynamic contrast-enhanced MRI. At baseline (top row), the multiple liver metastases (arrows) demonstrate intense contrast enhancement. Following treatment with the VEGF receptor protein kinase inhibitor PTK/ZK, the liver metastases demonstrate no visible contrast enhancement, indicating a marked decrease in vascular permeability or perfusion. Permission obtained from the American Society of Clinical Oncology © Morgan B et al. (2003) Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 21: 3955–3964.
Molecular imaging can be particularly helpful in dose-finding studies of anti-angiogenic agents because the genetic variability of the endothelial cells forming the blood supply to the tumor is likely to be smaller than that of the tumor cells. As a consequence, a relatively low interpatient variability of the drug concentration needed for endothelial cell target inhibition would be expected. By contrast, genetic variation of tumor cells can cause marked differences in their sensitivity to targeted drugs. For example, mutated forms of the EGFR found in some NSCLCs can be inhibited by EGFR kinase inhibitors at concentrations approximately 100-fold lower than those required to inhibit wild-type EGFRs.42 Such marked genetic variations in drug sensitivity can complicate the establishment of dose–response relationships in phase I studies.
Although DCE-MRI has shown encouraging data for imaging of target inhibition by PTK/ZK and other VEGF inhibitors, several technological challenges remain. Acquisition and analysis of DCE-MRI scans are technically demanding and not uniformly standardized. In clinical studies, the quality of data is frequently inadequate for quantitative analysis.43 There is also considerable intrapatient variability of the quantitative parameters; two pre-therapy baseline scans are currently recommended to assess the reproducibility of measurements in clinical trials.43 It is also unclear whether DCE-MRI will be as successful as existing imaging techniques for the assessment of the effects of drugs that are less potent inhibitors of vascular permeability than are VEGF inhibitors.
In the experimental setting, molecular imaging with genetically encoded reporters can provide additional insights into ligand–target interactions. In the ‘split protein’ strategy,44 a reporter enzyme is cleaved into N-terminal (Nr) and C-terminal (Cr) fragments, each of which is enzymatically inactive. Each fragment is fused to one of two interacting proteins (X and Y). A physical interaction between Nr-X and Cr-Y proteins reconstitutes the activity of the enzyme, leading to the generation of a signal after substrate administration. Thus, interactions between X and Y proteins mediated by drug administration can be studied in vivo. This approach has been used to study protein–protein interactions induced by the mTOR inhibitor rapamycin, which mediates the interaction of the FRB and FKBP12 proteins. The resulting FRB–rapamycin–FKBP12 complex inhibits mTOR, a serine–threonine kinase. To monitor this process in vivo, fragments of firefly luciferase were fused to FRB and FKBP12. Rapamycin-induced formation of the FRB and FKB12A complex restores the enzymatic function of luciferase; following injection of luciferin, light emission can be monitored noninvasively by optical imaging.45,46
Although studies involving genetically encoded reporters cannot be used in the clinical setting, they can provide fundamental information on the ability of new drugs to target protein–protein interactions in animal models. These reporters might be used for high-throughput screening of drugs in cell culture assays.
ASSESSMENT OF TUMOR RESPONSE TO THERAPY
FDG-PET has been used to assess the glycolytic responses of tumors to chemotherapy in patients with a wide variety of malignancies (Figure 5).47 Although the FDG-PET methodology and criteria for tumor responses varied in different studies, changes in FDG uptake by tumors after the first chemotherapy cycle correlate significantly with patient survival in these various studies.46 These data suggest that FDG-PET might become an early indicator for treatment efficacy in clinical studies.47
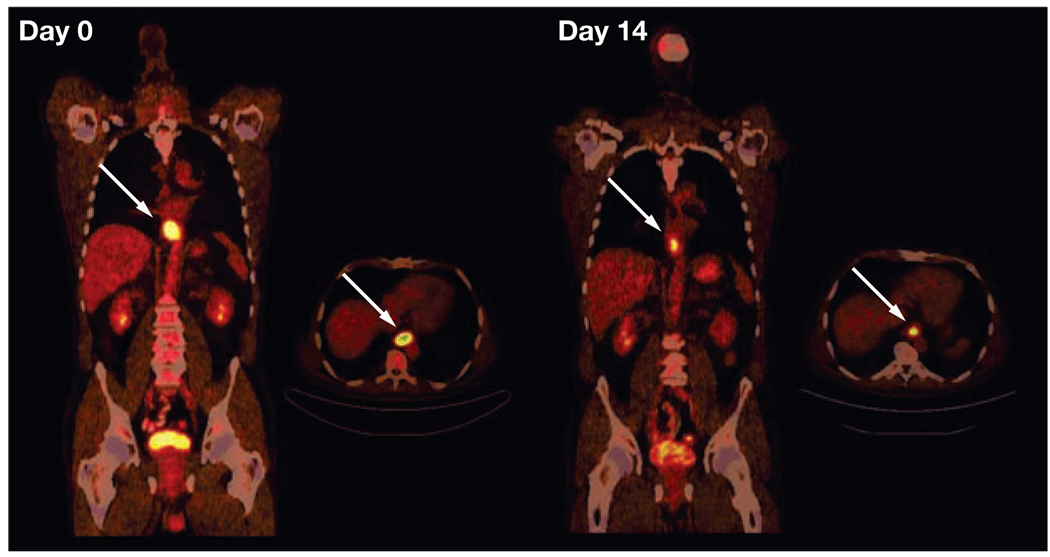
Figure 5.
Treatment monitoring with fluorodeoxyglucose (FDG) PET and CT in a patient with locally advanced distal esophageal cancer (arrows). In each image set, the image on the left is a longitudinal section from the neck to the pelvis and the image on the right is a cross-section through the plane containing the tumor. The tumor demonstrates intense FDG uptake before therapy (day 0). FDG uptake decreases markedly on day 14 of the first chemotherapy cycle. Quantitatively, FDG uptake by the tumor decreased from a standard uptake value of 9.2 to 4.2. After completion of preoperative chemotherapy, the tumor was resected. Histopathology demonstrated less than 10% of viable tumor cells in the resected specimen. Permission obtained from the American Society of Clinical Oncology © Weber WA (2006) Positron emission tomography as an imaging biomarker. J Clin Oncol 24: 3282–3292.
The National Cancer Institute has recently published guidelines for conducting clinical trials with use of FDG-PET to monitor therapies. These guidelines are a first step to standardization of imaging protocols across institutions.48 Such standardized protocols will greatly facilitate multicenter trials of cancer treatments that use FDG-PET imaging as a biomarker of tumor response. A consensus was reached recently by the Subcommittee of the International Harmonization Project regarding an optimal FDG-PET protocol for the assessment of treatment responses in lymphoma.49 Importantly, for the first time, guidelines for the timing and interpretation of post-treatment PET studies of a specific disease entity were established.
The Oncology Biomarkers Qualification Initiative, established in February 2006, is an agreement between the National Cancer Institute, FDA, and Centers for Medicare and Medicaid Services to collaborate on improving the development process for cancer therapies and the outcomes of cancer patients through the development and evaluation of biomarkers. The goal of this collaboration is to qualify biomarkers (i.e. to establish standard, broadly accepted criteria for biomarkers that have been accepted by this group) so that they can be used to evaluate new molecular diagnostics that will provide the necessary accuracy to shorten clinical trials, reduce the number of patients and resources required during the drug development process, improve the link between drug approval and drug coverage, and increase drug safety and the appropriateness of drug choices for patients with cancer. The first projects are focusing on standardizing and evaluating FDG-PET-based biomarkers through clinical trials. For each cancer setting studied, standardized protocols are being developed for image acquisition, data collection and analysis, and response criteria.
In patient studies, the most striking changes in tumor glycolysis have been reported for GIST lesions treated with imatinib.50 Changes in glycolysis preceded changes in tumor size by many weeks and were essential for documenting the activity of imatinib in GIST in clinical trials.50 Current studies are now using FDG-PET to monitor the development of resistance to imatinib and assess the efficacy of salvage therapy with second-generation targeted inhibitors of the mutated c-Kit receptor present in this subset of GIST lesions.
Marked changes in tumor glycolysis might also occur in response to other protein kinase inhibitors. Su et al. demonstrated a decrease in FDG uptake as early as 2 h after treatment with gefitinib in gefitinib-sensitive lung cancer cell lines.51 These metabolic alterations preceded changes in cell-cycle distribution, thymidine uptake and apoptosis, suggesting that changes in glycolysis are both an early and a sensitive marker of the response to treatment. These observations were then confirmed in micro-PET studies that detected up to a 55% decrease in FDG uptake in gefitinib-sensitive xenografts within 48 h after the start of treatment.51
[18F]3'-deoxy-3'-fluorothymidine (FLT), a marker of DNA replication and cell proliferation, was used in a study of glioblastoma patients who underwent bevacizumab treatment in combination with irinotecan. FLT uptake by tumor cells in vitro and in vivo was correlated to cell proliferation.52,53 Nineteen patients with recurrent malignant gliomas who were treated with biweekly cycles of bevacizumab and irinotecan were evaluated with FLT-PET at baseline, 1–2 weeks and 6 weeks after treatment. A decrease of at least 25% in FLT uptake by the tumor was defined as a metabolic response. Nine (47%) patients responded with more than a 25% decrease in FLT uptake, whereas 10 (53%) patients did not. Patients with an FLT-PET response survived significantly longer than those without a response (317 versus 152 days; P = 0.003). FLT-PET responses were stronger predictors of overall survival than the response predicted by MRI. In addition, responders were identified only 2 weeks after the start of treatment.54 If confirmed in larger study populations, this could result in substantial shortening of phase II trials in this disease.
CONCLUSIONS
Whole-body molecular imaging has the potential to answer several key questions crucial to the development and use of targeted cancer drugs. Where is the target protein of a drug expressed? To what level is the target expressed in the tumor? Where does the drug go following administration? Did the drug bind to the target? Did the drug occupy enough of the target sites to induce the desired pharmacologic response? At what dose did the drug induce this response? How long is the drug present in the tumor? How long is the drug able to modulate the target function? Did the tumor respond to therapy? Has a recurrent tumor acquired resistance to a previously efficacious therapy?
Molecular imaging can stratify patients with regard to the presence or absence of a drug target and provide tests to distinguish rapidly between responders and nonresponders to a drug. Several molecular imaging probes are already clinically available to address the questions above. Further validation for the use of these probes in drug development and for obtaining regulatory approval of new drugs is ongoing. Development of new molecular imaging probes for MRI, PET and optical imaging is a highly active area of research that will further extend the use of molecular imaging in drug development. Ultimately, molecular imaging will help to select the right drug for the right patient.
REVIEW CRITERIA.
The information for this Review was compiled by searching the PubMed and MEDLINE databases for articles published until 15 March 2007. Electronic early-release publications were also included. Only articles published in English were considered. The search terms used included “molecular imaging”, “positron emission tomography”, “bioluminescence imaging”, “fluorescence imaging”, “magnetic resonance imaging”, “drug development”, “targeted drugs”, and “protein kinase inhibitors”. Full articles were obtained and references were checked for additional material, as appropriate. References were chosen on the basis of the best clinical or laboratory evidence, especially if the work had been corroborated by published work from other centers. When possible, primary sources have been quoted. For several subtopics, the reader is referred to recent reviews to avoid redundancy and excessive referencing.
KEY POINTS.
There is an urgent need to develop and use assays that accelerate the drug development and evaluation processes and, at the same time, reduce the drugs’ costs
Radiolabeled drug analogs can be used to perform ‘phase 0’ (microdosing) studies at dose levels that have no significant toxic effects
Molecular imaging provides tools and assays that can address expression of the target protein, drug–target interactions, and tumor response in preclinical and clinical studies
The number of targets that can be studied noninvasively by molecular imaging is still limited
Development of new molecular imaging probes for MRI, PET and optical imaging is a highly active research area that will further extend the use of molecular imaging in the process of drug development
Acknowledgments
We thank the members of the Department of Molecular and Medical Pharmacology, Institute for Molecular Medicine, Crump Institute for Molecular Imaging, and Ahmanson Biological Imaging Division for their helpful conversations.
Footnotes
Competing interests
The authors declared no competing interests.
Contributor Information
Wolfgang A Weber, Professor and Director of Nuclear Medicine at the University of Freiberg, Germany..
Johannes Czernin, Professor of Molecular and Medical Pharmacology in the Ahmanson Biological Imaging Division, and Chief of the Ahmanson Biological Imaging Clinic, at the David Geffen School of Medicine, UCLA, Los Angeles, CA, USA..
Michael E Phelps, Chair of the Department of Molecular and Medical Pharmacology and Director of the Crump Institute for Molecular Imaging, UCLA, Los Angeles, CA, USA..
Harvey R Herschman, Director for Basic Research at the Jonsson Comprehensive Cancer Center, and Director of the In Vivo Cellular and Molecular Imaging Center, all at UCLA, Los Angeles, CA, USA..
References
- 1.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 2.Vogel CL, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 5.Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- 6.Workman P, et al. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J Natl Cancer Inst. 2006;98:580–598. doi: 10.1093/jnci/djj162. [DOI] [PubMed] [Google Scholar]
- 7.Perik PJ, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24:2276–2282. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 8.Ntziachristos V, et al. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 9.Cherry SR. The 2006 Henry N. Wagner Lecture: Of mice and men (and positrons)--advances in PET imaging technology. J Nucl Med. 2006;47:1735–1745. [PubMed] [Google Scholar]
- 10.Blodgett TM, et al. PET/CT: form and function. Radiology. 2007;242:360–385. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 11.Linden HM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 12.Beer AJ, et al. Biodistribution and pharmacokinetics of the alphavbeta3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 13.Mintun MA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 14.Larson SM, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 15.Hofmann M, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–1757. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 16.Schottelius M, et al. First (18)F-labeled tracer suitable for routine clinical imaging of sst receptor-expressing tumors using positron emission tomography. Clin Cancer Res. 2004;10:3593–3606. doi: 10.1158/1078-0432.CCR-03-0359. [DOI] [PubMed] [Google Scholar]
- 17.Haubner R, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]galacto-RGD. PLoS Med. 2005;2:244–252. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 19.Backer MV, et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–509. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, et al. Nanoparticles for bioimaging. Adv Colloid Interface Sci. 2006;123–126:471–485. doi: 10.1016/j.cis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 22.Ntziachristos V, et al. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson OC, et al. Pharmacokinetics of radiolabelled anticancer drugs for positron emission tomography. Curr Pharm Des. 2003;9:917–929. doi: 10.2174/1381612033455288. [DOI] [PubMed] [Google Scholar]
- 24.Jayson GC, et al. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst. 2002;94:1484–1493. doi: 10.1093/jnci/94.19.1484. [DOI] [PubMed] [Google Scholar]
- 25.Shani J, Wolf W. A model for prediction of chemotherapy response to 5-fluorouracil based on the differential distribution of 5-[18F]fluorouracil in sensitive versus resistant lymphocytic leukemia in mice. Cancer Res. 1977;37:2306–2308. [PubMed] [Google Scholar]
- 26.Hsueh WA, et al. Predicting chemotherapy response to paclitaxel with 18F-fluoropaclitaxel and PET. J Nucl Med. 2006;47:1995–1999. [PubMed] [Google Scholar]
- 27.Saleem A, et al. Metabolic activation of temozolomide measured in vivo using positron emission tomography. Cancer Res. 2003;63:2409–2415. [PubMed] [Google Scholar]
- 28.Saleem A, et al. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide in patients by positron emission tomography. J Clin Oncol. 2001;19:1421–1429. doi: 10.1200/JCO.2001.19.5.1421. [DOI] [PubMed] [Google Scholar]
- 29.Bergstrom M, et al. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol. 2003;59:357–366. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 30.Bading JR, et al. Kinetic modeling of 5-fluorouracil anabolism in colorectal adenocarcinoma: a positron emission tomography study in rats. Cancer Res. 2003;63:3667–3674. [PubMed] [Google Scholar]
- 31.Nunn AD. The cost of developing imaging agents for routine clinical use. Invest Radiol. 2006;41:206–212. doi: 10.1097/01.rli.0000191370.52737.75. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, et al. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science. 2005;310:1793–1796. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 33.Dehdashti F, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–56. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 34.Haubner R, et al. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 35.Bremer C, et al. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 36.Pal A, et al. Molecular imaging of EGFR kinase activity in tumors with 124I–labeled small molecular tracer and positron emission tomography. Mol Imaging Biol. 2006;8:262–277. doi: 10.1007/s11307-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Jones PM, et al. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–706. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 38.Smith-Jones PM, et al. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–796. [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor JP, et al. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–195. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas AL, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23:4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 41.Morgan B, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 42.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 43.Leach MO, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer. 2005;92:1599–1610. doi: 10.1038/sj.bjc.6602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massoud TF, et al. Reporter gene imaging of protein-protein interactions in living subjects. Curr Opin Biotechnol. 2007;18:31–37. doi: 10.1016/j.copbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulmurugan R, et al. Noninvasive imaging of protein-protein interactions in living subjects by using reporter protein complementation and reconstitution strategies. Proc Natl Acad Sci USA. 2002;99:15608–15613. doi: 10.1073/pnas.242594299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luker KE, et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA. 2004;101:12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol. 2006;24:3282–3292. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 48.Shankar LK, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 49.Juweid ME, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 50.Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs) Eur J Cancer. 2002;38 Suppl 5:S60–S65. doi: 10.1016/s0959-8049(02)80604-9. [DOI] [PubMed] [Google Scholar]
- 51.Su H, et al. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659–5667. doi: 10.1158/1078-0432.CCR-06-0368. [DOI] [PubMed] [Google Scholar]
- 52.Vesselle H, et al. In vivo validation of 3'deoxy-3'-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 53.Chen W, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46:945–952. [PubMed] [Google Scholar]
- 54.Chen W, et al. Predicting response of malignant brain tumors to bevacizumab and irinotecan therapy with FLT and FDOPA PET [abstract #78P] J Nucl Med. 2006;47 [Google Scholar]