Abstract
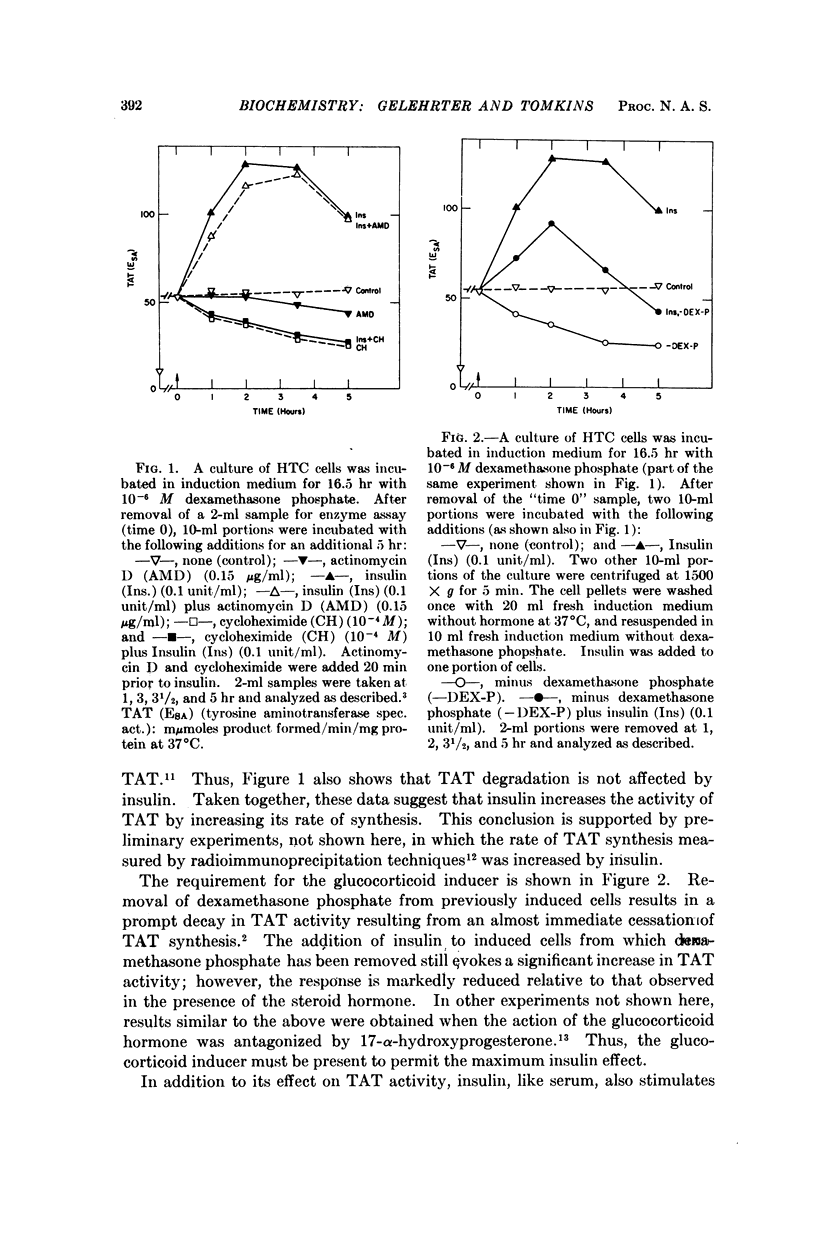
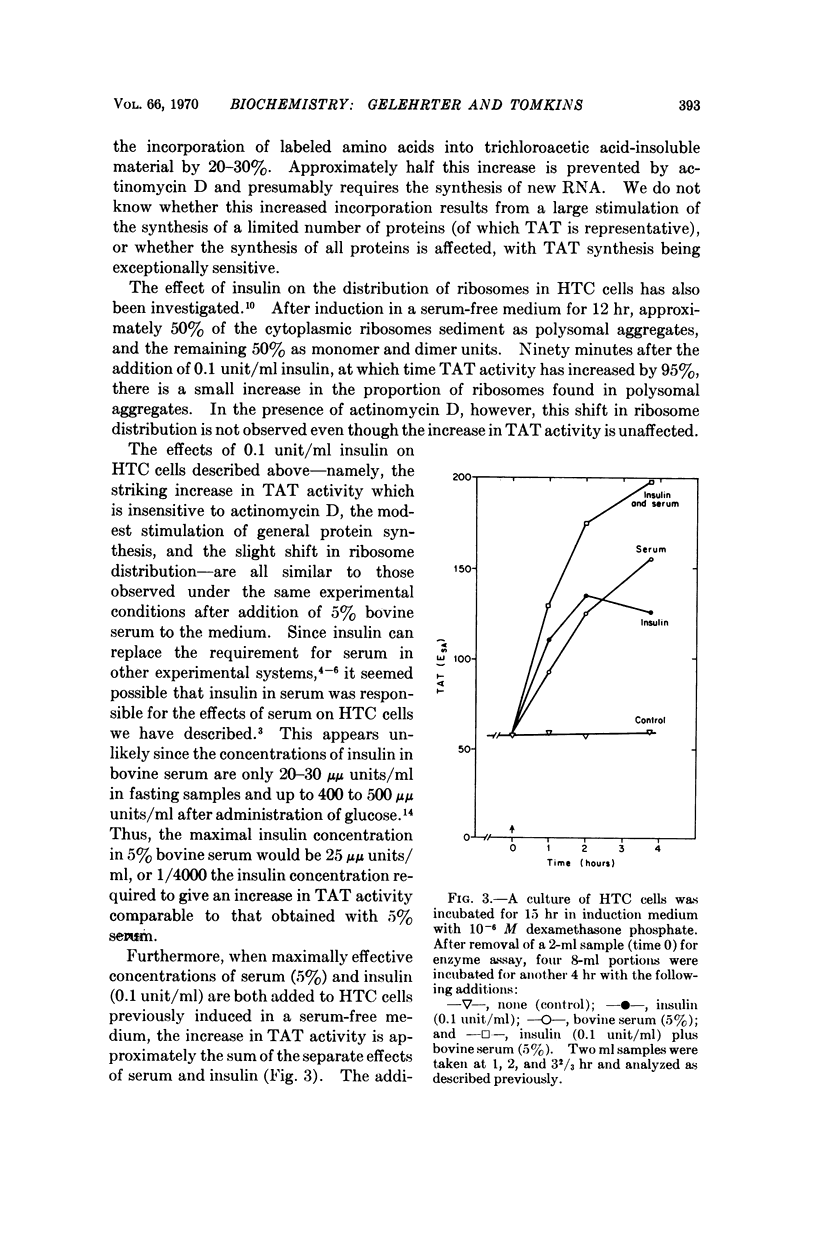
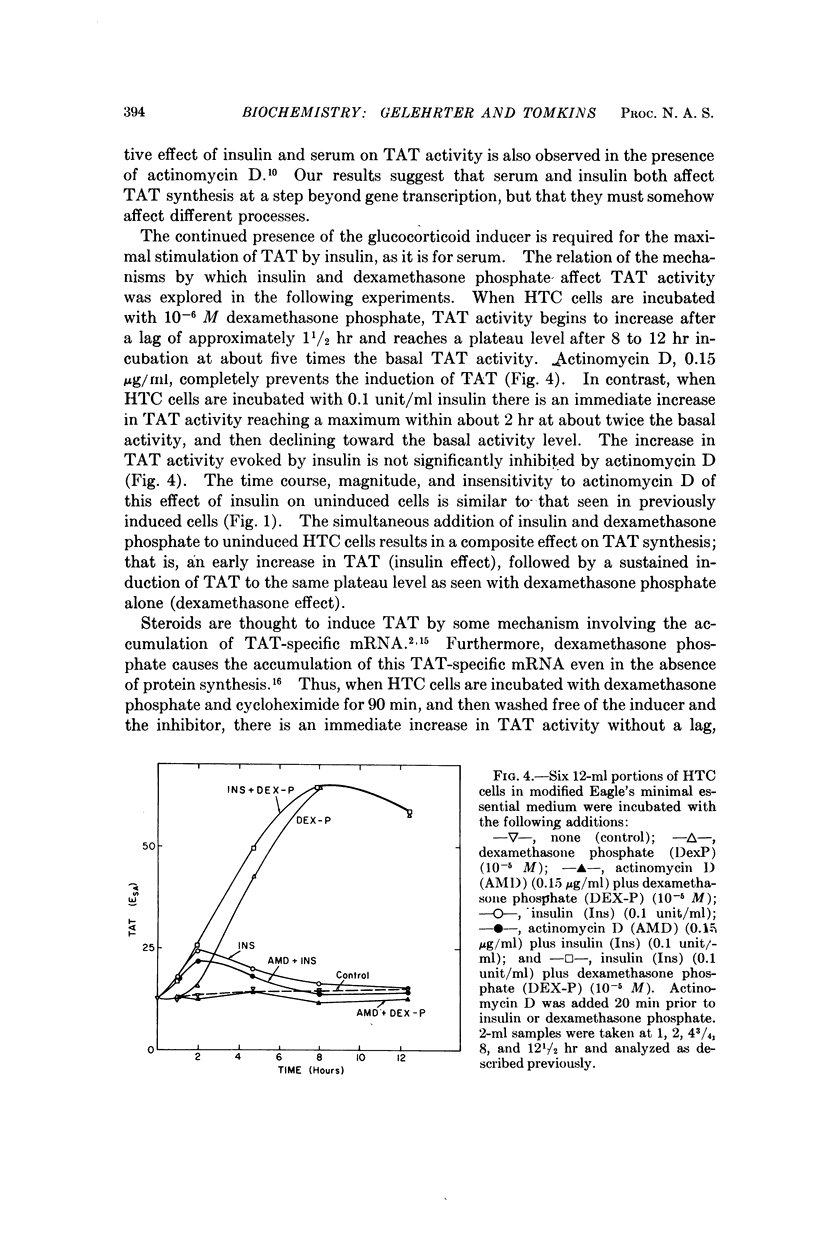
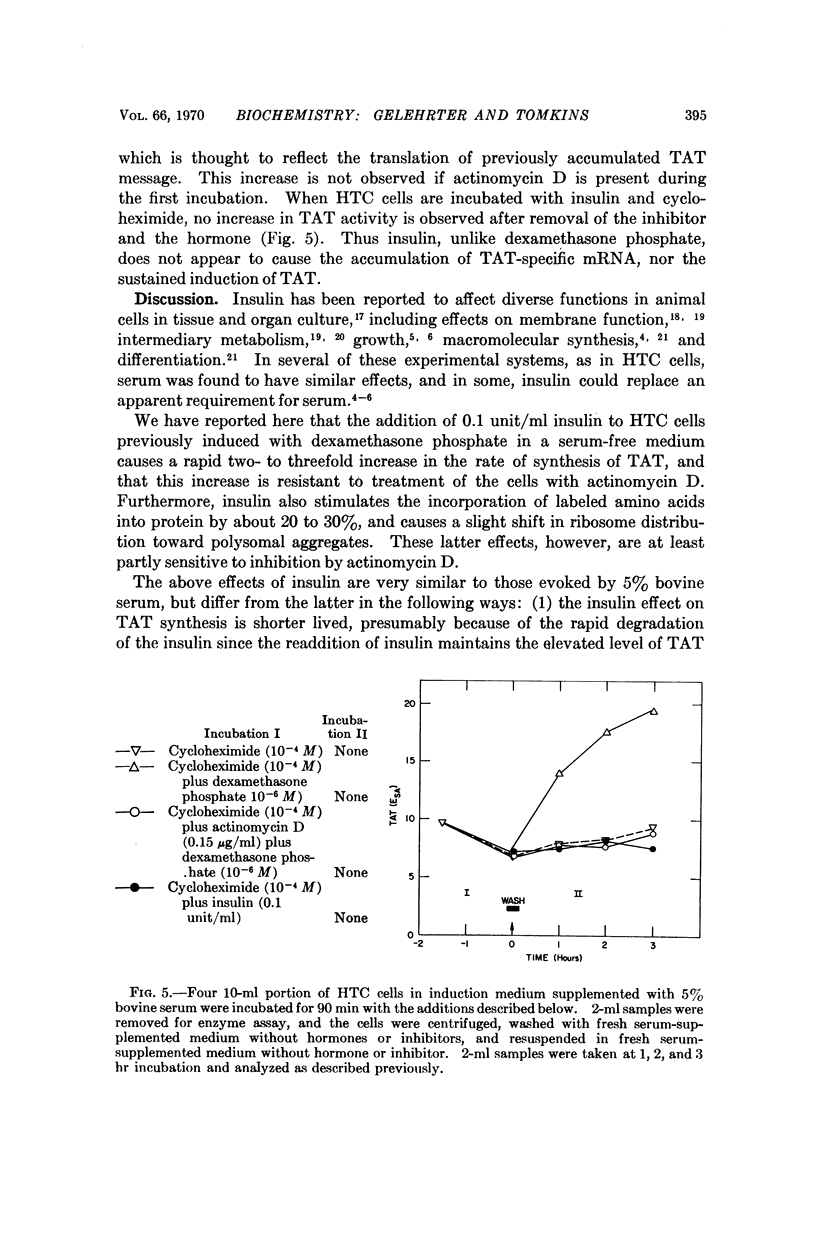
Adrenal steroid hormones induce the synthesis of tyrosine amino-transferase (TAT) in HTC cells, an established line of rat hepatoma cells in tissue culture. The addition of insulin to cells previously induced in a serum-free medium results in a rapid two- to threefold further increase in the rate of synthesis of TAT and a small increase in total amino acid incorporation. These changes do not require concomitant RNA synthesis, suggesting that insulin acts at a step in protein synthesis beyond that of gene transcription. Although the effects of insulin on HTC cells are similar to those caused by dialyzed bovine serum, evidence is presented that insulin and serum affect different aspects of TAT synthesis. Unlike the glucocorticoids, insulin does not cause the accumulation of TAT mRNA, nor the sustained induction of TAT. The continued presence of the inducing steroid is required to permit maximal expression of the insulin effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Martin D., Jr, Tompkins G. Control of degradation and synthesis of induced tyrosine aminotransferase studied in hepatoma cells in culture. Nature. 1969 Nov 22;224(5221):806–808. doi: 10.1038/224806b0. [DOI] [PubMed] [Google Scholar]
- Chambers J. W., Georg R. H., Bass A. D. Effect of hydrocortisone and insulin on uptake of alpha-aminoisobutyric acid by isolated perfused rat liver. Mol Pharmacol. 1965 Jul;1(1):66–76. [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. Control of tyrosine aminotransferase synthesis in tissue culture by a factor in serum. Proc Natl Acad Sci U S A. 1969 Oct;64(2):723–730. doi: 10.1073/pnas.64.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. The role of RNA in the hormonal induction of tyrosine aminotransferase in mammalian cells in tissue culture. J Mol Biol. 1967 Oct 14;29(1):59–76. doi: 10.1016/0022-2836(67)90181-7. [DOI] [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Hager C. B., Kenney F. T. Regulation of tyrosine-alpha-ketoglutarate transaminase in rat liver. VII. Hormonal effects of synthesis in the isolated, perfused liver. J Biol Chem. 1968 Jun 25;243(12):3296–3300. [PubMed] [Google Scholar]
- Holten D., Kenney F. T. Regulation of tyrosine alpha-ketoglutarate transaminase in rat liver. VI. Induction by pancreatic hormones. J Biol Chem. 1967 Oct 10;242(19):4372–4377. [PubMed] [Google Scholar]
- LESLIE I., FULTON W. C., SINCLAIR R. The metabolism of human embryonic and malignant cells and their response to insulin. Biochim Biophys Acta. 1957 May;24(2):365–380. doi: 10.1016/0006-3002(57)90207-x. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., OVE P. Growth factors for mammalian cells in culture. J Biol Chem. 1959 Oct;234:2754–2758. [PubMed] [Google Scholar]
- Lockwood D. H., Voytovich A. E., Stockdale F. E., Topper Y. J. Insulin-dependent DNA polymerase and DNA synthesis in mammary epithelial cells in vitro. Proc Natl Acad Sci U S A. 1967 Aug;58(2):658–664. doi: 10.1073/pnas.58.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- Martin D. W., Jr, Tomkins G. M., Bresler M. A. Control of specific gene expression examined in synchronized mammalian cells. Proc Natl Acad Sci U S A. 1969 Jul;63(3):842–849. doi: 10.1073/pnas.63.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL J., PEARSON E. S. The action of insulin on the metabolism of cell cultures. J Endocrinol. 1960 Dec;21:287–294. doi: 10.1677/joe.0.0210287. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Tomkins G. M. Evidence for the steroid-induced accumulation of tyrosine-aminotransferase messenger RNA in the absence of protein synthesis. Proc Natl Acad Sci U S A. 1968 May;60(1):222–228. doi: 10.1073/pnas.60.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G., Amos H. Insulin dependence of cells in primary culture: influence on ribo-some integrity. Nature. 1968 Sep 28;219(5161):1366–1367. doi: 10.1038/2191366a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Induction of tyrosine-alpha-ketoglutarate transaminase in fetal rat liver. J Biol Chem. 1968 Mar 10;243(5):900–906. [PubMed] [Google Scholar]



