Abstract
Obesity, diabetes and consequently atherosclerotic vascular disease have become major health and public health issues worldwide. The increasing and staggering prevalence of obesity might not only be explained by nutritional habits or the reduction of energy expenditure through decreased physical activity. In addition, recent studies have focused on intestinal microbiota as environmental factors that increase energy yield from diet, regulate peripheral metabolism and thereby increase body weight. Obesity is associated with substantial changes in composition and metabolic function of gut microbiota, but the pathophysiological processes driving this bidirectional relationship have not been fully elucidated. This review discusses the relationships between the following: composition of gut microbiota, energy extracted from diet, synthesis of gut hormones involved in energy homeostasis, production of butyrate and the regulation of fat storage.
Keywords: Gut microbiota, Insulin resistance, Obesity, Review, Type 2 diabetes
Introduction
The twin epidemics of obesity and type 2 diabetes mellitus have generated a wealth of literature regarding the intricate mechanisms of human metabolism in general and insulin resistance in particular. To date, many of the mechanistic studies have mainly focused on the biology of relationships between various human organs and cell systems. In contrast, geneticists have mainly focused on the human genome in their attempts to unravel the risk factors for type 2 diabetes mellitus. Nevertheless, there is an increasing body of literature that directs its attention to a possible third culprit: the gut microbiota [1–3]. These micro-organisms and thus their bacterial genome (also called the microbiome) are increasingly considered important pathogenic factors in various diseases ranging from gastrointestinal tract diseases such as inflammatory bowel disease to obesity [4]. With hindsight, this should hardly have come as a surprise, given the ubiquity of symbiotic processes in nature. The purpose of this review is to elaborate on how gut microbiota might contribute to the pathophysiology of obesity and type 2 diabetes mellitus.
Obesity is an increasingly common condition associated with an increased risk of type 2 diabetes mellitus and cardiovascular disease [5]. The causal role of obesity in type 2 diabetes is highlighted by the fact that preventive weight reduction is associated with a decreased incidence of diabetes mellitus in multiple studies [6, 7]. Moreover, weight loss following bariatric surgery in morbidly obese patients with type 2 diabetes leads to a rapid reversal of the pathophysiology and subsequent resolution of diabetes mellitus [7]. Interestingly, this glycaemic improvement actually occurs before weight loss. In addition, there is growing evidence that the increasing prevalence of obesity might not only be explained by nutritional habits or the reduction of energy expenditure through decreased physical activity. Thus even within families, some individuals seem more susceptible to diet-induced weight gain and hyperglycaemia, suggesting that other factors than those residing in the human genome are involved [8–10]. The gut microbiota could therefore be prime candidates to explain part of the residual differences in body weight between individuals.
The human gut microbiota
Until recently, our understanding of human gut microbiota was limited by technical issues. Although a large fraction of the dominant (anaerobic) gut microbiota still remains impossible to culture, the development of 16S ribosomal RNA gene-based approaches has facilitated the identification and classification of bacteria.
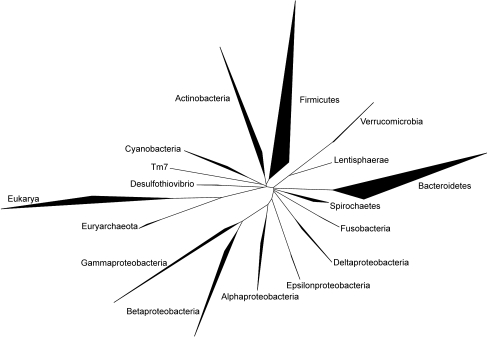
The human intestinal tract contains a large variety of micro-organisms, of which bacteria are the most dominant and diverse (Fig. 1). As a whole, the microbiome is more than 100 times larger than the human genome [11]. Thus, intestinal microbiota can be viewed as an ‘exteriorised organ’ that contributes to overall metabolism and plays a role in converting food into nutrients and energy. The community of at least 1014 bacteria is dominated by anaerobic bacteria and composed of 500 to 1000 different species [12]. Three bacterial divisions, the Firmicutes (gram-positive), Bacteroidetes (gram-negative) and Actinobacteria (gram-positive) dominate the adult human gut microbiota. The Firmicutes is the largest bacterial phylum and contains more than 200 genera, including Lactobacillus, Mycoplasma, Bacillus and Clostridium. The Bacteroidetes (including about 20 genera) and the Actinobacteria (gram-positive) also belong to the dominant gut microbiota, but the latter are frequently missed by RNA gene sequencing and can only be detected by fluorescent in situ hybridisation (FISH) [13].
Fig. 1.
Phylogenetic tree representing the groups of bacteria most frequently detected in human faeces using 16S rRNA gene sequencing. The extent of the bold areas indicate diversity and abundance of the bacterial groups
Fetuses are sterile in uteri, but in the first year of life the infant intestinal tract progresses from sterility to extremely dense colonisation with a mixture of microbes broadly similar to that found in the adult intestine [12]. During birth and rapidly thereafter, bacteria from the mother and the surrounding environment colonise the infant’s gut. After this inoculation, the microbiota changes rapidly, presumably under the influence of diet. At the age of 4 years, the gut microbiota in host individuals has fully matured. The final composition of the microbiota is influenced by the host genotype, colonisation history, the physiology of the host and an array of environmental factors [14]. Genetic makeup of the individual also influences the composition of the core microbiota, as was shown in several studies [10, 14]. For instance, the microbiota of monozygotic twins living separately, is notably more similar than the microbiota of unrelated individuals [10]. In contrast, the environment seems to be of less importance, since marital partners did not have a significantly greater similarity of bacterial communities than unrelated individuals, despite the fact that these partners lived in the same environment and had similar dietary habits [14].
The effect of diet on the composition of the infant microbiota is not clear; numerous studies report a lower abundance of bifidobacteria in the microbiota of formula-fed infants relative to breast-fed infants [15, 16], yet other reports have found no such differences [17]. The transformation to adult-type microbiota is probably triggered by multiple host and external factors [18]. After the transformation to adult-type microbiota, the gut microbiota remains remarkably constant until the 7th decade, fluctuating around an individual core of stable colonisers [13, 19, 20]. During life, the composition of these micro-organisms can be modulated by antibiotics. Short-term treatment in humans with a single dose of oral antibiotics affects the gut microbiota for as long as 4 weeks before it then tends to revert to its original composition [21]. In conclusion, each individual has his or her own personal and unique microbiota, with a large variability in microbiota between individuals. This impressive diversity complicates the studies that attempt to establish relationships between the host’s health and the presence of specific microbial populations [13, 19].
Gut microbiota and metabolism
A number of studies have revealed that specific relationships exist between intestinal microbiota and human metabolism (Fig. 2). Gordon and his colleagues pioneered the investigation of gut microbiota as an environmental factor influencing fat storage and obesity. They found that young conventionally reared mice have 42% more total body fat and 47% more gonadal fat than germ-free mice [22]. This was surprising, since the control mice had a lower energy intake than germ-free mice. The presence of microbiota in itself apparently increased the energy yield from the host organism’s diet. Following up on this observation, the authors demonstrated that colonisation of young germ-free mice with microbiota from conventionally reared mice produces a 60% increase in body fat mass that is associated with increased insulin resistance, despite lower energy intake. These same researchers also demonstrated that faeces transplantation with microbiota from obese mice (ob/ob) results in a significantly greater increase in total body fat than colonisation with microbiota from lean donors [23]. Again, these findings underscore, in obese individuals, the increased efficiency of microbiota in extracting energy from diet [22, 24].
Fig. 2.
Possible links between the gut microbiota and metabolism. Details, see main text. Continuous lines, likely pathway; dotted lines, putative pathway
Studies of germ-free and control mice have also revealed that microbiota direct the host to increased hepatic triacylglycerol and glucose production. In fact, microbial colonisation of the gut might suppress expression of the fasting-induced adipose factor (FIAF), leading to suppression of this lipoprotein lipase (LPL) inhibitor and hence to increased activity of LPL. Increased LPL activity promotes increased uptake of fatty acids and triacylglycerol accumulation in adipocytes. The physiological importance of FIAF was further established by studying germ-free Fiaf (also known as Angptl4)-knockout and wild-type mice. In contrast to the situation in wild-type mice, germ-free Fiaf-knockout mice are similarly obese to their conventionally reared counterparts, indicating that FIAF is a key modulator of the microbiota-induced increase in fat storage [22]. Furthermore, Backhed and colleagues have also demonstrated that germ-free mice have increased levels of phosphorylated AMP-activated protein kinase in muscle and liver, which would stimulate NEFA oxidation [25]. Therefore, germ-free animals seem protected from diet-induced obesity by two complementary, but independent mechanisms, which result in decreased fatty acid storage: (1) elevated levels of FIAF; and (2) increased AMP-activated protein kinase activity.
Another pathway affecting host energy storage is the bacterial fermentation of complex dietary carbohydrates to monosaccharides and short-chain fatty acids (SCFA). These SCFAs bind to G protein-coupled receptor 4 (GPR41), which is produced by enteroendocrine cells in the intestine. Activation of GPR41 enhances production of peptide YY (PYY), an enteroendocrine cell hormone that normally inhibits gut motility, increases intestinal transit rate and reduces extraction of energy (SCFAs) from the diet, thus affecting peripheral glucose utilisation [26].
In summary, a large body of evidence generated in animal models has revealed a relationship between gut microbiota composition and obesity; however, this hypothesis remains to be tested in the human setting.
Altered gut microbiota in obesity
It is now increasingly accepted that the composition of intestinal microorganisms may change with body weight. Ley et al. [27] recently analysed 5,088 bacterial 16S rRNA gene sequences from fat ob/ob mice, lean ob/+ mice and wild-type siblings. They demonstrated that obese animals have a 50% reduction in the abundance of Bacteroidetes and a proportional increase in Firmicutes. Ob/ob mice also harboured more methanogenic Archaea, which may increase the efficiency of bacterial fermentation [23]. Metagenomic analyses revealed that the caecal microbiota in the ob/ob mice were producing more SCFA through increased fermentation of dietary polysaccharides. The increased extraction of energy from dietary fibres may partly contribute to the excessive weight gain of ob/ob mice [23]. In this study, changes in Bacteroidetes and Firmicutes were noted across the division, i.e. they were not due to blooms or extinction of specific organisms. However, upon induction of obesity in mice, the increase in Firmicutes seemed mainly attributable to an increase in the Mollicutes class [28]. Similar to these animal experiments, Bacteroidetes tend to decrease and Firmicutes to increase in the faeces of obese compared with lean humans [19] (see also Fig. 2). Obese people harbour fewer Bacteroidetes and more Firmicutes than lean controls, whereas upon a carbohydrate- or fat-restricted low-energy diet, Bacteroidetes increased and Firmicutes decreased. These data suggest a relationship between obesity and the diversity of intestinal microbiota. Interestingly, the increase in Bacteroidetes was significantly correlated with weight loss achieved, but not with total energy intake, suggesting interactions between diet, gut microbiota and host metabolism [19, 29].
However, other human studies do not support these specific changes. Duncan et al. [3], using FISH to monitor faecal bacteria, could not confirm this ‘high Firmicutes/low Bacteroidetes’ hypothesis. In fact, they reported no differences in the proportion of Bacteroidetes measured in faecal samples between obese and non-obese participants. They also found no significant relationship between BMI and the proportion of Bacteroidetes. They did, however, confirm a significant diet-dependent reduction in Firmicutes in faecal samples from obese individuals on a low-carbohydrate diet. In accordance with this last study, Zhang et al. [30] found in a sequencing-based study that there was no difference between the fractions of Bacteroidetes in obese and non-obese participants. These authors showed that gastric bypass surgery (GBP) strongly altered gut microbiota and resulted in a large increase in Gammaproteobacteria (members of the family Enterobacteriaceae), a proportional decrease in Firmicutes and a loss of methanogens.
In line with this, a recent study by Schwiertz et al. [31] linked obesity as well as the composition of human microbiota with the production of SCFA. Moreover, the authors were unable to identify any correlation between obesity and higher proportions of Firmicutes; they also suggested that not the ratio of Firmicutes: Bacteroidetes is important, but rather the amount of SFCA produced. Finally, Kalliomaki et al. [32] examined whether early differences in faecal microbiota composition in children predict subsequent obesity. The bifidobacterial numbers in faecal samples during infancy, as assessed by FISH with additional flow cytometry, were lower in children with subsequent obesity, suggesting that variations in gut microbiota early in life may confer an increased risk of developing obesity in later life. Thus, the relationships between the various bacterial groups and obesity still remain a matter of debate.
Gut microbiota and inflammation
Obesity and diabetes are both characterised by low-grade inflammation of unclear origin. In in vitro and animal models an increase in proinflammatory cytokines, such as TNF-α, has led to tissue insulin resistance [33]. Cani et al. demonstrated that bacterial lipopolysaccharide (LPS) is a gut microbiota-related factor that triggers secretion of proinflammatory cytokines [34]. LPS is continuously produced in the gut through lysis of gram-negative bacteria. In fact, in the study mentioned above, continuous subcutaneous low-rate infusion of LPS led to excessive weight gain and insulin resistance in mice. Moreover, LPS receptor Cd14-knockout mice tend to be resistant to this chronic inflammatory state. Cani et al. [35] showed that a high-fat diet decreases the number of bifidobacteria and increases plasma LPS. They also demonstrated that modulation of gut microbiota, e.g. by antibiotic treatment or dietary intervention with oligofructoses, reduced glucose intolerance, decreased body weight gain and inhibited inflammation in mice [36, 37]. These findings suggest that changes in the gut microbiota could be responsible for increased endotoxaemia in response to a high-fat diet, which in turn would trigger the development of obesity and diabetes mellitus.
Another putative pathway linking microbiota to chronic inflammation could be butyrate bioavailability as obese participants are characterised by decreased plasma butyrate levels [38]. Apart from its well-known function as an essential energy source for colon epithelial cells, butyrate also has anti-inflammatory properties [39–41]. The two major butyrate-producing bacterial groups are the Roseburia/E. rectale species and F. prausnitzii (cluster of Firmicutes). Many studies show that the dietary intake of fermentable carbohydrates can influence butyrate production [42, 43]. Particularly, diets containing high levels of non-digestible carbohydrates stimulate the growth of particular butyrate-producing bacteria and therefore lead to elevated plasma levels of butyrate. Interestingly, older publications showed that increasing plasma levels of butyrate improve insulin sensitivity and increase energy expenditure in animal models of diet-induced obesity [44, 45]. Thus, the available evidence suggests that butyrate production from food glycans could be a contributing factor to obesity. Although the exact pathophysiological processes driving this bidirectional relationship are not yet elucidated [46], the potential involvement of this SCFA may open up interesting therapeutic avenues in the fight against obesity.
Gut microbiota and gut hormones
The gut communicates with areas in the hypothalamus that control energy balance by means of neural and endocrine pathways. Gut hormones are produced by specialised enteroendocrine cells scattered along the gastrointestinal tract from the stomach to the distal colon. Although these account for only 1% of the cells in the intestinal mucosa, the gastrointestinal tract can be regarded as a major endocrine organ.
Incretins are gut hormones that potentiate glucose-induced insulin secretion and may be responsible for up to 70% of postprandial insulin secretion [47]. The two main incretins are gastric inhibitory peptide (GIP) and glucagon like peptide-1 (GLP-1). GIP is secreted by K-cells and released from the upper small intestine (duodenum and proximal jejunum) in response to oral ingestion of carbohydrates and lipids. GLP-1 is mainly produced in mucosal L cells, located in the distal intestine (ileum and colon), but also in pancreatic alpha cells as well as in neurons from several brain areas. The secretion of GIP in type 2 diabetes is normal, whereas that of GLP-1 is reduced [47, 48]. GLP-1 secretion also seems to be reduced and delayed in obese patients compared with lean, healthy controls [49]. Therefore, different intestinal microbiota composition in obese humans may possibly contribute to this reduced GLP-1 secretion.
The secretion pattern of GLP-1 is biphasic, with an initial rapid rise that occurs 15 to 30 min after ingestion of a meal [50]. This quick rise occurs before nutrients can reach the ileum, where the GLP-1-producing L cells are located. These observations make it unlikely that GLP-1 release is only stimulated by direct action of nutrients on L cells. In line with the idea of a neuroendocrine loop, several peptides and neurotransmitters have been suggested to trigger GLP-1 secretion [51]. For example, GIP released when nutrients are present in the upper intestine was shown to be a potent stimulus of the first peak in GLP-1 secretion [51]. GLP-1 release is also believed to be triggered by the autonomic nervous system [51].The second peak of GLP-1 secretion is believed to occur as a result of direct interactions between nutrients and L cells, which also stimulate GLP-1 secretion. In this regard, different nutrient compositions of a meal result in different peptide release as glucose and fat have been found to be potent stimulators of GLP-1 secretion, whereas proteins do not appear to stimulate GLP-1 release [52]. Experiments with alpha-glucosidase inhibitors such as acarbose, which delays digestion and absorption of carbohydrates and causes a transfer of nutrients to distal segments of the intestine, support this action. Thus treatment with acarbose reduces GIP secretion, but augments GLP-1 secretion and improves glucose tolerance in diabetic participants [53].
The modulation of gut peptides involved in the control of glucose homeostasis could be one mechanism by which the modulation of gut microbiota via specific dietary fibres is associated with an improvement of metabolic disorders. For example, feeding rats with the prebiotic fibre oligofructose led to an increase in jejunal GIP concentrations and caecal GLP-1 [54]. Interestingly, oligofructose feeding in rats promotes L-cell differentiation in the proximal colon, contributing to higher endogenous GLP-1 production and subsequent improvement of diabetes induced by high-fat diet in mice [55]. Several studies have shown that prebiotics containing short-chain oligosaccharides decrease food intake, fat mass development and hepatic steatosis in normal and obese rats. In humans, the addition of non-digestible carbohydrates (oligofructose) to the diet also protects against body weight gain, fat mass development and serum triacylglycerol accumulation induced by a high-fat diet [56, 57]. Furthermore, oligofructose has been shown to promote satiety in healthy humans [57].
Similarly, GBP results in improvement of glycaemic control [58–60]. This effects seems to occur rapidly after surgery and often prior to substantial weight loss. The rapid effect of GBP on weight loss and metabolic improvement could be partly due to changes in gut hormone levels after surgery. Several studies have reported an increase in postprandial plasma levels of PYY and/or GLP-1 as early as 2 days after bypass [61]. The mechanism by which GBP causes increased gut hormone production still remains unclear, but it is obvious that the altered anatomy after GBP is the main raison for changes in gut hormone release. For example after GBP, nutrients reach the distal small intestine more rapidly, enhancing the release of GLP-1, which improves glucose metabolism. Altered microbial composition after GBP may also contribute to the increased release of gut hormones. Secretion of PYY, another hormone produced in the distal gut, was also found to be increased after GBP. This exaggerated beneficial response observed after GBP could be another reason that explains the improvement in glucose homeostasis after surgery [62]. In line with this, as the large gut microbiota population also shifts in patients after GBP [30], it cannot be excluded that motility disorders associated with long-standing obesity and diabetes mellitus (e.g. due to autonomic diabetic neuropathy) may affect small intestine gut microbiota composition, yet data on this topic are lacking. Thus, further research investigating the impact of intestinal anatomical alteration on gut microbiota and consequent changes in food ingestion and digestion is warranted.
Potential intervention in microbiota composition
The use of antibiotics to alter gut microbiota in genetically obese mice reduced body weight, and improved fasting glycaemia and glucose tolerance, suggesting that the gut microbiota could be a novel target for treating metabolic diseases [36, 63]. Concomitant reduction of LPS as well as increased adiponectin levels enhanced the glucose-lowering effects of antibiotics. Taking a reductionist approach, the composition of intestinal microorganisms could be influenced by changing exogenous dietary factors (e.g. butyrate supplementation, oligofructose) or by transplantation of human donor faeces [23, 44]. We are currently investigating the effect of donor faeces transplantation (derived from lean healthy participants) on glucose homeostasis and (intestinal) inflammation in otherwise healthy participants with metabolic syndrome (FATLOSE trial). This double-blind, randomised, controlled trial will hopefully provide more insight into microbiota and host physiology, as well as into the effectiveness of gut microbiota manipulation in humans.
Conclusions
Intestinal microbiota may play a pivotal role in converting nutrients into energy. Variations in the composition of microbiota are found in obese humans and mice. Increased energy yield from diet in obese mice and humans could be a contributing factor to obesity, although the pathophysiological processes driving this bidirectional relationship have not been fully elucidated. With the rapid developments in (relatively cheap) high-throughput techniques (involving phylogenetic microarrays based on small subunit rRNA sequences and metagenomics approaches exploiting rapid sequencing technologies), we might actually be able to unravel the endocrinological potential of gastrointestinal tract microbiota [64]. The last important clinical question then remains: Is it possible to modify the gut microbiota to reduce the impact of high-fat feeding on the occurrence of metabolic disease in humans?
Acknowledgements
The authors thank H. Smidt (Laboratory of Microbiology, Wageningen University, the Netherlands) for help with construction of the phylogenetic tree in Fig. 1 and T. Thörig for graphic support in Fig. 2. M. Nieuwdorp is supported by a ZonMW-VENI grant 2008 (016.096.044) and was awarded a personal unrestricted grant from the Netherlands Foundation for Cardiovascular Excellence in 2008.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- FIAF
Fasting-induced adipose factor
- FISH
Fluorescent in situ hybridisation
- GBP
Gastric bypass surgery
- GIP
Gastric inhibitory peptide
- GLP-1
Glucagon like peptide-1
- GPR41
G protein-coupled receptor 4
- LPL
Lipoprotein lipase
- LPS
Lipopolysaccharide
- PYY
Peptide YY
- SCFA
Short-chain fatty acids
References
- 1.Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–1483. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 2.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–469. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- 3.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 4.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 7.Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008;51:1781–1789. doi: 10.1007/s00125-008-1116-7. [DOI] [PubMed] [Google Scholar]
- 8.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 9.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dethlefsen L, Fall-Ngai M, Relman DA. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Gordon JI. Inaugural article: honor thy symbionts. Proc Natl Acad Sci USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 14.Zoetendal EG, Akkermans AD, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis. 2001;13:129–134. doi: 10.1080/089106001750462669. [DOI] [Google Scholar]
- 15.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Favier CF, Vaughan EE, de Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orrhage K, Nord CE. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl. 1999;88:47–57. doi: 10.1111/j.1651-2227.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 18.Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:1556–1573. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 20.Zoetendal EG, Akkermans AD, de Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:2383–2400. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 24.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 25.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2008;33:758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 32.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 34.Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729–734. doi: 10.1097/MCO.0b013e3282efdebb. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxaemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 36.Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 37.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 38.Vice E, Privette JD, Hickner RC, Barakat HA. Ketone body metabolism in lean and obese women. Metabolism. 2005;54:1542–1545. doi: 10.1016/j.metabol.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Saemann MD, Bohmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 40.Segain JP, de la Raingeard BD, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinolo MA, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct. 2009;27:48–55. doi: 10.1002/cbf.1533. [DOI] [PubMed] [Google Scholar]
- 42.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 43.Mahowald MA, Rey FE, Seedorf H, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci USA. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manns JG, Boda JM. Insulin release by acetate, propionate, butyrate, and glucose in lambs and adult sheep. Am J Physiol. 1967;212:747–755. doi: 10.1152/ajplegacy.1967.212.4.747. [DOI] [PubMed] [Google Scholar]
- 46.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 48.Knop FK, Vilsboll T, Hojberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56:1951–1959. doi: 10.2337/db07-0100. [DOI] [PubMed] [Google Scholar]
- 49.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rask E, Olsson T, Soderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 51.Herrmann-Rinke C, Voge A, Hess M, Goke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol. 1995;147:25–31. doi: 10.1677/joe.0.1470025. [DOI] [PubMed] [Google Scholar]
- 52.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 53.Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose) Scand J Gastroenterol. 1995;30:892–896. doi: 10.3109/00365529509101597. [DOI] [PubMed] [Google Scholar]
- 54.Kok NN, Morgan LM, Williams CM, Roberfroid MB, Thissen JP, Delzenne NM. Insulin, glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and insulin-like growth factor I as putative mediators of the hypolipidemic effect of oligofructose in rats. J Nutr. 1998;128:1099–1103. doi: 10.1093/jn/128.7.1099. [DOI] [PubMed] [Google Scholar]
- 55.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484–1490. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 56.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–37. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2006;60:567–572. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 58.Bose M, Olivan B, Teixeira J, Pi-Sunyer FX, Laferrere B. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg. 2008;19:217–229. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 63.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 64.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]