Abstract
AIMS
To evaluate inhibition of capsaicin-induced increase in dermal blood flow (DBF) following telcagepant (MK-0974), a potent and selective orally bioavailable calcitonin gene-related peptide (CGRP) receptor antagonist being developed for the acute treatment of migraine.
METHODS
A three-period crossover study in 12 healthy adult men. Each subject received a single oral dose of telcagepant 300 mg, telcagepant 800 mg or placebo at 0 h, followed 0.5 and 3.5 h later by two topical doses of 300 and 1000 µg capsaicin per 20 µl water–ethanol mixture. Capsaicin was applied at two sites on the volar surface of the subjects' left and right forearms. DBF was assessed by laser Doppler perfusion imaging immediately before (‘baseline’), and 0.5 h after each capsaicin application at 1 and 4 h. Plasma samples to determine telcagepant concentrations were collected immediately after laser Doppler perfusion imaging. A pharmacodynamic model was developed to explore the relationship between plasma concentration and inhibition of capsaicin-induced increase in DBF.
RESULTS
Geometric mean plasma concentrations after dosing with 300 mg and 800 mg telcagepant were 720 and 1146 nm, respectively, at 1 h, vs. 582 and 2548 nm, respectively, at 4 h. The pharmacodynamic model suggested that the EC90 for telcagepant inhibition of capsaicin-induced increases in DBF was 909 nm.
CONCLUSIONS
Telcagepant inhibits the increases in DBF induced by the topical application of capsaicin on the human forearm. This experimental medicine model may have utility to assist in dose selection for the development of CGRP receptor antagonists.
Keywords: blood flow, capsaicin, CGRP, migraine, telcagepant
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Calcitonin gene-related peptide (CGRP) was first described as a potent vasodilator.
CGRP is also increasingly recognized as a key player in the pathophysiology of migraine, and CGRP receptor antagonists potentially offer a new approach for treating migraine.
A novel pharmacodynamic assay to measure CGRP receptor antagonist activity non-invasively in humans has been developed, which involves measuring the increase in dermal blood flow induced by topical application of capsaicin on the forearm.
WHAT THIS STUDY ADDS
This study shows that the novel oral CGRP receptor antagonist, telcagepant, inhibits the increases in dermal blood flow induced by the topical application of capsaicin on the human forearm.
This experimental medicine model may have utility to assist in dose selection for the development of CGRP receptor antagonists.
Introduction
Calcitonin gene-related peptide (CGRP) was first described as a potent vasodilator [1, 2]. It acts primarily on vascular smooth muscle cells and may cause vasodilation via an endothelial-independent mechanism using adenylate cyclase [3, 4]. For some species, including humans, there are data available suggesting the possible contribution of an endothelial-dependent mechanism [5].
Apart from potent vasodilatory effects, the neuropeptide CGRP is also increasingly recognized as a key player in the pathophysiology of migraine [6, 7]. CGRP and its receptor are expressed in unmyelinated C-fibres and myelinated Aδ fibres and in multiple regions in the central nervous system, including areas associated with trigeminal circuits such as the hippocampus, amygdala, parabrachial nucleus, periaqueductal grey, trigeminal nucleus, and trigeminal ganglia [8, 9]. During a migraine headache, activation of the trigeminovascular system results in the release of CGRP into the cranial circulation leading to dilation of intracranial extracerebral arteries, including the middle cerebral artery [10].
The clinical efficacy of the triptans, so far the most successful class of acute antimigraine drugs, may be attributable to reversal of this vasodilation via 5-HT1B receptor activation as well as to inhibition of CGRP release (both peripheral and central) via activation of pre-junctional 5-HT1D receptors located on trigeminal fibres [6]. Indeed, 5-HT1D receptors have been shown to be co-localized with CGRP in human trigeminal ganglia [11].
These observations suggest that inhibition of CGRP-driven pathophysiological processes resulting from activation of the trigeminovascular system may yield a novel therapeutic approach to treating migraine. Blockade of these CGRP-dependent processes by the intravenous administration of the CGRP receptor antagonist olcegepant (BIBN4096BS) confirmed the efficacy of this new approach for reducing pain in migraine [12]. This confirmation fuelled the development of orally available CGRP receptor antagonists as well as research into pharmacodynamic models for the in vivo assessment of these compounds.
A novel pharmacodynamic assay to measure CGRP receptor antagonist activity non-invasively in vivowas first established and validated in the rhesus monkey [13]. Subsequently, the model was extended to humans as a reproducible model that can easily be incorporated in early clinical drug development studies [14]. In this model, changes in dermal blood flow (DBF) in the forearm are measured via laser Doppler imaging following the topical application of a capsaicin solution. Capsaicin activates the transient receptor potential vanilloid type 1 receptor (TRPV1), producing neurogenic inflammation and vasodilation via the local release of vasoactive mediators, including CGRP and substance P (SP), and possibly also by the activation of dorsal root reflexes. The resulting dermal vasodilation in the rhesus forearm is driven primarily by CGRP and can be blocked completely by CGRP receptor antagonists, thus permitting the assessment of antagonist potency in vivo against endogenously released CGRP [13]. In humans, the moderately potent CGRP antagonist CGRP8–37 also blocks a substantial part of the capsaicin-induced DBF increase, whereas indomethacin and L-NG-monomethyl arginine (inhibitors of cyclooxygenase and nitric oxide synthase, respectively) did not affect it [15].
Using the microvascular dynamics of the dermis as a pharmacodynamic marker, the aim of this study was to assess the ability of the novel oral CGRP receptor antagonist telcagepant [16–18] to block capsaicin-induced increases in DBF in humans and to establish a pharmacokinetic/pharmacodynamic relationship for the compound.
Methods
Ethics
The study protocol was reviewed and approved by the Independent Ethics Committee affiliated with the Katholieke Universiteit, Leuven, Belgium. Before enrolment, all participants gave informed consent in writing after a full verbal and written explanation of the study. The study was conducted in accordance with local law, the ethical principles of the Declaration of Helsinki, as well as the principles of Good Clinical Practice.
Subjects
Twelve healthy, male subjects between 18 and 45 years old were enrolled. Mean ± SD (range) for age, weight, and height were 22 ± 2 years (20–26), 74 ± 7 kg (61–85) and 181 ± 5 cm (176–188), respectively. All completed the study per protocol.
Design and procedure
This study (Merck Protocol 001) was a randomized, placebo-controlled, three-period crossover study. During each period, subjects received a single dose of one of the following treatments given orally as a liquid-filled capsule: telcagepant 300 mg, telcagepant 800 mg or placebo. An early experimental formulation of telcagepant was used that differed from that used in subsequent clinical trials in migraine patients [18–20] and the anticipated market formulation. The early experimental formulation had lower bioavailability than the later one. The sequence in which these treatments were administered over the course of the three periods was randomly assigned. Periods were separated by at least 1 week of washout.
Subjects received telcagepant 300 mg, telcagepant 800 mg, or placebo (time = 0 h) followed 0.5 and 3.5 h later by single topical doses of 300 and 1000 µg per 20 µl capsaicin solution (in ethanol/polysorbate 20/water) in 10-mm rubber ‘O’-rings at two sites on the volar surface of the subjects' left and right forearms. Capsaicin applications (300 and 1000 µg per 20 µl) were administered at two sites of the same forearm (right or left), and balanced by site (site 1 or site 2) on arm and by arm (right or left) per time point.
Laser Doppler scans were conducted immediately before (‘baseline’) and 0.5 h after each capsaicin application (i.e. at 1 and 4 h post telcagepant/placebo administration), allowing the maximal capsaicin-induced increase in DBF (i.e. 0.5 h post capsaicin application, as previously demonstrated [14]) to coincide with the appropriate time points. Plasma samples for the pharmacokinetic/pharmacodynamic analyses were collected at 1 and 4 h after telcagepant/placebo dosing, immediately after each laser Doppler DBF measurement. The timing of capsaicin applications and DBF assessments was based on the pharmacokinetic profile of the early formulation of telcagepant which, at doses up to 500 mg, demonstrated a Tmax of approximately 1–1.25 h and a plasma terminal half-life of 4–6 h, similar to that for the later formulation [21]. It was of interest to evaluate the ability of telcagepant to maintain activity over approximately 4 h post dose.
The skin perfusion of the test sites, i.e. the region delimited by the rubber O-rings, was mapped using a High Resolution Laser Doppler Perfusion Imager (HR-LDPI system, PeriScan PIM II®; Perimed, Järfälla, Sweden) as described by Van der Schueren et al.[14] (Figure 1). With the X-Y scanning laser probe, laser Doppler perfusion imaging allows two-dimensional mapping of blood flow variability over an extended skin surface. The method is noncontact, the laser beam being controlled by the computer-controlled rotation of a mirror about two perpendicular axes. The red light of a 633-nm helium-neon laser penetrates the skin variably to a depth of about 0.6 mm. The vasculature within these layers comprises vessels of variable size, orientation and function. For this reason, it is not meaningful to express the cutaneous flow in absolute terms, but rather as arbitrary ‘perfusion units’ (AU). The estimation of the average skin perfusion at the test sites was based on the measurement of perfusion values of approximately 60 individual sampling sites. These calculations were performed using the built-in statistical function of the HR-LDPI system (LDPIwin; Perimed). The methodology is fully described by Fullerton et al.[22].
Figure 1.
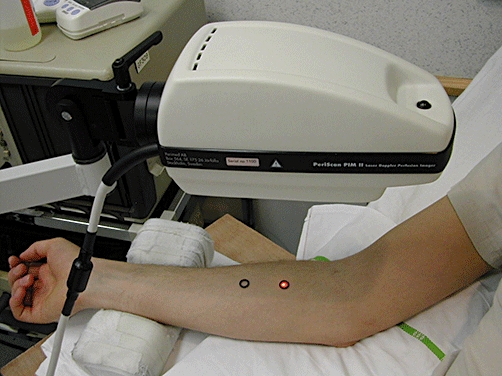
Assessment of dermal blood flow using laser Doppler following application of topical capsaicin to human forearm
Plasma samples were analysed for telcagepant concentrations. Both analyte and internal standard were isolated using on-line extraction from human plasma. The extracted analytes were analysed by high-performance liquid chromatography with tandem mass spectrometry detection [23]. The linear calibration curve ranged from 5 to 5000 nm. The lower limit of quantification for telcagepant in plasma was 5 nm.
Statistical analysis and pharmacodynamic modelling
Three analyses were performed. First, baseline DBF measurements were analysed to explore any treatment effects, since ‘baseline’ was prior to capsaicin application but after telcagepant administration. A mixed model with terms for period, site, arm, telcagepant dose and subject (as a random effect) was used for mean perfusion data at baseline.
Second, the percent difference from placebo in DBF at 1 and 4 h was calculated. This analysis did not take into account baseline data. Mean perfusion values were log transformed prior to analysis. An anova model appropriate for a three-period crossover design was used. The anova model included the following terms: subject (as a random effect), period, treatment, capsaicin dose, arm, site, treatment by capsaicin interaction, first-order carryover and within-subject error. A two-sided 90% confidence interval (CI) for the true difference (telcagepant–placebo) in log-scale capsaicin response was computed from the anova using the mean squared error and referencing a t-distribution for each telcagepant dose. The CI was back transformed to obtain a geometric mean and corresponding CI for the true mean ratio for fold difference from placebo in capsaicin response. The resulting geometric mean ratios and corresponding CIs were additionally transformed to percent difference from placebo using the following formula: percent difference from placebo = [1 – (fold difference from placebo)] × 100%. These estimates were calculated for each time point and capsaicin dose separately for both telcagepant 300 and 800 mg.
Finally, in addition to the descriptive analyses set out above, a pharmacodynamic model was developed. This model took into account baseline DBF. The model was fitted using NONMEM VI with Compaq Visual Fortran 6.6 (update C) using a first-order conditional model with interaction. The model used clinical data from a pilot study of DBF [14], a study using DBF assessment after dosing with a TRPV1 antagonist [24], and this present study. The model form is a double-Emax model (Equation 1). DBF is dermal blood flow (AU); E0 is the baseline blood flow for an individual prior to capsaicin application (AU); Emax, caps is the maximum blood flow increase due to capsaicin application (AU); Dcaps is the dose of capsaicin applied to the skin (µg per 20 µl); ED50, caps is the dose of capsaicin required to cause 50% of the maximal capsaicin-induced increase in DBF (µg per 20 µl); Emax, CGRP is the maximal inhibition of capsaicin-induced increase in DBF due to a CGRP antagonist (fraction); Ctelcagepant is the concentration of telcagepant (nm); and EC50, telcagepant is the concentration of telcagepant required to cause 50% inhibition of the current capsaicin-induced increase in DBF (nm). Log-normally distributed intersubject variability terms (η with standard deviation ω) were determined for the baseline blood flow (E0), maximum DBF change due to capsaicin application (Emax, caps), and 50% maximal change in DBF due to capsaicin application (ED50, caps). Covariates for additive or proportional changes in E0 (ΔE0) and Emax, caps (ΔEmax, caps) by study were assessed using forward substitution and backward elimination with α < 0.05 and 0.002, respectively. E0 and not Emax, caps covariates were significant for all studies, and in the model the TRPV1 study was considered the standard case.
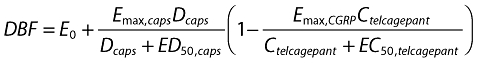 |
(1) |
Results
As expected, geometric mean plasma concentrations of telcagepant were higher after a dose of 800 mg than after 300 mg. Geometric mean plasma concentrations after dosing with 300 mg telcagepant and 800 mg telcagepant were 720 and 1146 nm, respectively, at 1 h, and 582 and 2548 nm, respectively, at 4 h (Table 1).
Table 1.
Individual plasma concentrations (nm) of telcagepant following administration of single oral doses of 300 and 800 mg to healthy male subjects (n= 12)
| Telcagepant 300 mg | Telcagepant 800 mg | |||
|---|---|---|---|---|
| Subject | C1 h (nm) | C4 h (nm) | C1 h (nm) | C4 h (nm) |
| 1 | 730.43 | 574.31 | 1125.2 | 989.83 |
| 2 | 1508.62 | 489.07 | 6803.51 | 5017.76 |
| 3 | 677.08 | 385.14 | 565.29 | 1607.59 |
| 4 | 1122.08 | 226.16 | 4587.8 | 1949.27 |
| 5 | 983.8 | 503.82 | 801.88 | 588.5 |
| 6 | 38.39 | 914.61 | 27.01 | 2523.28 |
| 7 | 1028.97 | 411.54 | 2741.02 | 4521.23 |
| 8 | 1061.97 | 969.62 | 1207.43 | 5316.89 |
| 9 | 1805.62 | 1506.9 | 5043.3 | 3151.16 |
| 10 | 2082.93 | 961.66 | 1942.43 | 8131.29 |
| 11 | 552.32 | 382.27 | 246.18 | 2037.17 |
| 12 | 268.2 | 604.52 | 1499.4 | 2578.26 |
| Geometric mean | 719.51 | 581.88 | 1146.28 | 2547.67 |
Analysis of ‘baseline’ DBF measurements performed 0.5 or 3.5 h after dosing with placebo or telcagepant, immediately before capsaicin administration, showed that neither dose of telcagepant had an intrinsic effect on DBF (Table 2).
Table 2.
Model-based means and standard errors for baseline pre-capsaicin dermal blood flow (DBF) (arbitrary units) and P-values assessing treatment effect (n= 12)
| Capsaicin dose* | ||
|---|---|---|
| Treatment | 300 µg per 20 µl | 1000 µg per 20 µl |
| Telcagepant 300 mg | 0.453 (0.0232) | 0.424 (0.0210) |
| Telcagepant 800 mg | 0.450 (0.0228) | 0.434 (0.0214) |
| Placebo | 0.449 (0.0228) | 0.414 (0.0210) |
| P-value† | 0.99 | 0.73 |
Baseline DBF scans were performed immediately before capsaicin application, at 0.5 h and 3.5 h after telcagepant or placebo.
P-value from model including terms for period, site, arm and treatment with subject as a random effect. Separate model used for 300 and 1000 µg per 20 µl capsaicin doses.
In the analysis of percent difference from placebo at 1 h and 4 h, which did not take into account baseline data, telcagepant produced a 37–68% difference (reduction) in mean perfusion relative to placebo depending on the dose (Table 3). Although CIs overlapped, greater mean percent differences (reductions) from placebo in perfusion were seen for the 800-mg dose of telcagepant compared with the 300-mg dose.
Table 3.
Capsaicin-induced dermal blood flow following telcagepant or placebo (n= 12)
| Time | Telcagepant dose, mg | Capsaicin dose | Mean perfusion (volt) Geometric mean* | Mean perfusion (active/ placebo) GMR* (90% CI) | % Difference† (90% CI) |
|---|---|---|---|---|---|
| 1 h | Placebo | 300 µg per 20 µl | 1.19 | ||
| 300 | 0.56 | 0.47 (0.34, 0.64) | 53.10 (36.15, 65.54) | ||
| 800 | 0.47 | 0.40 (0.29, 0.54) | 60.20 (45.83, 70.77) | ||
| Placebo | 1000 µg per 20 µl | 1.67 | |||
| 300 | 0.69 | 0.41 (0.30, 0.56) | 58.70 (43.78, 69.66) | ||
| 800 | 0.53 | 0.32 (0.23, 0.43) | 68.22 (56.74, 76.66) | ||
| 4 h | Placebo | 300 µg per 20 µl | 1.00 | ||
| 300 | 0.62 | 0.63 (0.48, 0.82) | 37.34 (17.63, 52.32) | ||
| 800 | 0.52 | 0.52 (0.40, 0.69) | 47.65 (31.19, 60.17) | ||
| Placebo | 1000 µg per 20 µl | 1.44 | |||
| 300 | 0.86 | 0.60 (0.45, 0.78) | 40.38 (21.64, 54.64) | ||
| 800 | 0.62 | 0.43 (0.33, 0.56) | 57.23 (43.78, 67.46) |
Geometic mean ratio (GMR), computed from least squares estimates from anova performed on the natural-log transformed values.
Transformed from mean perfusion GMR.
Using the semi-mechanistic pharmacodynamic model represented in Equation 1 (which did account for baseline data), with parameters shown in Table 4, the interactions between CGRP receptor antagonism and capsaicin-induced increase in DBF became apparent. Figure 2 illustrates the concentration–response curves for telcagepant. The EC50,telcagepant was found to be 101 nm, yielding an EC90 of 909 nm, but owing to a paucity of data at and below the EC50, this should be viewed as an approximation for the EC50.
Table 4.
Parameters fitted to the pharmacodynamic model
| Parameter | Parameter estimate | % RSE for parameter | ω Estimate | % RSE for ω estimate |
|---|---|---|---|---|
| E0 (AU) | 0.544 | 4.17 | 0.019 | 41.2 |
| Emax,caps (AU) | 2.56 | 18.4 | 0.257 | 49.8 |
| Emax,CGRP (fraction) | 0.921 | 2.76 | NA | NA |
| ED50, caps (µg per 20 µl) | 430 | 46.5 | 3.57 | 44.5 |
| EC50, telcagepant | 101 | 37.3 | NA | NA |
| ΔE0, pilot | 0.416 | 11.2 | NA | NA |
| ΔE0, telcagepant | −0.115 | 21.8 | NA | NA |
| Proportional residual error | 0.0678 | 10.5 |
AU, arbitrary units; RSE, relative standard error. See text for definition of parameters.
Figure 2.
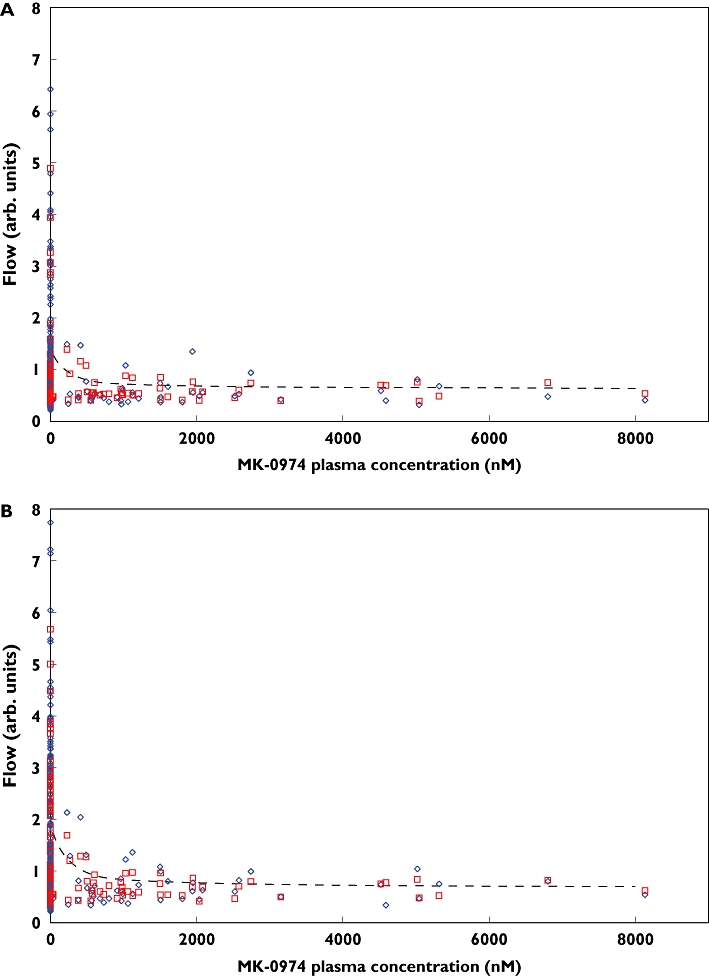
Dermal blood flow (DBF) vs. telcagepant (MK-0974) plasma concentration. Panels (a) and (b) indicate the DBF at 300 and 1000 µg per 20 µl capsaicin, respectively. The blue diamonds show the measured DBF, red squares show the individual predicted values, and the dashed lines indicate the population predicted mean values. Actual Flow (◊); Individual Predicted (□); Population Predicted (---)
Discussion
The present study has clearly demonstrated that the novel CGRP receptor antagonist telcagepant is able to inhibit capsaicin-induced increases in DBF. Both 300 and 800 mg of telcagepant caused significant inhibition of capsaicin-induced increases in DBF after 300 or 1000 µg capsaicin at 1 and 4 h post telcagepant intake. Analysis of the pharmacokinetic/pharmacodynamic relationship suggests that telcagepant engages the CGRP receptor with an EC90 of approximately 900 nm. However, because of the paucity of data at lower plasma concentrations, the certainty of the EC50 is not clear. Additional studies at lower concentrations may help to delineate this part of the curve further.
The relatively flat concentration–response curve above 900 nm indicates that at or above this plasma concentration, telcagepant is maximally blocking the peripheral CGRP receptor in healthy men. Of note, the early formulation of telcagepant used in the present study had lower bioavailability than the formulation subsequently used in the acute migraine clinical trials. In the clinical trials, both 150- and 300-mg doses of the later formulation were effective [19, 20]. Interestingly, the mean plasma concentrations achieved by these doses are approximately two- to four-fold higher than 900 nm, suggesting that larger doses than those producing maximal peripheral CGRP receptor inhibition are necessary for antimigraine efficacy. It could be the case that in a migraine population, higher doses are needed to inhibit maximally the peripheral CGRP receptor in a majority of patients. Another possibility is that peripheral CGRP receptor inhibition may not be sufficient for antimigraine efficacy and that central engagement may also be required. Further research is needed to investigate these possibilities.
Additional studies with other CGRP receptor antagonists will help delineate the relationship between capsaicin-induced dermal vasodilation and acute migraine efficacy. Studies using intravenous bolus administration of a close analogue of olcegepant in the rhesus monkey have shown that the doses required to inhibit capsaicin-induced DBF increase by 47% and 100% were 3 and 30 µg kg−1, respectively [13]. These doses were similar to those reported for comparable levels of inhibition of facial blood flow in response to trigeminal nerve stimulation in the marmoset, the invasive animal model used to guide clinical dose selection for olcegepant [25]. The clinically effective dose reported in the olcegepant proof-of-concept study in migraine patients was 2.5 mg intravenously, which in 50–80-kg adults approximates to 32–50 µg kg−1. This dose of olcegepant, in a separate study, resulted in a geometric mean Cmax of 210 ng ml−1[26], supporting the clinical relevance of this pharmacodynamic model in the marmoset.
As noted in the Introduction, binding of capsaicin to the TRPV1 receptor provokes neurogenic inflammation and vasodilation through the release of bioactive mediators, and possibly also by activation of dorsal root reflexes. In human skin CGRP, SP, neurokinin A, nitric oxide (NO), histamine and prostaglandins are among the mediators thought to contribute to this response [27]. Nevertheless, our previous study using this DBF model [15] suggested that prostaglandins, NO and SP did not make a substantial contribution to the changes in microvascular perfusion accompanying neurogenic inflammation. This is in contrast to findings in rodent skin, where SP is probably an important mediator of the response [28]. One of the key parameters in the pharmacodynamic model presented here is Emax,CGRP, the fraction of the maximal inhibition of capsaicin-induced increase in DBF that can be ascribed to a CGRP antagonist. The best fit was found with a value of 92.1%, suggesting that other mediators may indeed play a minor role. The pharmacodynamic modelling approach employed in the present study is a more robust data analysis method than previous graphical methods due to the fact that it allows more accurate accounting for baseline variability and allows the biological mechanism to be detailed in a mathematically consistent manner. Future studies will be important to determine the identity of the mediators involved as well as their contribution.
Although CGRP is one of the most potent endogenous vasodilators in the human body, it seems minimally involved in the maintenance of basal peripheral vascular tone [29–32]. In humans, high doses of the peptide CGRP receptor antagonist CGRP8–37 did not decrease resting forearm blood flow [33]. It has also been reported that the small molecule CGRP receptor antagonist olcegepant has no effects on cerebral and systemic haemodynamics or on temporal, radial and middle cerebral artery diameter in healthy subjects [34]. Likewise, in the present study telcagepant did not affect baseline dermal perfusion. Taken together, these data suggest that CGRP receptor antagonists may be relatively free of cardiovascular side-effect liability under resting conditions. As a result, in the treatment of acute migraine headache, CGRP antagonists may offer advantages over current 5-HT1B/1D receptor agonists (triptans), where cardiovascular liabilities are a major perceived risk. However, notwithstanding these reassuring data, given the role of CGRP in myocardial ischaemia and heart failure [35, 36], the vascular effects of this new class of compounds should be assessed more extensively in vivo in humans.
In summary, the present study has shown that telcagepant (MK-0974) inhibits the increases in DBF induced by the topical application of capsaicin on the human forearm without affecting basal dermal perfusion. This in vivo human experimental medicine model may provide useful information to be taken into account when considering dose selection for the development of CGRP receptor antagonists.
Competing interests
S.R.S., S.A.K., A.X., K.J.W., J.B., I.d.L., Y.X., L.H., W.S.D., C-C.L., J.P., M.G.M. and T.W.H. are employees of Merck and own stock or stock options in Merck. B.J.V.S., F.H.M.V. and J.N.H. have received research funding from Merck. M.D. and A.V.H. have no conflicts to disclose.
This study was funded by Merck Research Laboratories. The authors would like to acknowledge the contributions of Christopher Lines and Rebecca Blanchard in helping to draft the manuscript.
REFERENCES
- 1.Evans RM, Amara S, Rosenfield MG. Molecular events in developmental regulation of neuroendocrine genes: characterization of the novel neuropeptide CGRP. Cold Spring Harb Symp Quant Biol. 1983;48:413–7. doi: 10.1101/sqb.1983.048.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 3.Edvinsson L, Fredholm BB, Hamel E, Jansen I, Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985;58:213–7. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- 4.Uddman R, Edvinsson L, Ekblad E, Håkanson R, Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986;15:1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 5.de Hoon JN, Pickkers P, Smits P, Struijker-Boudier HA, Van Bortel LM. Calcitonin gene-related peptide: exploring its vasodilating mechanism of action in humans. Clin Pharmacol Ther. 2003;73:312–21. doi: 10.1016/s0009-9236(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 6.Edvinsson L, Hargreaves R. CGRP involvement in migraines. In: Olesen J, editor. The Headaches. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins Publishing; 2006. pp. 289–300. [Google Scholar]
- 7.Goadsby PJ. Calcitonin gene-related peptide (CGRP) antagonists and migraine: is this a new era? Neurology. 2008;70:1300–1. doi: 10.1212/01.wnl.0000309214.25038.fd. [DOI] [PubMed] [Google Scholar]
- 8.Sexton PM. Central nervous system binding sites for calcitonin and calcitonin gene-related peptide. Mol Neurobiol. 1991;5:251–73. doi: 10.1007/BF02935550. [DOI] [PubMed] [Google Scholar]
- 9.Christopoulos G, Sexton P, Paxinos G, Huang X-F, Beaumont K, Toga A. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can J Physiol Pharmacol. 1995;73:1037–41. doi: 10.1139/y95-146. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 11.Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci. 2001;13:2099–104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- 12.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 13.Hershey JC, Corcoran HA, Baskin EP, Salvatore CA, Mosser S, Williams TM, Koblan KS, Hargreaves RJ, Kane SA. Investigation of the species selectivity of a nonpeptide CGRP receptor antagonist using a novel pharmacodynamic assay. Regul Pept. 2005;127:71–7. doi: 10.1016/j.regpep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Van der Schueren BJ, de Hoon JN, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR. Reproducibility of the capsaicin-induced dermal blood flow response as assessed by laser Doppler perfusion imaging. Br J Clin Pharmacol. 2007;64:580–90. doi: 10.1111/j.1365-2125.2007.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Schueren BJ, Rogiers A, Vanmolkot FH, Van Hecken A, Depré M, Kane SA, De Lepeleire I, Sinclair SR, de Hoon JN. Calcitonin gene-related peptide8-37 antagonizes capsaicin-induced vasodilation in the skin: evaluation of a human in vivo pharmacodynamic model. J Pharmacol Exp Ther. 2008;325:248–55. doi: 10.1124/jpet.107.133868. [DOI] [PubMed] [Google Scholar]
- 16.Paone DV, Shaw AW, Nguyen DN, Burgey CS, Deng JZ, Kane SA, Koblan KS, Salvatore CA, Mosser SD, Johnston VK, Wong BK, Miller-Stein CM, Hershey JC, Graham SL, Vacca JP, Williams TM. Potent, orally bioavailable calcitonin gene-related peptide receptor antagonists for the treatment of migraine: discovery of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide (MK-0974) J Med Chem. 2007;50:5564–7. doi: 10.1021/jm070668p. [DOI] [PubMed] [Google Scholar]
- 17.Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324:416–21. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- 18.Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 19.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 20.Connor K, Shapiro R, Diener H-C, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–7. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han TH, Blanchard R, Palcza J, De Lepeleire I, Laethem M, Martucci A, Willson K, Xu Y, Boyle J, Butterfield K, Mahon C, Lewis N. The pharmacokinetics, safety and tolerability of telcagepant, a novel calcitonin gene related peptide (CGRP) receptor antagonist, in healthy subjects and migraineurs. Clin Pharmacol Ther. 2009;85(Suppl. 1):S39–40. Abstract PII-11. [Google Scholar]
- 22.Fullerton A, Stücker M, Wilhelm KP, Wårdell K, Anderson C, Fischer T, Nilsson GE, Serup J European Society of Contact Dermatitis Standardization Group. Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact Dermatitis. 2002;46:129–40. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Willson KW, Anderson MDG, Miller-Stein CM, Woolf EJ. Elimination of diastereomer interference to determine Telcagepant (MK-0974) in human plasma using on-line turbulent-flow technology and off-line solid-phase extraction coupled with liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2009;877:1634–42. doi: 10.1016/j.jchromb.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Denney WS, Hang Y, Dockendorf MF, Li C-C, Eid SR, Valesky R, Laethem T, Van Hoydonck P, De Lepeleire I, de Hoon JNJM, Crutchlow M, Blanchard R. Modeling and simulation for determination of the therapeutic window of MK-2295: a TRPV1 antagonist. 2009. PAGE 18 Abstract 1507. Available at http://www.page-meeting.org/?abstract=1507 (last accessed 2 October, 2009)
- 25.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–3. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iovino M, Feifel U, Yong CL, Wolters JM, Wallenstein G. Safety, tolerability and pharmacokinetics of BIBN 4096 BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia. 2004;24:645–56. doi: 10.1111/j.1468-2982.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 27.Wallengren J. Vasoactive peptides in the skin. J Investig Dermatol Symp Proc. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- 28.Grant AD, Gerard NP, Brain SD. Evidence of a role for NK1 and CGRP receptors in mediating neurogenic vasodilatation in the mouse ear. Br J Pharmacol. 2002;135:356–62. doi: 10.1038/sj.bjp.0704485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, Hughes B. Human alpha-calcitonin gene-related peptide (CGRP)-(8-37), but not -(28-37), inhibits carotid vasodilator effects of human alpha-CGRP in vivo. Eur J Pharmacol. 1991;199:375–8. doi: 10.1016/0014-2999(91)90504-j. [DOI] [PubMed] [Google Scholar]
- 30.Arulmani U, Schuijt MP, Heiligers JP, Willems EW, Villalón CM, Saxena PR. Effects of the calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS on alpha-CGRP-induced regional haemodynamic changes in anaesthetised rats. Basic Clin Pharmacol Toxicol. 2004;94:291–7. doi: 10.1111/j.1742-7843.2004.pto940606.x. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor K, Arulmani U, Heiligers JP, Willems EW, Doods H, Villalón CM, Saxena PR. Effects of BIBN4096BS on cardiac output distribution and on CGRP-induced carotid haemodynamic responses in the pig. Eur J Pharmacol. 2003;475:69–77. doi: 10.1016/s0014-2999(03)02082-x. [DOI] [PubMed] [Google Scholar]
- 32.Shen YT, Pittman TJ, Buie PS, Bolduc DL, Kane SA, Koblan KS, Gould RJ, Lynch JJ., Jr Functional role of alpha-calcitonin gene-related peptide in the regulation of the cardiovascular system. J Pharmacol Exp Ther. 2001;298:551–8. [PubMed] [Google Scholar]
- 33.Vanmolkot FH, Van der Schueren BJ, de Hoon JN. Calcitonin gene-related peptide-induced vasodilation in the human forearm is antagonized by CGRP8-37: evaluation of a human in vivo pharmacodynamic model. Clin Pharmacol Ther. 2006;79:263–73. doi: 10.1016/j.clpt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, Olesen J. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–47. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 35.Mair J, Lechleitner P, Längle T, Wiedermann C, Dienstl F, Saria A. Plasma CGRP in acute myocardial infarction. Lancet. 1990;335:168. doi: 10.1016/0140-6736(90)90040-c. [DOI] [PubMed] [Google Scholar]
- 36.Gennari C, Nami R, Agnusdei D, Fischer JA. Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc Res. 1990;24:239–41. doi: 10.1093/cvr/24.3.239. [DOI] [PubMed] [Google Scholar]


