Abstract
AIMS
Antihypertensive (AH) agents have been shown to reduce the risk of cardiovascular events, including coronary artery disease (CAD). Previous surveys have shown that a substantial number of patients with diagnosed hypertension remain uncontrolled. Non-adherence to AH agents may reduce the effectiveness. The aim was to evaluate the impact of better adherence to AH agents on the occurrence of CAD in a real clinical setting.
METHODS
A cohort of 83 267 patients was reconstructed using the Régie de l'assurance maladie du Québec databases. Patients were eligible if they were between 45 and 85 years of age without indication of cardiovascular disease, and had been newly treated with AH agents between 1999 and 2004. A nested case–control design was used to study the incidence of CAD. Every case of CAD was matched for age and duration of follow-up to up to 15 randomly selected controls. The adherence level was measured by calculating the medication possession ratio. Cases' adherence was calculated from the start of follow-up to the time of the CAD (index date). For controls, adherence was calculated from the start of follow-up to the time of selection (index date). Rate ratios of CAD were estimated by conditional logistic regression adjusting for covariables.
RESULTS
The mean patient age was 65 years, 37% were male, 8% had diabetes and 18% had dyslipidaemia. High adherence level (96%) to AH therapy compared with lower adherence level (59%) was associated with a relative risk reduction of CAD events (rate ratios 0.90; 0.84, 0.95). Risk factors for CAD were male gender, diabetes, dyslipidaemia and developing a cardiovascular condition disease during follow-up.
CONCLUSION
Our study suggests that better adherence to AH agents is associated with a risk reduction of CAD. Adherence to AH agents needs to be improved so that patients can benefit from the full protective effects of AH therapies.
Keywords: adherence to therapy, antihypertensive agents, coronary artery disease
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Non-adherence is probably an important source of preventable cardiovascular morbidity and mortality.
However, until now there have been very few large effectiveness studies assessing the relationship between adherence levels to antihypertensive medication and major cardiovascular outcomes for primary prevention of cardiovascular disease.
WHAT THIS STUDY ADDS
The study results suggest that there is an association between better adherence to antihypertensive agents and a relative risk reduction of coronary artery disease.
Adherence to antihypertensive agents needs to be improved so that patients can benefit from the full protective effects of antihypertensive therapies.
Introduction
Cardiovascular diseases (CVD) are responsible for the highest healthcare utilization costs in most industrialized countries [1–3]. Many risk factors are involved in the development of the disease [4–6]. Hypertension represents a modifiable risk factor for coronary artery disease (CAD). The potential of antihypertensive (AH) agents, including diuretics, β-blockers, angiotensin converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs) and angiotensin receptor blockers (ARBs), to reduce CVD mortality and morbidity is well established [7–10].
Systematic overviews of trials showed that reductions in blood pressure of about 10–12 mmHg systolic and 5–6 mmHg diastolic conferred relative reductions in the risk of CAD of 16–19% within a few years of beginning treatment [7, 11]. Current hypertension guidelines strongly endorse the concept that the reduction of hypertension-related complications depends more on the extent of blood pressure reduction than on the choice of any specific drug class as first-line therapy for those patients without compelling indications for a specific agent [12–14].
Despite all the available evidence, there is still a large discrepancy between guideline recommendations and clinical practice [15, 16]. Non-adherence to AH therapy is acknowledged as a major contributor to this discrepancy [17]. Non-adherence is probably an important source of preventable cardiovascular morbidity and mortality. However, until now there have been very few large effectiveness studies assessing the relationship between adherence levels to AH medication and major cardiovascular outcomes for primary prevention of CVD. A study of self-reported adherence (dichotomized variable) in the Second Australian National Blood Pressure Study in primary prevention reported that older subjects who adhered to their medication were less likely to present major CVD events or death [18]. The present study was designed to estimate the use and impact of being adherent to AH therapy in a real-life setting with a large number of patients from an unselected cohort on the rate of CAD among 45- to 85-year-old patients without a history of CVD.
Methods
Data sources
This population-based study used the databases of the Régie de l'assurance maladie du Québec (RAMQ) and MedEcho, which administers public healthcare insurance programmes in Quebec, Canada. The RAMQ databases contain three types of health-related data. The demographic information file contains the age, gender, postal code and year of death for all registered individuals. The medical services file comprises claims for all inpatient or ambulatory medical services and includes data such as the type of medical procedure, date and site (office, emergency room, hospital) of the procedure, and the diagnostic code [19]. Diagnosis is coded based on the International Classification of Disease (ICD-9). The procedure codes are also enclosed and are defined by the Canadian classification of diagnostic, therapeutic and surgical procedures [20]. These codes are linked to the doctor's payments and are audited carefully. The pharmaceutical file contains data on all prescriptions for covered drugs prescribed to patients living in the community whose medications are insured by RAMQ. The file includes the name, dose and quantity of the drug as well as the date and duration of therapy, as indicated by the pharmacist. The MedEcho database contains data on acute care hospitalizations, such as date of admission, length of stay and primary and secondary diagnoses. All these files contain also the individual's health insurance number, which serves as a link.
The first two datasets contain information on all residents covered by the provincial health insurance plan, which is the entire Quebec population. Data from the prescription claims dataset are from beneficiaries of the provincial prescription drug insurance plan, which represents about 40% of the Quebec population aged 45–64 years, and about 94% of those ≥ 65 years old [21]. The three databases are linked by the medical insurance number of the patients. The prescription claims database has been used in pharmacoepidemiological studies and is an accurate means of determining drugs dispensed to individuals [22]. Validity studies have been performed specifically for the medical services claims of Quebec administrative databases [23, 24]. The validity of the diagnostic codes identifying episodes of hospitalization for acute myocardial infarction was assessed among patients >65 years old [25]. Compared with the information in the patient hospital discharge summary, the sensitivity and specificity of the diagnostic code identifying the first hospital stay for acute myocardial infarction were 81.1% and 98.8%, respectively.
Cohort study
All subjects aged 45–85 years who were newly treated with either diuretics (excluding high ceiling diuretics), β-blockers, ACEIs, CCBs, ARBs or a combination between 1 January 1999 and 31 December 2004, and had at least a diagnosis with essential hypertension (ICD-9 code 401) were identified. Patients were considered newly treated if they had not taken any AH agent in the 2 years prior to entry into the cohort, which was defined as the date of the first prescription. Only patients who had filled at least three AH prescriptions within the 6 months after their entry into the cohort were included. In addition, in order to ensure that the patients required pharmacological treatment, they had to have had a medical visit with their doctor and to have filled at least one AH prescription for each period of 1.5 years.
In order to be eligible, subjects must not have had any indication of a CVD as evidenced by the absence of a related diagnosis or medical procedure in the last 5 years, and any vascular drug marker in the 2 years prior to the cohort entry date. Patients had to be free of any marker of CVD such as: (i) CAD: diagnosis of myocardial infarction or angina (ICD-9 codes 410–414); vascular medical procedure, e.g. coronary artery bypass grafting, angiography, or angioplasty or stent, or use of nitrate, including nitroglycerin; (ii) cerebrovascular disease: diagnosis (430–438) or vascular medical procedures or use of nimodipine; (iii) peripheral arterial disease: diagnosis of a peripheral vascular disease (440–447), medical procedure of noncoronary angioplasty or use of pentoxifylline; (iv) chronic heart failure (CHF): diagnosis of CHF (398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or the use of furosemide alone or with digoxin, ACEIs, spironolactone or β-blockers; (v) arrhythmia: diagnosis (426–427), a medical procedure involving a pacemaker or the use of drugs for cardiac arrhythmias; or (vi) valvular heart disease (393–398). We also excluded patients with diseases such as a renal disease (580–589), a related medical procedure, or drugs that may have caused secondary hypertension. The RAMQ drug database was also used to exclude patients who received other drugs such as antiplatelets (excluding a low dose of aspirin), or anticoagulants during the 2 years preceding the cohort entry date.
The study cohort included 83 267 individuals who were followed from the date of issuance of the first prescription of an AH agent until the first CAD or the end of the study period (30 June 2005). Individuals who lost coverage under the RAMQ drug insurance, died during follow-up or developed renal insufficiency were censured. The total death rate was assessed in the cohort. Subjects were followed for a minimum of 6 months and a maximum of 6.5 years.
Nested case–control study
The nested case–control model was used to estimate the rate ratio (RR) of the first nonfatal or fatal CAD with respect to adherence level to AH agents. A nonfatal or fatal CAD was defined by a composite end-point: diagnosis of myocardial infarction or angina (ICD-9 codes 410–414), a medical procedure (coronary artery bypass grafting, angiography, angioplasty, stent), use of nitrate including nitroglycerin or death. All cases of CAD were identified, and up to 15 controls were randomly selected from the risk set for each case using density sampling (i.e. by matching with respect to age ± 1 year and the same follow-up time) [26].
Exposure assessment
Within each risk set, adherence level was measured by calculating the medication possession ratio (MPR), defined as the number of days' supply of medication received divided by the length of follow-up [27, 28]. The MPR corresponded to the total number of days' supply of medication dispensed divided by the length of follow-up [29]. Cases' adherence was calculated from the start of follow-up to the time of the CAD (index date). For controls, the adherence was calculated from the start of follow-up to the time of selection (index date). The MPR was dichotomized, setting a threshold of MPR <80% to identify non-adherent patients, consistent with literature data [30].
Covariables
The social assistance status at the cohort entry date was determined using data in the file of the beneficiary in the RAMQ database. During follow-up, the fact of developing cerebrovascular disease, CHF, peripheral arterial disease or any other cardiovascular events (cardiac arrhythmia, pulmonary circulation problems or miscellaneous heart illnesses or being known for taking anticoagulant drugs) was also included in the model as covariables.
Diabetes and dyslipidaemia were identified in the year prior to cohort entry and during follow-up. They were defined as follows: diabetes by ICD-9 code 250 or by the use of insulin or an antidiabetic agent, and dyslipidaemia by ICD-9 code 272 or by the use of lipid-lowering drugs. Patients with diabetes or dyslipidaemia diagnosed only in the year preceding the index date were considered newly treated. With respect to the other patients, the use of antidiabetic or dyslipidaemia agents in the year before the index date was dichotomized: high adherence, indicated by having filled >80% of the prescribed doses, and low adherence, by having filled <80%. Patients who were diagnosed with diabetes mellitus or dyslipidaemia but who were never treated were defined as such. The reference categories were individuals with no dyslipidaemia or diabetes.
Dichotomic variables were used for respiratory diseases, the use of antidepressant or anxiolytic agents. Finally, an updated patient chronic disease score (CDS) was calculated in the year preceding the index date. The CDS is a comorbidity index that uses drugs dispensed as surrogate markers for chronic illnesses instead of using clinical diagnoses [31]. Scores are weighted according to the number of different chronic diseases under treatment [31]. The CDS was dichotomized into two levels: CDS ≥4 and <4.
Statistical analysis
Characteristics of cases and controls were compared using the t-test for continuous variables and the χ2 test for categorical variables. We used Kaplan–Meier analysis to compare the time to first CAD event between the high- and low-adherence groups based on first-year period adherence. In multivariable analysis, a conditional logistic regression model was constructed to evaluate the association between AH adherence and all nonfatal CAD. When studying exposure that varies with time, as in our study (adherence to AH), an additional level of complexity is introduced based upon the need to account for time-dependent exposure in the analysis; this can be accomplished through a cohort analysis using Cox regression including time-dependent covariates. Alternatively, a nested case–control approach can be used provided the exposure and covariate information for controls reflects the values corresponding to the time of selection of their respective case. Nested case–control analyses have been found to yield results similar to those from the Cox regression on the full cohort when studying time-dependent exposures, with the advantage of superior computational efficiency with the conditional logistic regression, given that only a sample of all possible controls is included in the risk set of each case [32].
Multivariable models were constructed to adjust maximally for confounding and included all previously described variables. RRs and 95% confidence intervals were calculated for each independent variable in the multivariable models. The possible effect of time was taken into account by stratifying the analysis in accordance with the time of case occurrence: cases occurring in the first year of follow-up and those after 1 year of follow-up.
Residuals from regression models were assessed for violations of the assumptions of multicollinearity or deviance [33, 34]. All analyses used a precision threshold of 5% and were done using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Ethical considerations
No identifiers related to patients or physicians were provided to the researchers. The Research and Ethics Committee of the University of Montreal approved the study.
Results
Characteristics of patients
After applying the inclusion and exclusion criteria (Figure 1), the cohort consisted of 83 267 patients with a mean age at entry of 65 years, 37% of whom were male, 12% welfare recipients, 8% diabetics and 18% had dyslipidaemia (Table 1). As first AH monotherapy treatment, diuretics (27%) were used the most often, followed by ACEIs (25%). Combined therapy was less likely to be prescribed at the start of therapy (4.3%). As shown in Table 1, there were no major differences in baseline characteristics across the five AH drug classes. However, ACEIs appeared to be preferentially prescribed to sicker patients, particularly those with diabetes. Table 2 provides demographic and clinical characteristics at the cohort entry of the patients with respect to adherence level. We observed no major clinically significant difference between the groups, except for dyslipidaemia and male gender.
Figure 1.
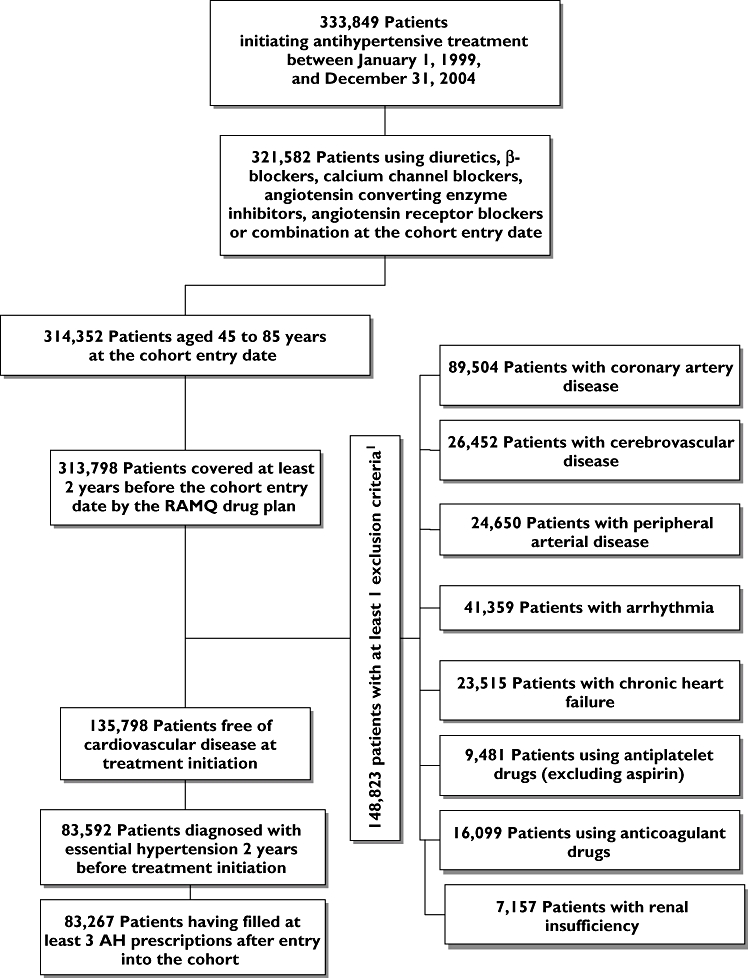
Flow chart of inclusion and exclusion criteria. Exclusion criteria were assessed in the 5 years preceding the cohort entry for diagnoses and medical procedures and 2 years prior the cohort entry for the medication
Table 1.
Characteristics of patients initiating a new antihypertensive treatment in Quebec RAMQ Database in 1999–2004
| Antihypertensive drug class* | ||||||
|---|---|---|---|---|---|---|
| Diuretics | BBs | CCBs | ACEIs | ARBs | Combined | |
| No. patients | 21 664 | 8635 | 11 307 | 21 600 | 16 703 | 3682 |
| Follow-up time (continuous) | 1 051 (±630) | 1112 (±664) | 1 069 (±634) | 1 084 (±629) | 1 000 (±587) | 861 (±542) |
| Mean age (continuous)* | 66 (±9.7) | 62 (±9.8) | 66 (±9.8) | 64 (±9.7) | 64 (±9.6) | 63 (±9.7) |
| Male sex (%)* | 28 | 34 | 41 | 42 | 41 | 43 |
| Social assistance (%)* | 11 | 15 | 11 | 12 | 10 | 13 |
| Diabetes mellitus (%)† | 3.2 | 3.5 | 4.4 | 19.2 | 7.7 | 6.2 |
| Dyslipidaemia (%)† | 17.0 | 16.8 | 16.4 | 23.7 | 20.7 | 18.7 |
| Antiplatelet drugs (%)† | 13 | 14 | 15 | 20 | 16 | 16 |
| Respiratory diseases (%)† | 10 | 5 | 12 | 9 | 10 | 9 |
| Antidepressant drugs (%)† | 7 | 8 | 6 | 7 | 7 | 6 |
| Anxiolytic drugs (%)† | 26 | 28 | 25 | 23 | 24 | 20 |
| Chronic disease score ≥4 (%)† | 12.6 | 12.3 | 13.9 | 11.3 | 11.5 | 10 |
At treatment initiation.
ICD-9 or pharmacological treatment in the year preceding the cohort entry. BBs, β-blockers; CCBs, calcium channel blockers; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
Table 2.
Characteristics of patients at the cohort entry with respect to adherence level
| Low adherence | High adherence | ||
|---|---|---|---|
| <80% | ≥80% | ||
| Number of subjects | 28 908 | 157 627 | |
| Mean age (continuous)* | 66 ± 9.6 | 66 ± 9.1 | <0.0001 |
| Sex (% male)* | 38 | 35 | <0.0001 |
| Social assistance (%)* | 11.0 | 9.0 | <0.0001 |
| Diabetes (%)† | 8.0 | 8.9 | <0.0001 |
| Dyslipidaemia (%)† | 16.2 | 19.5 | <0.0001 |
| Respiratory diseases (%)† | 10 | 9.4 | 0.0008 |
| Antidepressant drugs (%)† | 6.7 | 6.1 | <0.0001 |
| Anxiolytic drugs (%)† | 24.3 | 25.5 | <0.0001 |
| Chronic disease score (≥4) (%)† | 10.9 | 12.7 | <0.0001 |
At treatment initiation.
ICD-9 or pharmacological treatment in the year preceding the cohort entry.
During follow-up, 14.5% of patients had had CAD (4.4/100 persons-years), 2.2% cerebrovascular disease (0.7/100 persons-years), 1.8% CHF (0.6/100 person-years), 1.5% a peripheral artery disease condition (0.5/100 persons-years), 7.5% other cardiovascular condition (2.4/100 persons-years) and 5.8% had had more than two cardiovascular events (1.9/100 person-years). The percentage of death during follow-up was 2.0% (0.7 per 100 persons-years). As shown in Table 3, the proportion of men, welfare recipients, patients with diabetes, dyslipidaemia or respiratory diseases, and users of antidepressant or anxiolytic agents was statistically higher among the cases. In addition, cases had experienced more cardiovascular conditions during follow-up than controls, and presented a higher chronic disease score. The characteristics of patients with CAD and their matched controls by level of adherence after 1 year's follow-up are presented in Table 4. Finally, in the year prior to the index date, 70% of patients were using monotherapy, 27% were on bitherapy and 3% were using tritherapy.
Table 3.
Characteristics of patients with coronary artery disease and their matched controls
| Cases occurring in the first year of follow-up and their controls | Cases occurring after 1 year's follow-up and their controls | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Numbers | 4720 | 70 634 | 6878 | 102 151 |
| Age (continuous) (years)† | 66 (±9) | 66 (±9) | 67 (±9) | 67 (±9) |
| Statin adherence (%)‡ | ||||
| 1–19 (%) | 0.2 | 0.1 | 0.1 | 0.1 |
| 20–39 (%) | 2 | 1 | 2 | 2 |
| 40–59 (%) | 3 | 3 | 5 | 4 |
| 60–79 (%) | 7.2 | 6.6 | 11 | 10 |
| ≥80 (%) | 88 | 89 | 82 | 83 |
| Sex (% male)† | 43 | 37 | 40 | 33 |
| Social assistance (%)† | 12 | 10 | 12 | 10 |
| Cerebrovascular disease during follow-up (%)§ | 2 | 1 | 4 | 2 |
| Chronic heart failure during follow-up (%)¶ | 2 | 0.4 | 4 | 1 |
| Peripheral arterial disease during follow-up (%)†† | 1.7 | 0.4 | 3.0 | 1.6 |
| Other cardiovascular events during follow-up (%)‡‡ | 12 | 2 | 17 | 7 |
| Having ≥2 cardiovascular events (%) | 4 | 0.3 | 9 | 2 |
| Diabetes (%)§§ | 13 | 10 | 19 | 16 |
| Diagnosed and nontreated (%)§§ | 4 | 3 | 7 | 6 |
| Newly treated for diabetes mellitus (%)§§ | 4 | 3 | 2 | 1 |
| Antidiabetic agents adherence <80% (%)§§¶¶ | 1.4 | 0.9 | 3 | 2 |
| Antidiabetic agent adherence ≥80% (%)§§¶¶ | 4 | 3 | 8 | 7 |
| Dyslipidaemia (%)§§ | 29 | 24 | 40 | 34 |
| Diagnosed and nontreated (%)§§ | 3 | 3 | 4 | 4 |
| Newly treated for dyslipidaemia (%)§§ | 15 | 10 | 8 | 5 |
| Lipid-lowering agent adherence <80% (%)§§¶¶ | 3 | 3 | 9 | 7 |
| Lipid-lowering agent adherence ≥80% (%)§§¶¶ | 8 | 9 | 19 | 18 |
| Respiratory diseases (%)§§ | 16 | 11 | 25 | 17 |
| Antidepressant drugs (%)§§ | 9 | 8 | 15 | 12 |
| Anxiolytic drugs (%)§§ | 32 | 28 | 42 | 36 |
| Chronic disease score (≥4) (%) | 11 | 8 | 14 | 10 |
At treatment initiation.
Proportion of days covered (%).
Diagnosis of cerebrovascular disease (ICD-9 codes 430–438) or medical procedures.
Diagnosis of chronic heart failure (ICD-9 codes 398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or a prescription of furosemide alone or with digoxin, angiotensin converting enzyme inhibitors, spironolactone or β-blockers.
Diagnosis of peripheral arterial disease: diagnosis (ICD-9 codes 440–447, medical procedure of noncoronary angioplasty, or use of pentoxifylline).
Diagnosis of other cardiovascular disease: arrhythmia: diagnosis (ICD-9 code 427), a medical procedure using a pacemaker and the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone, or sotalol); or valvular heart disease; or anticoagulants; other cardiopathy.
ICD-9 or pharmacological treatment.
Proportion of days covered (%) in the year before the index date.
Table 4.
Characteristics of Patients with coronary artery disease and their matched controls by level of adherence after 1 year's follow-up
| Cases occurring after 1 year of follow-up and their controls | ||||
|---|---|---|---|---|
| Cases among low adherence | Controls among low adherence | Cases among high adherence | Controls among high adherence | |
| Numbers | 1396 | 18 707 | 5616 | 85 462 |
| Age (continuous) (years)† | 67 (±10) | 67 (±10) | 67 (±9) | 67 (±9) |
| Sex (% male)† | 43 | 37 | 39 | 33 |
| Social assistance (%)† | 13 | 12 | 11 | 9 |
| Cerebrovascular disease during follow-up (%)§ | 4 | 2 | 4 | 2 |
| Chronic heart failure during follow-up (%)¶ | 4 | 2 | 3 | 1 |
| Peripheral arterial disease during follow-up (%)†† | 3 | 1 | 3 | 2 |
| Other cardiovascular events during follow-up (%)‡‡ | 16 | 8 | 17 | 7 |
| Having ≥2 cardiovascular events (%) | 10 | 2 | 9 | 2 |
| Diabetes (%)§§ | 19 | 15 | 20 | 16 |
| Diagnosed and nontreated (%)§§ | 6 | 6 | 7 | 6 |
| Newly treated for diabetes mellitus (%)§§ | 2 | 2 | 2 | 1 |
| Antidiabetic agents adherence <80% (%)§§¶¶ | 6 | 4 | 2 | 2 |
| Antidiabetic agent adherence ≥80% (%)§§¶¶ | 5 | 3 | 9 | 7 |
| Dyslipidaemia (%)§§ | 38 | 31 | 40 | 35 |
| Diagnosed and nontreated (%)§§ | 4 | 4 | 4 | 4 |
| New treated for dyslipidaemia (%)§§ | 7 | 4 | 8 | 5 |
| Lipid-lowering agent adherence <80% (%)§§¶¶ | 17 | 14 | 7 | 6 |
| Lipid-lowering agent adherence ≥80% (%)§§¶¶ | 10 | 9 | 21 | 20 |
| Respiratory diseases (%)§§ | 26 | 18 | 24 | 17 |
| Antidepressant drugs (%)§§ | 16 | 12 | 15 | 12 |
| Anxiolytic drugs (%)§§ | 43 | 36 | 42 | 36 |
| Chronic disease score (≥4) (%) | 15 | 10 | 14 | 10 |
At treatment initiation.
Diagnosis of cerebrovascular disease (ICD-9 codes 430-438) or medical procedures.
Diagnosis of chronic heart failure (ICD-9 codes 398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or a prescription of furosemide alone or with digoxin, angiotensin converting enzyme inhibitor, spironolactone or β-blockers.
Diagnosis of peripheral arterial disease: diagnosis (ICD-9 codes 440-447, medical procedure of noncoronary angioplasty, or use of pentoxifylline).
Diagnosis of other cardiovascular disease: arrhythmia: diagnosis (ICD-9 code 427), a medical procedure using a pacemaker and the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone, or sotalol); or valvular heart disease; or anticoagulants; other cardiopathy.
ICD-9 or pharmacological treatment.
Proportion of days covered (%) in the year before the index date.
Impact of adherence level on CAD and risk of CAD
The mean high adherence level to AH agents was around 99% during the first year and 96% after 1 year's follow-up; and those values were, respectively, at 60% and 59% for the low adherence level. As shown in Figure 2, there was a significant difference in time to first CAD event between high- and low-adherence groups (P < 0.0001).
Figure 2.

Kaplan–Meier analysis for rate of coronary artery disease (CAD) among high and low adherence levels; the groups of adherence were based on first-year adherence (>80% vs. <80%)
In the multivariate model, the CAD rate decreased by 10% in the group with a high adherence level compared with the reference group for the total follow-up (RR 0.90; 0.86, 0.95). When the analysis was stratified with respect to the time of case presentation, we found that high adherence had an association with the relative risk reduction of CAD after at least 1 year following the initiation of AH agents (RR 0.90; 0.84, 0.95) (Table 5). Risk factors such as diabetes and hypertension increased the incidence of CAD by 16–56%. In the multivariate model, social assistance, being male, respiratory disease, antidepressant and anxiolytic drug use had a higher risk of developing CAD. Developing a cerebrovascular disease, CHF, peripheral artery disease or another CVD condition during follow-up increase significantly the risk of CAD from 2.8 to 6.0, and those estimates were even higher when a CVD condition occurred during the first year of follow-up. Again, subjects having experienced more than one CVD event had a much higher risk of CAD. Finally, having a high chronic disease score was associated with a higher CAD occurrence.
Table 5.
Rate ratio of coronary artery disease
| Rate ratio (95% CI) | ||||
|---|---|---|---|---|
| Cases occurring in the first year of follow-up and their controls | Cases occurring after 1 year's follow-up and their controls | |||
| Crude | Adjusted | Crude | Adjusted | |
| Antihypertensive adherence (%)‡ | ||||
| <80 | Reference | Reference | Reference | Reference |
| ≥80 | 0.92 (0.84, 1.00) | 0.93 (0.85, 1.03) | 0.88 (0.83, 0.94) | 0.90 (0.84, 0.95) |
| Sex (male vs. female)† | 1.31 (1.24, 1.39) | 1.28 (1.20, 1.36) | 1.33 (1.27, 1.40) | 1.33 (1.26, 1.40) |
| Social assistance† (yes vs. no)† | 1.21 (1.09, 1.34) | 1.05 (0.94, 1.17) | 1.29 (1.18, 1.40) | 1.10 (1.01, 1.21) |
| Monotherapy of diuretics or BBs | Reference | Reference | Reference | Reference |
| Monotherapy of ACEIs | 1.03 (0.95, 1.11) | 0.94 (0.86, 1.01) | 1.12 (1.01, 1.20) | 1.02 (0.95, 1.09) |
| Monotherapy of CCBs | 1.18 (1.08, 1.28) | 1.11 (1.01, 1.21) | 1.11 (1.03, 1.20) | 1.05 (0.98, 1.13) |
| Monotherapy of ARBs | 0.89 (0.82, 0.97) | 0.87 (0.80, 0.95) | 1.06 (0.99, 1.13) | 1.02 (0.95, 1.09) |
| Bitherapy | 1.52 (1.41, 1.64) | 1.35 (1.25, 1.46) | 1.24 (1.18, 1.31) | 1.14 (1.08, 1.20) |
| Tritherapy | 2.49 (2.01, 3.07) | 1.80 (1.43, 2.26) | 1.58 (1.42, 1.75) | 1.23 (1.10, 1.38) |
| Having cerebrovascular disease during follow-up§ | 3.72 (3.01, 4.60) | 4.79 (3.85, 5.95) | 1.99 (1.75, 2.26) | 2.81 (2.46, 3.20) |
| Having chronic heart failure during follow-up¶ | 5.21 (4.10, 6.62) | 6.45 (5.04, 8.25) | 2.50 (2.18, 2.86) | 3.61 (3.13, 4.16) |
| Having peripheral artery disease during follow-up†† | 3.62 (2.84, 4.62) | 4.65 (3.63, 5.96) | 2.05 (1.78, 2.37) | 2.99 (2.58, 3.47) |
| Having another CVD condition during follow-up‡‡ | 6.38 (5.75, 7.07) | 7.60 (6.83, 8.45) | 2.71 (2.54, 2.91) | 3.62 (3.37, 3.89) |
| Having ≥2 CVD events | 11.95 (9.78, 14.61) | 14.22 (11.56, 17.51) | 4.68 (4.27, 5.13) | 5.97 (5.41, 6.58) |
| No diabetes | Reference | Reference | Reference | Reference |
| Diabetes diagnosed and nontreated§§ | 1.22 (1.05, 1.42) | 1.10 (0.94, 1.29) | 1.23 (1.16, 1.35) | 1.04 (0.94, 1.15) |
| Newly treated for diabetes mellitus§§ | 1.35 (1.15, 1.58) | 1.03 (0.87, 1.22) | 1.29 (1.06, 1.57) | 1.01 (0.82, 1.23) |
| Antidiabetic agent adherence <80%§§¶¶ | 1.49 (1.16, 1.92) | 1.24 (0.95, 1.63) | 1.51 (1.31, 1.74) | 1.16 (1.00, 1.34) |
| Antidiabetic agent adherence ≥80%§§¶¶ | 1.13 (0.97, 1.32) | 1.02 (0.87, 1.21) | 1.30 (1.19, 1.42) | 1.08 (0.98, 1.19) |
| No dyslipidaemia | Reference | Reference | Reference | Reference |
| Dyslipidaemia diagnosed and nontreated§§ | 0.89 (0.74, 1.07) | 0.92 (0.76, 1.12) | 1.11 (0.98, 1.25) | 1.11 (0.98, 1.27) |
| Newly treated for dyslipidaemia§§ | 1.73 (1.59, 1.88) | 1.40 (1.28, 1.54) | 1.90 (1.73, 2.09) | 1.56 (1.41, 1.72) |
| Lipid-lowering agent adherence <80%§§¶¶ | 1.19 (1.01, 1.41) | 1.13 (0.95, 1.35) | 1.46 (1.34, 1.59) | 1.24 (1.14, 1.36) |
| Lipid-lowering agent adherence ≥80%§§¶¶ | 0.99 (0.88, 1.10) | 0.96 (0.86, 1.08) | 1.19 (1.12, 1.27) | 1.07 (1.00, 1.14) |
| Respiratory diseases (yes vs. no)§§ | 1.56 (1.44, 1.69) | 1.38 (1.27, 1.51) | 1.55 (1.46, 1.64) | 1.30 (1.22, 1.38) |
| Antidepressant drugs (yes vs. no)§§ | 1.23 (1.11, 1.36) | 1.11 (0.99, 1.25) | 1.35 (1.26, 1.44) | 1.11 (1.03, 1.20) |
| Anxiolytic drugs (yes vs. no)§§ | 1.21 (1.13, 1.28) | 1.18 (1.10, 1.26) | 1.28 (1.22, 1.35) | 1.18 (1.12, 1.25) |
| Chronic disease score (≥4 vs. <4) | 1.39 (1.26, 1.53) | 1.13 (1.02, 1.26) | 1.52 (1.42, 1.63) | 1.19 (1.10, 1.28) |
The model was adjusted for all those covariables described in Table 3.
At treatment initiation.
Proportion of days covered (%).
Diagnosis of cerebrovascular disease (ICD-9 codes 430-438) or medical procedures.
Diagnosis of chronic heart failure (ICD-9 codes 398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or a prescription of furosemide alone or with digoxin, angiotensin converting enzyme inhibitor, spironolactone or b-blockers.
Diagnosis of peripheral arterial disease: diagnosis (ICD-9 codes 440-447, medical procedure of noncoronary angioplasty, or use of pentoxifylline).
Diagnosis of other cardiovascular disease: arrhythmia: diagnosis (ICD-9 code 427), a medical procedure using a pacemaker and the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone, or sotalol); or valvular heart disease; or anticoagulants; other cardiopathy.
ICD-9 or pharmacological treatment.
Proportion of days covered (%) in the year before the index date. BBs, β-blockers; CCBs, calcium channel blockers; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CVD, cardiovascular disease.
Sensitivity analysis
The risk reduction of myocardial infarction (ICD-9410) was 20% (RR 0.78; 0.67, 0.91). Subgroup analysis revealed that among subjects <65 years old the risk reduction for CAD (RR 0.89; 0.81, 0.99) presented similar results compared with subjects >65 years old (RR 0.90; 0.83, 0.98). The fact of being adherent for >80% decreased the risk of CAD among patients having dyslipidaemia or diabetes diagnosis (RR 0.87; 0.78, 0.96). Similar results were seen among those without dyslipidaemia and diabetes (RR 0.91; 0.83, 0.99).
Discussion
Non-adherence is probably an important source of preventable cardiovascular morbidity and mortality. However, until now there have been very few large studies assessing the relationship between adherence levels to AH medication and major cardiovascular outcomes for primary prevention of CVD in the real clinical setting. A study of self-reported adherence (dichotomized variable) in the Second Australian National Blood Pressure Study in primary prevention reported that older subjects who adhered to their medication were less likely to present major CVD events or death [18]. The present study was designed to assess the use and impact of being adherent to AH therapies in a real-life setting on the occurrence of CAD in primary prevention. Using a large, population-based cohort, we have demonstrated that a high level of adherence to AH therapy is associated with a decreasing risk of developing CAD. This finding builds on recent work that demonstrates a positive relationship between adherence and clinical outcomes [35]. However, to our knowledge this is the first study to show in actual clinical practice that adherence of at least 80% (mean at 96%) compared with an adherence level <80% (mean at 59%) is significantly associated with a 10% decrease in the risk of CAD among newly treated hypertensive patients in a primary prevention setting. This association was not significant among patients exposed for <1 year, suggesting that the efficacy of AH agents in CAD prevention may take some time before becoming apparent.
The risk reduction observed in our study is in relation to the results reported in randomized clinical trials in a primary prevention setting where the control arms were placebo. For example, a meta-analysis of randomized clinical trials found that blood pressure lowering with diuretics or β-blockers regimens reduced the incidence of CAD by 16% [7]. The Blood Pressure Lowering Treatment Trialists' Collaboration reached similar conclusions for ACEIs, CCBs and ARBs [8]. Indeed, ACEIs and CCBs reduced the risk of CAD compared with placebo by approximately 20% [8].
The coefficients associated with CAD risk factors such as dyslipidaemia and socioeconomic status agreed with findings of other studies [5, 6]. Previous observational studies have revealed that patients with dyslipidaemia have 1.5–2 times the risk of CAD of individuals without dyslipidaemia, and individuals with low or middle incomes have a 1.2 times greater risk of those with higher incomes [5, 6]. The coefficient associated with diabetes is in the same direction as the previously reported coefficient, but is lower. Indeed, in previous reports it has been stated that the risk of CAD was two to four times higher among patients with diabetes [5, 6, 36].
Patients without clinical CAD are at high risk of CAD on the basis of atherosclerosis disease in other vascular beds and the presence of multiple other risk factors. The association between the development of cerebrovascular disease, peripheral artery disease or CHF agrees with literature data [37]. For example, in asymptomatic individuals >50 years old, a number of studies have demonstrated an up to fivefold increase in future CAD risk in those with intimal medial thickness of >1 mm of the carotid [38]. Again, epidemiological data show that subjects with peripheral artery disease are four times more likely to develop myocardial infarction than those without this disease [39].
The current study has attempted to overcome some methodological problems. Due to concern about the treatment for selection bias, we used only incident users. As with all observational studies of the effects of medications, the potential for confounding by indication should be carefully evaluated [40]. First, we could not control for all patient characteristics that may influence the choice of doctor. Unmeasured comorbidities as well as unavailable clinical data for blood pressure could have residual confounding effects. However, there is no reason to believe that the type of AH agents prescribed to patients would be strongly influenced by blood pressure. The analysis of available baseline characteristics did not suggest preferential prescribing of a particular AH agent to sicker patients except for ACEIs.
Second, we controlled for cardiovascular risk factors and the development of cardiovascular conditions during follow-up by including relevant variables in the regression model to decrease the bias further. The covariables were the fact of having dyslipidaemia or being a diabetic patient, or of developing a cardiovascular condition after the initiation of AH treatment. Although a fully time-dependent measure would have produced a more precise point estimate of effectiveness, it would not have precisely addressed the impact of changes in drug pattern of use. Third, this study population was large and representative of diverse sociodemographic characteristics. Nevertheless, residual confounding effects due to incomplete or inaccurate measurement of covariates or unmeasured confounders cannot be excluded. For example, patients who do not adhere may have other traits that contribute to worsened outcomes, including factors such as depression, lower socioeconomic status and associated adverse health behaviours [30], but we were able to adjust in part for these factors. Again, based on a recent publication on the differential class effects of drug adherence on long-term survival, it has been suggested that adherence-related benefits associated with evidence-based pharmacotherapies are mediated by drug effects more than by healthy adherer behaviours [41].
Our study has other limitations. First, administrative databases provide no information on clinical data and thus do not permit adjustment for clinical severity of diseases. Severely hypertensive patients often require more than an AH agent to achieve blood pressure control [12]. These patients may also be less likely to be adherent to therapy, which may lead to bias [42]. In the current study, we identified patients on monotherapy, bitherapy or tritherapy in the year prior to the index date to adjust for hypertension severity. Second, we could not adjust for glycaemia and cholesterol level, a well-known risk factor of CAD; however, if individuals were using medication for diabetes or dyslipidaemia, we defined the categories of adherence level for these therapies to take into account the adherence level and risk reduction of CAD, giving a partial adjustment. Third, some subjects may have had a previous CVD condition that did not appear in the information collected during the 5-year period prior to the cohort entry date. Therefore, it is reasonable to believe that our subjects may not have had a symptomatic CVD, given that no drug marker was used in the 2 years before the cohort entry date.
Finally, the analysis used prescription refill patterns to assess exposure and thus does not ascertain whether the dispensed medication was actually taken by the patient. However, data suggest good correlation between pharmacy dispensing records and cumulative drug exposure [43]. Despite these limitations, administrative databases offer many advantages, mainly the ability to identify a large population of users of medications in a timely and efficient manner as well as the capacity to reflect usage in a real-world setting. Moreover, a recently published systematic review reported that administrative databases are particularly suited for the evaluation of drugs intended for long-term therapy [44].
In summary, this study of real-world drug utilization patterns in a Canadian setting has shown an association with significant benefit linked with good adherence to AH medication in the context of primary prevention. The assessment of medication adherence and behaviours should be incorporated into routine clinical practice. Interventions in this area are essential so that the therapeutic benefits translate into clinical practice.
Competing interests
None to declare.
The Canadian Institutes Health Research (CIHR) supported this work. S.P., L.L., L.B. and A.B. are research scholars who receive financial support from the Fonds de Recherche en Santé du Québec. We thank Ms Marie-Claude Giguère from the Régie de l'assurance maladie du Québec and Ms Louise Légaré from the Ministère de la Santé et des Services sociaux du Québec for assistance with the data.
REFERENCES
- 1.Fondation des maladies du Coeur du Canada (Fondation des maladies du Coeur du Canada. Le fardeau croissant des maladies cardiovasculaires et des accidents vasculaires cérébraux au Canada, 2003. Report No.: 1-89624-32-4.
- 2.Writing Group Members. Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Mark DB, Van de Werf FJ, Simes RJ, White HD, Wallentin LC, Califf RM, Armstrong PW for the VIGOUR Group. Cardiovascular disease on a global scale: defining the path forward for research and practice. Eur Heart J. 2007;28:2678–84. doi: 10.1093/eurheartj/ehm411. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. Eur Heart J. 2002;23:620–6. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lana F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 7.Collins R, MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Br Med Bull. 1994;50:272–98. doi: 10.1093/oxfordjournals.bmb.a072892. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 10.Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;355:1955–64. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 11.Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 12.Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR, Leiter LA, Lewanczuk RZ, Schiffrin EL, Hill MD, Arnold M, Moe G, Campbell TS, Herbert C, Milot A, Stone JA, Burgess E, Hemmelgarn B, Jones C, Larochelle P, Ogilvie RI, Houlden R, Herman RJ, Hamet P, Fodor G, Carruthers G, Culleton B, Dechamplain J, Pylypchuk G, Logan AG, Gledhill N, Petrella R, Tobe S, Touyz RM Canadian Hypertension Education Program. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II—Therapy. Can J Cardiol. 2006;22:583–93. doi: 10.1016/s0828-282x(06)70280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 14.Rosendorff C, Black H, Cannon C, Gersh B, Gore J, Izzo J, Kaplan N, O'Connor C, O'Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–88. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 15.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, Poulter N, Primatesta P, Stegmayr B, Thamm M. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 16.Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10(10)(1):1097–102. doi: 10.1016/s0895-7061(97)00224-0. Pt. [DOI] [PubMed] [Google Scholar]
- 17.Krousel-Wood M, Thomas S, Muntner P, Morisky D. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–62. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MR, Reid CM, Ryan P, Willson K, Yelland L. Self-reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2) Med J Aust. 2006;185:487–9. doi: 10.5694/j.1326-5377.2006.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 19.International Classification of Diseases. Manual of the International Statistical Classification of Diseases, Injuries, and Cause of Death. 9th. Geneva: World Health Organization; 1977. edn. Publication no PHS 80-1260. [Google Scholar]
- 20.Canada: Supply and Services. Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures. 2nd edn. Ottawa, Canada: Statistics Canada Health Division; 1986. [Google Scholar]
- 21.RAMQ, Principales variables selon la catégorie de personnes assurées. Studies and Statistics 2005. Available at http://www.ramq.gouv.qc.ca (last accessed 20 September 2006.
- 22.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 23.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 24.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–94. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 25.Monfared A, Rahme E, LeLorier J. Accuracy of ICD-9 diagnosis code ‘410’ to identify episodes of hospitalizations for acute myocardial infarction in RAMQ. First Canadian Therapeutics Congress 2004. Halifax, Canada, 2004.
- 26.Lubin J, Gail M. Biased selection of controls for case control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 27.Peterson AM, Nau DP, Cramer JA, Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 28.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–57. [PubMed] [Google Scholar]
- 29.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–8. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 30.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 31.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 32.Essebag V, Platt R, Abrahamowicz M, Pilote L. Comparison of nested case–control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5:5–11. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene WH. Econometric Analysis. 3rd edn. Upper Saddle River, NJ: Prentice-Hall; 1997. p. 552. [Google Scholar]
- 34.Belsley DA, Kuy E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New York: John Wiley & Sons; 1981. [Google Scholar]
- 35.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 36.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 37.Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol. 2006;97:28A–32A. doi: 10.1016/j.amjcard.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 38.O'Leary DH, Polak JF, Kronmal RA, Manolia TA, Burke GL, Wolfson SK for The Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older patients. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 39.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 40.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiology terminology. Am J Epidemiol. 1999;149:981–3. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 41.Daskalopoulo SS, Delaney JAC, Filion KB, Brophy JM, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population-based study. Eur Heart J. 2008;29:2083–91. doi: 10.1093/eurheartj/ehn346. [DOI] [PubMed] [Google Scholar]
- 42.Payne KA, Esmonde-White S. Observational studies of antihypertensive medication use and compliance: is drug choice a factor in treatment adherence? Curr Hypertens Rep. 2000;2:515–24. doi: 10.1007/s11906-996-0035-6. [DOI] [PubMed] [Google Scholar]
- 43.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]


