Abstract
Aims/hypothesis
Variants in the TCF7L2 gene remain the strongest genetic associations with increased risk of type 2 diabetes. Recently, we identified a unique splicing form of TCF7L2 expressed in pancreatic islets, pancreas and colon and detected by assay ‘ex13-13b’. The expression of ex13-13b strongly correlated with proinsulin in glucose-stimulated pancreatic islets, suggesting a potential role for this form in the development of type 2 diabetes. The goal of this study was to further characterise this unique TCF7L2 splicing form in human tissues.
Methods
We used a panel of 34 human tissues and 80 human cell lines to measure the expression of assay ex13-13b with use of quantitative RT-PCR.
Results
The highest expression of assay ex13-13b was detected in several areas of the brain (hypothalamus/thalamus, occipital lobe) and in neuronal cell line SHS5Y5. Low expression was confirmed in pancreatic islets, small intestine, pancreas and colon, while no expression was detected in other human tissues and cell lines. The expression of assay ex13-13b correlated with the gene for cocaine- and amphetamine-regulated transcript (CART, also known as CARTPT) in a panel of human tissues (n = 12, r = 0.85, p = 0.00046), pancreatic islets (n = 23, r = 0.62, p = 0.0016) and colon (n = 98, r = 0.54, p < 0.0001).
Conclusions/interpretation
The significant correlation between expression of a unique splicing form of TCF7L2, named here TCF7L2-NE, and CART, the gene for an anorexigenic neurohormone expressed in the central and peripheral nervous system, suggests that these transcripts may share neuroendocrine functions important for brain, gut and pancreatic islets.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-009-1640-0) contains supplementary material, which is available to authorised users.
Keywords: Brain–gut–islets axis, CART, Gene expression, Neuroendocrine regulation, TCF7L2, Type 2 diabetes
Introduction
Transcription factor 7-like 2 (TCF7L2) (also known as TCF-4) plays a central role in the WNT pathway and is involved in a variety of biological processes. Non-coding genetic variants within TCF7L2 gene remain the strongest genetic association with increased risk of type 2 diabetes and dysfunction of pancreatic islets [1, 2]. However, the molecular and cellular mechanisms through which TCF7L2 contributes to these associations are still unclear.
Previously, we described a tissue-specific splicing diversity of the gene resulting from the use of three alternative promoters and six alternative exons [3]. We detected a unique splicing form defined by the presence of an alternative C-terminal exon, 13b, expressed in pancreatic islets, colon and pancreas [3]. Expression of this form strongly correlated with insulin expression in glucose-stimulated pancreatic islets and was decreased in islets of carriers of the type 2 diabetes-associated single nucleotide polymorphisms of TCF7L2, rs7903146 and rs12255372 [3].
In the current study we aimed to further characterise the TCF7L2 splicing form containing the alternative exon 13b. We provide evidence for a neuroendocrine pattern of expression of this form, named TCF7L2-NE, in human tissues and cell lines. We show that expression of TCF7L2-NE correlated with expression of the gene for cocaine- and amphetamine-regulated transcript (CART, also known as CARTPT), also found in the brain, islets and gut. CART has been shown to play a critical role in glucose sensing, stimulation of hormone secretion, cell survival, thermoregulation and appetite suppression. Based on strong correlation between expression of TCF7L2-NE and CART in a panel of human tissues, islets and colon, these data suggest that TCF7L2-NE may have important neuroendocrine functions.
Methods
Samples, cDNA preparation and expression analysis
Samples of total RNA from human tissues were purchased from Clontech (Mountain View, CA, USA) and BioChain Institute (Hayward, CA, USA). Samples of human pancreatic islets, monocytes, colon, pancreas and lymphoblastoid cell lines were previously described [3]. RNA from the NCI-60 cell lines was provided by the Molecular Targets Team, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI/NIH. All other cell lines were purchased from ATCC (Manassas, VA, USA) or Asterand (Detroit, MI, USA). Total RNA was prepared with a MirVana kit (Ambion, Austin, TX, USA) and the quality was evaluated with a Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). cDNA from all samples was prepared from 1 μg total RNA, as previously described [3]. All reactions were run in duplicate in 5 μl volumes in 384-well optical plates on an SDS7900 (Applied Biosystems, Foster City, CA, USA). Negative controls and genomic DNA controls were used for all assays. The expression of endogenous control genes, beta-2 microglobulin (B2M) and cyclophilin A (PPIA), measured in separate reactions but in the same cDNA samples was used for normalisation of expression. All assays were tested before analysis and showed comparable amplification efficiencies. The list of expression assays is provided in the Electronic supplementary material (ESM) Table 1.
Statistical analysis
Average values for technical duplicates were normalised by geometric means of endogenous controls according to the ΔCt method of relative quantification, as previously described [3]. Pearson’s correlation coefficients and two-sided p values were calculated with SPSS 16.0 software (SPSS, Chicago, IL, USA). p values were not adjusted for multiple tests.
Results
Expression of the TCF7L2 splicing form with exon 13b in human tissues and cell lines
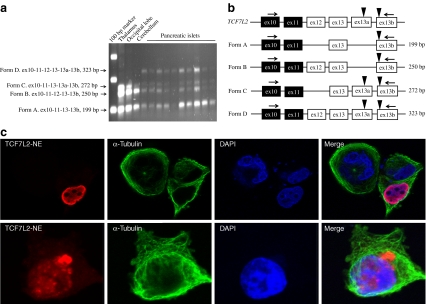
Initially, the expression of TCF7L2 assay ex13-13b was detected in pancreatic islets, pancreas and colon [3]. We then tested the expression of this assay in 34 human tissues and 80 cell lines (60 cell lines from the NCI-60 set and 20 additional cell lines; ESM Tables 2 and 3). High expression of assay ex13-13b was detected in several areas of the brain (thalamus, hypothalamus, occipital lobe) and in SHS5Y5 neuroblastoma cell line, but not in other brain-derived cell lines (a human neuronal epithelioma cell line SK-N-MC and glioblastoma and astrocytoma cell lines SF_268, SF_295, SF_539, SNB_19, U251 and SNB_75). Expression was also detected in pancreatic islets, small intestine, colon and pancreas, but not in other human tissues, NCI-60 and additional cell lines (Table 1). We PCR amplified a fragment of TCF7L2 gene between a constitutive exon 10 and an alternative exon 13b in cDNA from human brain and pancreatic islets (Fig. 1a,b). There were four possible combinations of alternative exons 12, 13, 13a and 13b in splicing forms with exon 13b. The splicing forms that include both exons 13a and 13b have two alternative stop codons separated by 37 bp (Fig. 1a,b). Expression of these forms (assay 13a-13b) was found to be very low in brain and islets and was not studied further (data not shown). However, the expression of assay ex13-13b was up to 750 times higher in the brain than in pancreas (Table 1).
Table 1.
Expression of TCF7L2 assay ex13-13b and neurohormone genes
| Tissue | TCF7L2 ex13-13b | ex13-13b (fold vs pancreas) | CART | CHRA | NPY | GHRE | VIP | AGRP | SST | POMC | GIP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thalamus | −4.25 | 750 | −6.13 | −4.50 | −2.67 | −12.83 | −8.58 | −14.49 | −4.55 | ND | ND |
| Occipital lobe | −5.42 | 333 | −5.15 | −0.23 | −1.00 | −10.97 | −2.80 | −12.75 | −0.86 | ND | ND |
| Hypothalamus | −6.49 | 159 | −6.06 | −5.57 | −3.98 | −13.39 | −8.58 | ND | −2.94 | ND | ND |
| Cerebellum | −7.82 | 63 | −5.13 | −6.53 | −8.85 | −12.74 | −8.64 | −11.73 | −14.85 | ND | ND |
| Pituitary gland | −8.58 | 37 | −9.15 | −0.33 | ND | −13.76 | −14.13 | ND | −10.74 | ND | ND |
| SHS5Y5 neuroblastoma cell line | −8.75 | 33 | −10.50 | NT | NT | NT | NT | NT | NT | NT | NT |
| Hippocampus | −8.99 | 28 | −6.57 | −3.51 | −3.85 | −10.85 | −6.68 | −13.05 | −5.54 | ND | −14.76 |
| Small intestine | −11.36 | 5.4 | −9.49 | −3.83 | −7.02 | −9.26 | −7.01 | ND | −5.83 | ND | −6.91 |
| Pancreatic islets | −11.84 | 3.9 | −12.05 | 0.76 | −3.14 | −5.79 | −11.48 | −16.50 | 3.29 | −13.18 | ND |
| Colon tumour | −13.24 | 1.5 | −10.90 | −9.47 | ND | ND | −8.13 | ND | −14.09 | ND | ND |
| Colon | −13.84 | 1.2 | −10.77 | −6.04 | −10.98 | ND | −6.80 | ND | −8.14 | ND | ND |
| Pancreas | −13.80 | 1.0 | −11.95 | −3.31 | −7.27 | −11.12 | −7.62 | ND | −2.59 | ND | ND |
| Correlation (r) | – | 0.85 | 0.21 | 0.66 | −0.57 | 0.10 | 0.45 | 0.11 | NS | NS | |
| p value | 0.00046* | NS | 0.06 | NS | NS | NS | NS |
Expression values are normalised to endogenous controls B2M and PPIA. Pearson’s correlation coefficients (r) are for expression of TCF7L2 assay ex13-13b and each neurohormone
Each tissue is represented by one sample or a pool of samples from two or three individuals; expression of assay ex13-13b was not detected in the following tissues: liver, lung, spleen, kidney, skeletal muscle, heart, blood, monocytes, lymphoblasts, breast, parotid, thyroid, ovary, oesophagus, stomach, bladder, prostate, ovarian cancer, lung cancer, kidney cancer, breast cancer, adipose and in NCI-60 cell lines and additional cell lines 293T, HepG2, HeLa, MDA435, MCF10, PANC1, SK-N-MC, prostate cell lines OPCN1, OPCN2, OPCN3, OPCT1, OPCT2, OPCT3, RWPE-1, RWPE-2, PWR1E, bladder cancer cell lines HTB-5, HTB-9, HT-1376 and J-82
*p value will be significant even after adjustment for multiple tests
ND, expression tested but not detected; NS, no significant correlation based on samples with detectable expression for both assays; NT, expression not tested
Fig. 1.
C-terminal alternative exons of TCF7L2 in transcripts expressed in total human brain and pancreatic islets and cellular localisation of TCF7L2 splicing form detected by assay ex13-13b (TCF7L2-NE). a Results of PCR amplification with primers ex10 Forw and ex13b Rev of TCF7L2 (ESM Table 1) in cDNA from total human brain and pancreatic islets. cDNA prepared from 10 ng total RNA was used for all PCR reactions. The PCRs were performed with Phusion DNA polymerase (New England Biolabs, Ipswich, MA, USA) and the PCR products were resolved on a 2% (wt/vol.) agarose gel. Four types of PCR fragments (forms A–D) were observed based on the combination of alternative exons 12, 13, 13a and 13b. b Schematic representation of C-terminal exons of TCF7L2. Constitutive exons are represented by black rectangles and alternative exons by white rectangles. Black triangles mark alternative translation stops. Arrows indicate positions of PCR primers. Forms A and B use a stop codon within exon 13b and forms C and D use a stop codon within exon 13a. In the forms with alternative exons 13a and 13b (C and D) two in-frame stop codons are separated only by 37 bp. Expression of these forms (assay ex13a-13b) was very low in brain and islets and was not studied further. c Confocal imaging of cellular localisation of the recombinant TCF7L2-NE in human PANC-1 cell line (pancreatic cancer). Upper and lower panels represent images of PANC-1 cells transfected with the TCF7L2-NE Halo-tag expression construct (GenBank FJ010169) [3]. Non-transfected cells serve as controls for specificity of detection. For the expression construct a full-length cDNA for TCF7L2-NE splicing form was cloned into a pFC8A expression vector with a C-terminal Halo-tag (Promega, Madison, WI, USA). The endotoxin-free plasmid was prepared with a EndoFree plasmid Maxi kit (Qiagen, Gaithersburg, MD, USA). For transfection, human PANC-1 (pancreatic cancer) cells were plated on chamber slides (Thermo Fisher Scientific, Rochester, NY, USA) and transfected next day with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After 48 h, the cells were fixed and incubated with primary rabbit anti-HaloTag antibodies (Promega) and with mouse anti-α-tubulin antibodies ab7291-100 (Abcam, Cambridge, MA, USA). Secondary donkey anti-rabbit and anti-mouse antibodies labelled with Alexa fluor 594 and 488 were used for imaging (Invitrogen). The anti-fade ProLong Gold mounting media with DAPI (Invitrogen) was used to mount the cover slides. The imaging was performed with a confocal microscope LSM 510 Meta (Carl Zeiss Microimaging, Thornwood, NY, USA) with ×63 magnification
Coexpression of TCF7L2 assay ex13-13b with brain–islet–gut neurohormones
The pattern of expression of TCF7L2 splicing form detected by assay ex13-13b suggested that it might be expressed in neuroendocrine cells of brain, islets and gut. Based on the literature, we selected a number of neurohormones that can be expressed in the brain and enteric nervous system [4]. We tested for correlation in expression of assay ex13-13b with several neurohormone genes, neuropeptide Y (NPY) and agouti-related peptide (AGRP) expressed in glucose-inhibited neurons, pro-opiomelanocortin (POMC) and CART expressed in glucose-activated neurons, a marker of endocrine function chromogranin A (CHRA, also known as CHGA), and several other neuropeptides: ghrelin (GHRE, also known as GHRL), vasoactive intestinal peptide (VIP), gastric inhibitory polypeptide, also known as glucose-dependent insulinotropic peptide (GIP) and somatostatin (SST).
In a panel of 34 human tissues and 80 cell lines (NCI-60 and 20 additional cell lines) the most similar pattern of tissue distribution and level of expression was observed between TCF7L2 assay ex13-13b and CART (r = 0.85, p = 0.00046, Table 1). Both these transcripts were expressed highly in the brain and in neuronal cell line SHS5Y5, low in small intestine, islets, colon and pancreas and not expressed in all other tissues and cell lines tested, even though the expression of endogenous controls B2M and PPIA was equally high in all these samples (data not shown). Based on the observed pattern of expression, we designated the splicing form detected by assay ex13-13b as a neuroendocrine splicing form, TCF7L2-NE. Expression of TCF7L2-NE also showed correlation with CART in human pancreatic islets (n = 23, r = 0.62, p = 0.0016) and colon (n = 98, r = 0.54, p < 0.0001). Expression of both TCF7L2-NE and CART in pancreas (n = 44) was low and not detectable in all samples and was not analysed (data not shown).
The protein encoded by this splicing form is C-terminally truncated and lacks binding sites for the C-terminal binding protein (CtBP) that regulates TCF7L2 post-translationally [5]. Alternative exon 13b contributes only two amino acids before the termination of transcription, making it impossible to distinguish TCF7L2-NE from other TCF7L2 forms at the protein level. We tested cellular localisation of this form by transfecting an expression construct for the full-length TCF7L2-NE splicing form (GenBank FJ010169) [3] into human PANC-1 cells. The localisation of this form was clearly nuclear (Fig. 1c), as was expected based on the presence of nuclear localisation signals and the role of TCF7L2 as a transcription factor.
Discussion
Disturbances in brain–gut–islet regulation of satiety and glucose metabolism are associated with development of obesity and type 2 diabetes [6, 7]. In this study we provide evidence that the unique splicing form of TCF7L2 that we named a ‘neuroendocrine form, TCF7L2-NE’, might be a part of this regulation. TCF7L2-NE exhibits a neuronal pattern of expression, as it is highly expressed in the thalamic, hypothalamic and occipital regions of the brain, as well as in a neuronal cell line SHS5Y5. Comparatively lower levels of expression were found in islets and gut (up to 750-fold lower than in brain), while no expression was found in all other human tissues and cell lines tested here. Expression of a similar murine splicing form of TCF7L2 (designated as a form with exons 12-13-14-17-18) was recently located to postmitotic neurons of mouse midbrain [8].
TCF7L2-NE is highly expressed in the brain areas that contain neurons of the hypothalamic arcuate (ARC) nucleus that regulate energy metabolism and satiety. Glucose-sensing neurons express CART and POMC [4]. In the panel of human tissues used in this study, expression of TCF7L2-NE correlated with CART but not with POMC, possibly marking a subpopulation of glucose-activated neurons activated by blood glucose, insulin and leptin [9]. Expression of TCF7L2-NE did not correlate with NPY and AGRP (markers of glucose-inhibited neurons activated by ghrelin and inhibited by glucose and leptin [4]). In sum, expression of TCF7L2-NE and CART strongly correlated in a panel of human tissues (n = 12, r = 0.85, p = 0.00046), in islets (n = 23, r = 0.62, p = 0.0016) and colon (n = 98, r = 0.54, p < 0.0001) with p values being significant even after adjustment for multiple tests.
CART has been shown to play a critical role in glucose sensing, stimulation of hormone secretion in pancreatic islets, neuronal survival, thermoregulation and appetite suppression [4, 10, 11]. The functional relationship between TCF7L2-NE and CART is not clear. Based on the strong correlation between expression of TCF7L2-NE and CART, we suggest that these transcripts may have similar functions related to the brain–islet–gut regulation of metabolism. Further studies will be needed to identify the functional role of TCF7L2-NE in different metabolic conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
List of expression assays and primers (PDF 53 kb)
NCI-60 cell lines that showed absence of expression of TCF7L2 assay ex13-13b and CART (PDF 75 kb)
Additional cell lines that showed absence of expression of TCF7L2 assay ex13-13b and CART (PDF 52 kb)
Acknowledgements
We are grateful to the Molecular Targets Team, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis (DCTD), National Cancer Institute (NCI)/National Institutes of Health (NIH) for providing RNA samples from NCI-60 cell lines, to the Islet Cell Resource Centers and the National Disease Research Interchange Network for human islets, to F. S. Collins and M. R. Erdos (National Human Genome Research Institute [NHGRI]/NIH) for sharing samples of pancreatic islets and helpful comments, and to S. Bowell for help with procurement of tissues from the University of Minnesota Tissue Procurement Facility. We would also like to thank M. Carlson and C. Welch for their help in preparing samples for analysis. The study was supported by the intramural research program of NCI/NIH and by the NIH grant 1R21DK078029-01 (J. L. Hall).
Duality of interest
The authors declare that there is no duality of interests associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- TCF7L2
Transcription factor 7-like 2
- TCF7L2-NE
Neuroendocrine splicing form of TCF7L2
References
- 1.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 2.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prokunina-Olsson L, Welch C, Hansson O, et al. Tissue-specific alternative splicing of TCF7L2. Hum Mol Genet. 2009;18:3795–3804. doi: 10.1093/hmg/ddp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mountjoy PD, Rutter GA. Glucose sensing by hypothalamic neurones and pancreatic islet cells: AMPle evidence for common mechanisms? Exp Physiol. 2007;92:311–319. doi: 10.1113/expphysiol.2006.036004. [DOI] [PubMed] [Google Scholar]
- 5.Fang M, Li J, Blauwkamp T, Bhambhani C, Campbell N, Cadigan KM. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J. 2006;25:2735–2745. doi: 10.1038/sj.emboj.7601153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity: a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Nazwar TA, Glassmann A, Schilling K. Expression and molecular diversity of Tcf7l2 in the developing murine cerebellum and brain. J Neurosci Res. 2009;87:1532–1546. doi: 10.1002/jnr.21989. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Liu X, Hentges ST, et al. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 10.Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: a putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci USA. 1999;96:13506–13511. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wierup N, Sundler F. CART is a novel islet regulatory peptide. Peptides. 2006;27:2031–2036. doi: 10.1016/j.peptides.2006.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of expression assays and primers (PDF 53 kb)
NCI-60 cell lines that showed absence of expression of TCF7L2 assay ex13-13b and CART (PDF 75 kb)
Additional cell lines that showed absence of expression of TCF7L2 assay ex13-13b and CART (PDF 52 kb)