Abstract
Background
Complementary and alternative medicines are increasingly used for the treatment of asthma in Western countries. A novel three-herb antiasthma herbal medicine intervention (ASHMI™; Sino-Lion Pharmaceutical Company; Shan Dong China) was demonstrated to be effective and safe in a murine model of asthma and in a preliminary clinical study in China.
Objective
The objective of this study was to evaluate the safety and tolerability of ASHMI in adult subjects with allergic asthma.
Design
Randomized, double-blind, placebo-controlled, dose escalation, phase I trial aimed at developing a botanical drug under the United States Food and Drug Administration Investigational New Drug title.
Interventions
Subjects received one of three doses of ASHMI or placebo: 600 mg (2 capsules); 1200 mg (4 capsules); or 1800 mg (6 capsules) twice daily for 1 week. Four (4) ASHMI and 2 placebo subjects were treated at each dose level. Subjects continued to use their conventional asthma medications for the duration of the study.
Outcome measures
Vital signs, physical examination, laboratory data, and electrocardiogram data were monitored throughout the study to assess occurrence of adverse events (AEs). Immunomodulatory studies were performed to evaluate the effect of ASHMI on cytokine, chemokine, and growth factor levels.
Results
Twenty (20) nonsmoking, allergic subjects with asthma were included in the study. Eight (8) subjects (4 ASHMI and 4 placebo) reported mild gastrointestinal symptoms. No grade 3 AEs were observed during the study period. Vital signs, electrocardiogram findings, and laboratory results obtained at pre- and post-treatment visits remained within normal range. No abnormal immunologic alterations were detected.
Conclusion
In this phase I study, ASHMI appeared to be safe and well tolerated by subjects with asthma. These findings allowed initiation of a larger phase II study to assess the efficacy of ASHMI.
Introduction
Asthma is a major public health problem worldwide, particularly in Westernized societies, affecting an average annual 20 million persons in the United States in recent years.1 Inhaled corticosteroids (ICS) are the first-line treatment for persistent asthma;2 however, some patients remain symptomatic despite long-term use of these medications. Additionally, many patients with asthma are concerned about the side-effects of ICS, a factor associated with poor patient adherence.3,4 Newer asthma drugs, including leukotriene inhibitors and an anti-immunoglobulin E (IgE) medication, have also been shown to be effective but have not replaced the use of ICS.2 For these reasons, in spite of the uncertainty of its benefits due a lack of well-controlled scientific studies, patients have increasingly turned to complementary and alternative medicine (CAM) for treatment of their asthma.5 A recent study showed that up to 30% of adults and 60% of children use some form of CAM to treat their asthma.6
We recently developed a novel, nonsteroidal Chinese herbal formula (antiasthma herbal medicine intervention or ASHMI™; see Materials and Methods, Study medication) for the treatment of asthma. ASHMI is composed of three herbs: Ling-Zhi (Ganoderma lucidum), Ku-Shen (Sophora flavescens), and Gan-Cao (Glycyrrhiza uralensis), and was derived from a Traditional Chinese Medicine (TCM) 14-herb formula.7 In an animal model of asthma, ASHMI decreased airway hyperreactivity and eosinophilic inflammation, and resulted in a downregulation of the Th2 response; results were comparable to the classic 14-herb formulation.8–11 No cytotoxicity was detected at the highest effective dose tested.11,12 A preliminary study in China, which included 91 subjects with moderate-to-severe persistent asthma, showed that ASHMI significantly improved lung function and reduced symptom scores.12 These studies suggest that ASHMI may be an effective novel medication for asthma, particularly for patients with an allergic component.
On the basis of these observations, we initiated a phase I study to evaluate the tolerability and safety of ASHMI in subjects with allergic asthma as a United States Food and Drug Administration (U.S. FDA) Investigational New Drug (IND) botanical drug product.
Materials and Methods
Subjects
Nonsmoking subjects with asthma ages 18–55 were screened after informed consent was obtained according to institutional guidelines. In order to be eligible, subjects had to have physician-diagnosed asthma and positive skin tests or serum specific IgE [UniCAP; Phadia, Uppsala, Sweden] to at least two of the following allergens: Dermatophagoides pteryonyssinus, D. farinae, cat, dog, German cockroach, molds, trees, or ragweed. Additional inclusion criteria included the following: (1) no acute infection, (2) lack of clinically significant abnormalities on screening electrocardiogram or laboratory data, and (3) no history of systemic diseases. Subjects with uncontrolled asthma, defined as those having an acute exacerbation requiring systemic steroids, were excluded from the study. Additional exclusion criteria included (1) pregnancy or breastfeeding; (2) abnormal hepatic, bone marrow, or renal function; (3) drug or alcohol abuse; and (4) participation in another research protocol within the last 30 days.
Study design
This study was a randomized, double-blinded, placebo-controlled, dose-escalation, phase I trial. Three (3) doses of ASHMI were studied: 600 mg (2 capsules); 1200 mg (4 capsules); or 1800 mg (6 capsules) twice a day for 1 week. Selection of dose range was based on previous experience with ASHMI in animal models and humans. Four (4) ASHMI and 2 placebo subjects were treated at each dose level. ASHMI doses were increased after a review of safety data from the 6 subjects receiving the lower dose of medication. If at any point a subject needed to be replaced and blinding was able to be maintained, the subject was replaced by 1 additional subject. If blinding was unable to be maintained, 2 additional subjects (1 ASHMI and 1 placebo) were enrolled.
Initial evaluation consisted of a thorough medical history and physical examination, vital signs, prick skin testing, or in vitro allergen-specific IgE testing to environmental allergens, baseline peak expiratory flow (PEF) and spirometry measurements, electrocardiogram, urinalysis, and routine laboratory blood tests (complete blood count, serum chemistries, renal function, liver function tests, and pregnancy test for female participants).
After initial screening, subjects were started on ASHMI or placebo twice daily with meals for 7 days. Subjects continued their conventional asthma medications for the duration of the study, but were asked to refrain from other herbal medication use. A daily diary was kept to record morning and evening medication doses and peak flows, as well as any new symptoms. Investigators were in direct telephone contact with each subject on two occasions during the study period to reinforce medication compliance and assess potential adverse events (AEs). During the final visit, the intercurrent medical history was reviewed and physical examination, PEF, spirometry, electrocardiogram, and laboratory testing were repeated.
Study medication
ASHMI is manufactured by a good manufacturing practice facility at Sino-Lion Pharmaceutical Company (Shan Dong, China; U.S. FDA IND 71,526). The quality, safety, and consistency of ASHMI were established according to FDA guidance under botanical drug title (Chemical, Manufacturing, and Control Data [21 CFR 312.23(a) (7)])13 and are summarized below.
Raw medicinal herbs
Herbs used in this study were of Chinese origin. All were inspected for identity and quality by pharmacists trained and licensed in identification and processing of herbal medicines14,15 and only herbs meeting quality and safety standards were used.15 General information, including time of harvesting, processing, and potential residual pesticide level, is listed in Table 1. Based on the organoleptic, macroscopic, and microscopic examination, the raw herbal materials used in ASHMI were identified as the fruiting body of Ling-Zhi, the roots of Ku-Shen, and the roots and rhizome of Gan-Cao (Fig. 1). Voucher specimens of the raw herbs are archived in our laboratory.
Table 1.
Basic Information on Raw Materials Used in ASHMI™
| Properties | Ling-Zhi (Ganoderma lucidum) | Ku-Shen (Sophora flavescens) | Gan-Cao (Glycyrrhiza uralensis) |
|---|---|---|---|
| Harvest time | August and September | May, June, and July | Autumn |
| Processing | Clean and dry in the shade or in oven at 40–50°C | Remove the remains of rootstock and rootlet, wash clean, soak briefly cut into thick slices, and dry | Eliminate foreign matter, wash clean, soften thoroughly, cut into thick slices, stir-bake the slices until they become yellow to deep yellow and not sticky to the fingers, remove and cool in the air |
| Storage | Preserve in a dry place, protected from molds and moths | Preserve in a dry place | Preserve in a well-ventilated dry place, protected from moths |
| Heavy metals | |||
| Leada (mg/kg) | <0.85 | <0.87 | <0.23 |
| Mercuryb (mg/kg) | <0.1 | <0.1 | <0.1 |
| Pesticides | |||
| Cypermethrinc (mg/kg) | 0.047 | 0.02 | 0.01 |
| Dichlorvosd (mg/kg) | 0.031 | 0.007 | 0.01 |
Recommended limits <5.0 mg/kg (Green Trade Standards of Importing & Exporting Medicinal Plants & Preparations, Published by Ministry of Foreign Trade and Economic Cooperation, People's Republic of China, 2001.
See online document at: www.cas.ac.cn/html/Dir/2001/04/23/5754.htm)
Recommended limits <0.2 mg/kg (Green Trade Standards of Importing & Exporting Medicinal Plants & Preparations, Published by Ministry of Foreign Trade and Economic Cooperation, People's Republic of China, 2001. See online document at: www.cas.ac.cn/html/Dir/2001/04/23/5754.htm)
Recommended limits 0.5 mg/kg. (Maximum Residue Limit in the European Union. See online documents at: www.nda.agric.za/docs/plantquality/MRL%20Export%20lists/table%20grapes.pdf and www.upasitearesearch.org/news/26.pdf).
Recommended limits <0.1 mg/kg. (Maximum Residue Limit in the European Union. See online documents at: www.nda.agric.za/docs/plantquality/MRL%20Export%20lists/table%20grapes.pdf and www.upasitearesearch.org/news/26.pdf)
ASHMI™ is manufactured by Sino-Lion Pharmaceutical Company, Shan Dong, China.
FIG. 1.
Samples of raw herb materials used in ASHMI™ (Sino-Lion Pharmaceutical Company, Shan Dong, China).
Manufacturing process
Ninety-six (96) kg of raw materials containing 62.5% of Ling-Zhi (60 kg), 28.1% of Ku-Shen (27 kg), and 9.4% of Gan-Cao (9 kg) were soaked in 10 times volume of water for 1 hour, boiled for 2 hours, and the decoction was collected. The residue was boiled again with 10 times volume of water for 2 hours. The decoctions were combined, concentrated on reduced pressure (60°C, P 0.08 MPa), and dried by thermal spray to yield 11 kg (11.5%) of ASHMI extract (substance). The substance was granulated and encapsulated at 0.3 g per capsule. Levels of heavy metals and microbial and pesticide residues were well below the recommended limit.15–18
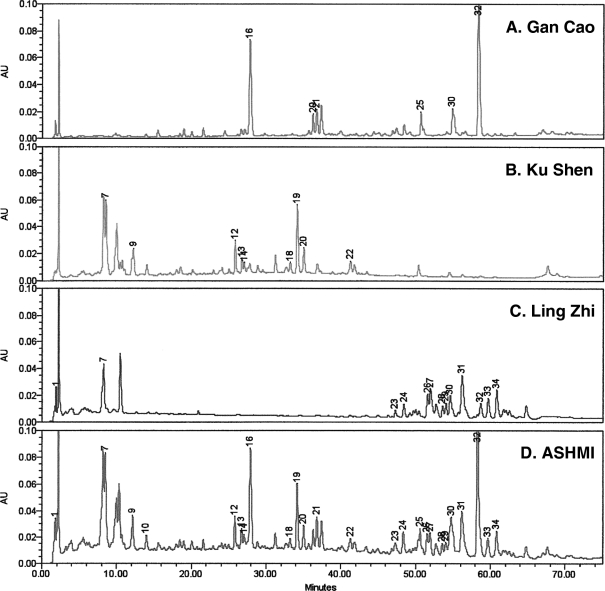
High-pressure liquid chromatographic fingerprint of ASHMI
We also generated a high-pressure liquid chromatography (HPLC) fingerprint of ASHMI as well as the individual herbs in ASHMI as a means of standardization according to FDA's guidance for industrial botanical drug products.13 Gan-Cao 9.4 mg, 20.9 mg of Ku-Shen, 16.4 mg of Ling-Zhi, and 50 mg of ASHMI extracts were dissolved in 10 mL of H2O. The solution was transferred to a separatory funnel, and extracted with 5 mL of n-butanol three times. The combined extracts were dried and dissolved in 50% aqueous methanol. The solution was then transferred to a 2-mL volumetric flask. The resulting solution was filtered through a 0.2-μm filter (Whatman Inc., Sanford, ME). The 10-μL solution was then analyzed by using Waters Alliance 2695 HPLC system with photodiode array detector (2996) (Waters Corp., Milford, MA). Data were acquired and processed with Waters' Empower software. The separation was achieved on Zorbax SBC18 column (150×4.6 mm; 5-μm particle size) from Agilent Technologies (Santa Clara, CA) and an Agilent's Zorbax C18 Guard column was also installed on it. The mobile phases include 0.15% H3PO4 (A) and acetonitrile (B). The separation gradient started at 2% of B to 48% for 75 minutes and further increased to 70% in 4 minutes. The total chromatographic run time was 80 minutes. The flow rate was adjusted to 1.0 mL/min and the column temperature was set to be 27°C. All the chemicals and solvent used are HPLC grade from Fisher Scientific (Fisher Scientific, Pittsburgh, PA).
Figure 2 shows the 2D-HPLC profiles (at 254 nm) of each herb in parallel with that of the entire ASHMI formula. By comparing the online ultraviolet spectra and retention time (tR), most diagnostic peaks in the fingerprint of ASHMI™ were correlated with individual herbal medicines. Peaks 16, 21, and 32 originated from Gan-Cao, peaks 12, 13, 14, 18, 19, 20, and 22 from Ku-Shen, and peaks 23, 24, 27, 28, 29, 31, 32, and 33 from Ling-Zhi. Two batches of ASHMI were used in the study; HPLC was similar for both batches.
FIG. 2.
High-pressure liquid chromatography (HPLC) chromatogram of individual herbs and ASHMI™ (Sino-Lion Pharmaceutical Company, Shan Dong, China) product: A–C: HPLC fingerprints of individual herbs. D: The HPLC fingerprint of ASHMI. HPLC conditions: column, Agilent Zorbax SB-C18 column (150×4.6 mm i.d.; 5 μm particle size); flow rate, 1mL/minute; wavelength, 254 nm; column temperature, 27°C; mobile phase A, 0.15% H3PO4, mobile phase B, acetonitrile. Data were processed using Waters Empower software.
Placebo capsules
Placebo capsules were identical in appearance but contained cornstarch, a nonallergenic excipient, manufactured by the same company as ASHMI.
Immunological studies
Peripheral blood samples for immunologic studies were obtained from participants in the highest dose level cohort. To assess the effect of ASHMI on serum cytokines, chemokines, adhesion molecules, and growth factors, the samples were evaluated using 23- and 27- Plex Bio-Plex human cytokine assays panels (Bio-Rad Laboratories, Inc., Hercules, CA). The assays, which utilize multiple enzyme-linked-immunosorbent serologic assays, were performed according to the manufacturer's instructions. Briefly, 50 μL per well of capture antibody-coated beads were added to a 96-well filter plate. The plate was washed between steps with a vacuum manifold and 50 μL per well of serum samples at a dilution of 1:4 or standards were incubated with the beads for 30 minutes at room temperature with constant shaking at 300 rpm. Thereafter, the plate was washed as in the earlier step and 25 μL per well of detection antibody was added and incubated with shaking for 30 minutes at room temperature. After washing, streptavidin–phycoerythrin was added and the samples were incubated for 10 minutes at room temperature with shaking. The plate was washed, 125 μL of Bio-Plex assay buffer was added, and after quick shaking at 300 rpm for 30 seconds, the plate was read using the Bio-Plex 200 Suspension Array system (Bio-Rad). Concentrations of various analytes were calculated using the Bio-Plex Manager software version 4.1 (Bio-Rad).
Statistical data analysis
Formal statistical methods were not used to calculate the sample size. The target number of subjects was expected to minimize risk by not exposing a large number of subjects to treatment, while allowing assessment of safety and tolerability as approved by the U.S. FDA.
Descriptive statistics are presented as means and standard deviation. Baseline laboratory values were defined as the pretreatment values obtained during the baseline screening visit.
Safety monitoring
Safety monitoring for AEs was ongoing throughout the study. Table 2 shows our criteria, which were adapted from the World Health Organization Recommendations for Grading of Acute and Subacute Toxicity19 and were approved by the FDA for our ongoing phase I and II ASHMI clinical trials.
Table 2.
Grading of Adverse Effects
| Type of adverse event | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Hematologic | ||||
| Hemoglobin (g/dL) | ≥11.0 | ≥11.0 | ≥11.0 | <11.0 |
| Leukocytes (× 103/μL) | ≥4.0 | ≥4.0 | ≥4.0 | <4.0 |
| Granulocytes (× 103/μL) | ≥1.9 | ≥1.9 | ≥1.9 | <1.9 |
| Platelets (× 103/μL) | ≥100 | ≥100 | ≥100 | <100 |
| Metabolic/gastrointestinal | ||||
| Hyperglycemia (mg/dL) | <120 | <120 | <120 | ≥120 |
| Hypoglycemia (mg/dL) | ≥60 | ≥60 | ≥60 | <60 |
| Hypercalcemia (mg/dL) | <11.0 | <11.0 | <11.0 | ≥11.0 |
| Hypocalcemia (mg/dL) | ≥8.4 | ≥8.4 | ≥8.4 | <8.4 |
| Bilirubin | ≤1.25×Na | ≤1.25×Na | ≤1.25×Na | >1.26×Na |
| SGOT/SGPT | ≤1.25×Na | ≤1.25×Na | ≤1.25×Na | >1.26×Na |
| Alkaline phosphatase | ≤1.25×Na | ≤1.25×Na | ≤1.25×Na | >1.26×Na |
| Abdominal distention | None | Mild | Mild to moderate, not requiring treatment | Moderate-severe, requiring treatment |
| Nausea/vomiting | None | None | Loss of appetite, nausea, transient vomitingb | Vomiting >3 days >3 times/day |
| Diarrhea | None | None | Transient, <3 days, <3 times/day | >3 days >3 times/day |
| Renal | ||||
| Blood urea nitrogen | <1.25×Na | <1.25×Na | <1.25×Na | >1.26×Na |
| Creatinine | <1.25×Na | <1.25×Na | <1.25×Na | >1.26×Na |
| Proteinuria | None | None | None | >1+(<3g/L) |
| Hematuria | None | None | None | Microscopic uropathy |
| Allergic | None | None | None | Drug rash or drug fever |
| Neurologic | None | None | None | Any perceived change in sensation, motor skills, mood |
| Dermatologic | None | None | None | Any dermatologic changes or alopecia |
| Cardiovascular | ||||
| Hypotension | None | None | None | Mild hypotension |
| Ischemia/arrhythmia | None | None | None | Any detectable EKG or symptomatic changes |
N indicates the normal value.
<3 days <3 times/day.
SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; EKG, electrocardiogram.
According to the study protocol, any AE of grade 3 in an ASHMI-treated subject would result in immediate discontinuation of the study medication in that subject and required 3 additional subjects (2 ASHMI, 1 placebo) to be added to that dose cohort. Dose escalation would be delayed until the additional 3 subjects completed the day 7 safety evaluation. If a grade 3 AE was observed in 2 ASHMI subjects in any dose level, the maximum tolerated dose was defined to be one dose level lower.
Results
Subject characteristics
A total of 35 subjects with asthma underwent an initial screening evaluation for the study. Of these, 14 subjects were excluded as they were found to be ineligible at baseline. One (1) subject did not complete the study after the initial visit and was withdrawn from the study after randomization. A total of 20 subjects (12 subjects on ASHMI and 8 on placebo) completed the study and were included in the analyses evaluating the tolerability and safety of ASHMI. Although the protocol specified an enrollment of 18 subjects in the study (6 per dose level), there was an error in the randomization procedures and in order to complete the middle dose cohort while maintaining blinding, we had to enroll 2 additional subjects. Baseline characteristics of study subjects are shown in Table 3. There are no clinically significant differences between the ASHMI and placebo groups at baseline. Study medication compliance was 98% in the ASHMI group and 96% in the placebo group as determined by self- report.
Table 3.
Baseline Characteristics of Study Subjects
| ASHMI™ (n = 12) | Placebo (n = 8) | |
|---|---|---|
| General characteristics | ||
| Age (years), mean ± SD | 32 ± 7 | 31 ± 9 |
| Female gender (%) | 75 | 63 |
| Race/ethnicity (%) | ||
| White | 25 | 75 |
| Black | 25 | 0 |
| Hispanic | 42 | 25 |
| Asian | 8 | 0 |
| Other | 0 | 0 |
| History | ||
| Age at asthma diagnosis (years), mean ± SD | 12 ± 11 | 15 ± 15 |
| Current use of inhaled corticosteroids (%) | 83 | 63 |
| History of emergency department visits for asthma (%) | 17 | 25 |
| History of hospitalizations for asthma (%) | 17 | 25 |
| Prior intubation for asthma (%) | 8 | 0 |
| Baseline pulmonary function test (% predicted) | ||
| FEV1 | 88 ± 10 | 97 ± 6 |
| FVC | 92 ± 13 | 109 ± 18 |
| FEV1/FVC | 98 ± 8 | 82 ± 35 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
ASHMI™ is manufactured by Sino-Lion Pharmaceutical Company, Shan Dong, China.
Adverse events
No grade 3 AEs occurred in subjects treated with ASHMI (Table 4). Three subjects receiving placebo had a grade 3 AE (1 patient had an elevated serum glutamic pyruvic transaminase and 2 subjects had electrocardiogram changes that were not considered to be clinically significant). Results of laboratory testing performed during the study are shown in Table 5. Laboratory values obtained at baseline and after completing ASHMI treatment were within acceptable range. Three (3) subjects had a minor decrease in bicarbonate levels (2 treated with ASHMI and 1 in the placebo group).
Table 4.
Prevalence and Severity of Adverse Events Among Study Participants
| |
Grade 2 or lower Number of total (%) |
Grade 3 Number of total (%) |
||
|---|---|---|---|---|
| Adverse event | ASHMI™ (N = 12) | Placebo (N = 8) | ASHMI™ (N = 12) | Placebo (N = 8) |
| Gastrointestinal symptomsa | 4 (33) | 4 (50) | 0 (0) | 0 (0) |
| Vomiting | 0 (0) | 1 (13) | 0 (0) | 0 (0) |
| Upper respiratory tract infection | 1 (8) | 2 (25) | 0 (0) | 0 (0) |
| Increased thirst | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Body achesb | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Elevated SGPT | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Decreased total serum bicarbonate level | 2 (17) | 1 (13) | 0 (0) | 0 (0) |
| Back muscle injury | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Transient upper extremity itchiness | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Electrocardiogram changes | 0 (0) | 0 (0) | 0 (0) | 2 (25) |
| Trichomonas in urine | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Insomnia | 0 (0) | 1 (13) | 0 (0) | 0 (0) |
Includes heartburn, transient nausea, loss of appetite, abdominal fullness or abdominal discomfort.
Symptoms associated with subject's typical premenstrual syndrome.
SGPT, serum glutamic pyruvic transaminase.
ASHMI™ is manufactured by Sino-Lion Pharmaceutical Company, Shan Dong, China.
Table 5.
Summary of Laboratory Results for Subjects Treated with ASHMI™ or Placebo
| |
|
ASHMI™ (N = 12) |
Placebo (N = 8) |
||
|---|---|---|---|---|---|
| Characteristic | Normal values | Baseline | Post-treatment | Baseline | Post-treatment |
| Glucose (mg/dL), mean ± SD | 60–120 | 81 ± 12 | 79 ± 9 | 75 ± 14 | 71 ± 7 |
| Sodium (mEq/L), mean ± SD | 135–145 | 139 ± 2 | 140 ± 2 | 139 ± 3 | 139 ± 1 |
| Potassium (mEq/L), mean ± SD | 3.5–5.0 | 4 ± 0.3 | 4 ± 0.4 | 4 ± 0.4 | 4 ± 0.2 |
| Chloride (mEq/L), mean ± SD | 96–108 | 103 ± 2 | 103 ± 1 | 102 ± 1 | 102 ± 2 |
| CO2 (mEq/L), mean ± SD | 22.0–32.0 | 24 ± 3 | 23 ± 2 | 24 ± 2 | 24 ± 2 |
| Urea (mg/dL), mean ± SD | 10–30 | 13 ± 3 | 13 ± 3 | 16 ± 4 | 17 ± 5 |
| Creatinine (mg/dL), mean ± SD | 0.7–1.4 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.2 |
| SGPT (U/L), mean ± SD | 1–53 | 19 ± 8 | 20 ± 8 | 21 ± 9 | 20 ± 9 |
| SGOT (U/L), mean ± SD | 1–50 | 20 ± 4 | 20 ± 7 | 24 ± 8 | 24 ± 10 |
| Eosinophil (× 103/μL), mean ± SD | 0–0.8 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.4 |
| WBC (× 103/μL), mean ± SD | 4.5–11.0 | 7 ± 2 | 7 ± 1 | 7 ± 2 | 7 ± 1 |
| Hemoglobin (g/dL), mean ± SD | 13.9–16.3 | 13 ± 0.9 | 13 ± 1 | 14 ± 2 | 14 ± 1 |
| Platelet (× 103/μL), mean ± SD | 150-450 | 292 ± 81 | 238 ±123 | 281 ± 60 | 273 ± 64 |
SD, standard deviation; SGPT, serum glutamic pyruvic transaminase; SGOT, serum glutamic oxaloacetic transaminase; WBC, white blood cells.
ASHMI™ is manufactured by Sino-Lion Pharmaceutical Company, Shan Dong, China.
Gastrointestinal complaints were the most common AEs reported during the study. Four (4) (33%) subjects treated with ASHMI and 4 (50%) placebo-treated subjects reported these symptoms. Most gastrointestinal complaints were of grade 2 (mild to moderate gastrointestinal symptoms not requiring treatment). Additionally, 1 patient receiving placebo reported grade 2 vomiting (transient vomiting <3 days and <3 times a day). There were no acute asthma exacerbations during the study period.
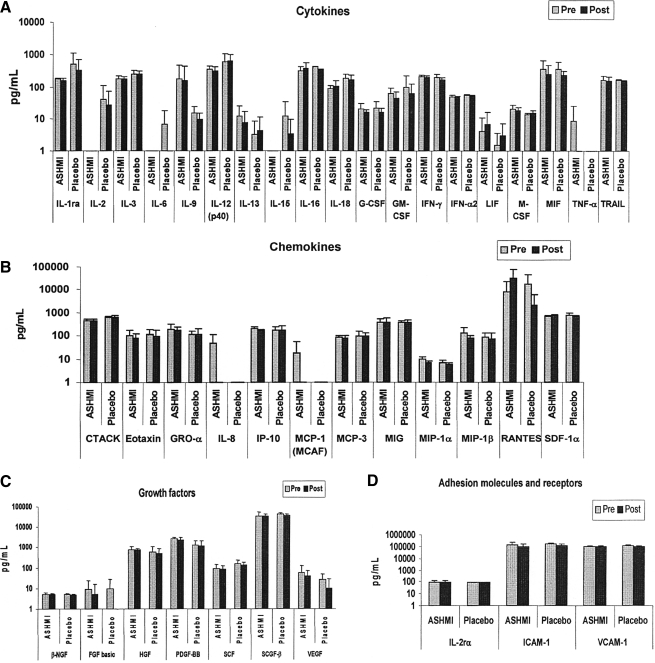
Immunological outcomes
Cytokine, chemokine, and growth factor production remained normal after 1 week of treatment with ASHMI (Fig. 3). Pro-inflammatory cytokines such as tumor necrosis factor (TNF)-a, interleukin (IL)-1, and IL-6 were not increased by ASHMI, suggesting that there was no harmful impact on the immunologic response in the short term.
FIG. 3.
Levels of various cytokines, chemokines, growth factors, adhesions molecules, and receptors in serum samples pre- and post-treatment with ASHMI™ (Sino-Lion Pharmaceutical Company, Shan Dong, China) (N = 4) or placebo (N = 3). Peripheral blood samples for immunologic studies were obtained from some participants and evaluated using 23- and 27- Plex Bio-Plex human cytokine assays. Of the 50 analytes tested, 41 were within the detectable range, and these are divided into cytokines (A), chemokines (B), growth factors (C), and adhesion molecules and receptors (D). IL-1a, 1b, 4, 5, 7, 10, 12 (p70), 17, and TNF-β levels were below the detection range and hence not shown in the graphs. IL, interleukin; r, receptor; G, growth; CSF, colony-stimulating factor; GM, granulocyte macrophage; IFN, interferon; LIF, leukemia inhibitory factor; M, macrophage; MIF, macrophage migration inhibitory factor; TNF, tumor necrosis factor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; CTACK, cutaneous T-cell attracting chemokine; GRO, growth-regulated protein; IP, interferon-γ inducible protein; MCP, macrophage chemoattractant protein; MCAF, monocyte chemotactic and activating factor; MIG, monokine-induced interferon gamma; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T expressed and secreted; SDF, stromal cell derived factor; NGF, nerve growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; PDGF platelet derived growth factor; SCF, stem cell factor; SCGF, stem cell growth factor; VEGF, vascular endothelial growth factor; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule.
Discussion
Prior reports suggest that complementary and alternative medicines may have some therapeutic effects on allergic asthma.11 This phase I study was conducted to determine the safety and clinical and hematologic tolerability of ASHMI, a novel herbal formula, in subjects with asthma. There were no serious AEs among subjects treated with active drug at all doses tested. Similarly, biochemical and hematologic parameters remained within acceptable range. The most frequent AE was minor gastrointestinal discomfort. However, these symptoms were reported equally by subjects on placebo and ASHMI, and there was no association with doses tested. Our results show that ASHMI was safe and well tolerated in allergic asthmatic patients. A larger phase II trial is now being initiated to assess the clinical effectiveness of ASHMI.
Although there is strong public interest and increased use of CAMs, including TCM, there are few phase I studies of CAM in the biomedical literature. A phase I study is the first step required by the U.S. FDA for developing new botanical drugs. Recently, the significance of early phase clinical studies in CAM research has been reinforced.20–22 Our study employed a double-blind, placebo-controlled, dose escalation design as required by the FDA. The dose escalation approach ensures the safety and minimizes potential risks. A double-blind, placebo-controlled approach allowed us to exclude non-drug-related AEs. Our results support the usefulness of this design for testing new CAMs.
Research on botanicals involves unique challenges because plant source materials frequently vary in their chemical content and may contain unwanted pesticides, heavy metals, contaminant plant species, or other adulterants. Additionally, TCM formulas frequently contain many unknown components. The U.S. FDA has issued guidelines for industrial botanical products, focusing on the safety and efficacy of products.13 However, information on active ingredients in the botanical products being tested in early phase studies is not required. Our group is the first to obtain a U.S. FDA IND approval to investigate a botanical product to treat asthma. Standardization of the raw herbs, the manufacturing process, and the final product, as well as the safety data on limiting testing of pesticides, heavy metals, and microbials, were key components in obtaining this approval. HPLC is highly recommended by the U.S. FDA to be used as a means of monitoring the consistency of raw herbs and the final product. Studies are under way to identify the active compounds, to further enhance quality control, and to perform a phase II study, which will include evaluation of the drug's pharmacokinetics.
The Bio-Plex multiplexing immunoassay system detects an array of cytokines and chemokines in a single assay and is a powerful, novel technique that has been used in several recent studies.23–25 TNF-α, IL-1, IL-6, B cells, and T cells are involved in immune responses to pathogens and other stimuli and participate in the inflammatory cascade of both allergy and autoimmune diseases. Our study suggests that 1 week of ASHMI treatment did not increase expression of these cytokines. Th2 cytokines play a central role in the pathogenesis of asthma. Our previous studies in an animal model showed that ASHMI significantly reduced Th2 and increased Th1 cytokine production in cultured splenocytes.10,26 A clinical study of 4 weeks' duration conducted in China found that ASHMI significantly reduced serum IL-5 and IL-13 and increased interferon-γ levels, suggesting an immunomodulatory effect, but not overall immune suppression.12 In this phase I study, the small sample size and short duration do not allow us to look for statistically significant effects in Th1/Th2 responses. Additional data from a larger number of participants in the phase II study will be important to assess the immunomodulatory effect of ASHMI.
Conclusions
The present study demonstrated the safety and tolerability of ASHMI. Additional research will be required to determine the therapeutic potential of this new oral IND botanical drug for asthma. A randomized, double-blind, placebo-controlled, phase II study in adults with moderate-to-severe allergic asthma is currently being initiated.
Acknowledgments
This publication was made possible by Grants 1 P01 AT002625-01 from the National Center for Complementary and Alternative Medicine and MO1-RR00071 from the National Center for Research Resources. We would like to thank Sally Noone, Beth Robinson, and Linda Lurslurchachai, whose help was invaluable to this study. We also would like to thank Drs. Ming-Chun Wen, Bolleddula Jayaprakasam, Zhong Mei Zou, Brian Schofield, and Joseph Goldfarb for their helpful discussion and technical support for ASHMI quality control.
Disclosure Statement
Drs. Kelly-Pieper, Patil, Busse, Yang, Kattan, and Wisnivesky have no competing financial interests to disclose. Drs. Sampson and Li hold U.S. Patent Application PCT/US05/08600 on ASHMI.
References
- 1.Moorman JE. Rudd RA. Johnson CA, et al. National surveillance for asthma: United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Apter AJ. Boston RC. George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: It's not just black and white. J Allergy Clin Immunol. 2003;111:1219–1226. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 4.George M. Freedman TG. Norfleet AL, et al. Qualitative research-enhanced understanding of patients' beliefs: Results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol. 2003;111:967–973. doi: 10.1067/mai.2003.1459. [DOI] [PubMed] [Google Scholar]
- 5.Bielory L. Russin J. Zuckerman GB. Clinical efficacy, mechanisms of action, and adverse effects of complementary and alternative medicine therapies for asthma. Allergy Asthma Proc. 2004;25:283–291. [PubMed] [Google Scholar]
- 6.Slader CA. Reddel HK. Jenkins CR, et al. Complementary and alternative medicine use in asthma: Who is using what? Respirology. 2006;11:373–387. doi: 10.1111/j.1440-1843.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 7.Li XM. Huang CK. Zhang TF, et al. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106:660–668. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- 8.Busse PJ. Wen MC. Huang CK, et al. Therapeutic effects of the Chinese herbal formula, MSSM-03d, on persistent airway hyperreactivity and airway remodeling [abstr] J Allergy Clin Immunol. 2004;113:S220. [Google Scholar]
- 9.Busse PJ. Zhang T. Srivastava K, et al. Prophylactic effects of the Chinese herbal formula, ASHMI, in a murine model of late onset atopic asthma [abstr] J Allergy Clin Immunol. 2007;119:S247. [Google Scholar]
- 10.Li XM. Zhang TF. Sampson H, et al. The potential use of Chinese herbal medicines in treating allergic asthma. Ann Allergy Asthma Immunol. 2004;93(suppl 1):S35–S44. doi: 10.1016/s1081-1206(10)61485-8. [DOI] [PubMed] [Google Scholar]
- 11.Li XM. Traditional Chinese herbal remedies for asthma and food allergy. J Allergy Clin Immunol. 2007;120:25–31. doi: 10.1016/j.jaci.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen MC. Wei CH. Hu ZQ, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116:517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration (FDA), and Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. Rockville, MD: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 14.Bensky D. Gamble A. Chinese Herbal Medicine: Materia Medica. Seattle: Eastland Press; 1993. [Google Scholar]
- 15.The State Pharmacopoeia Commission of The People's Republic of China. Pharmacopoeia of the People's Republic of China Beijing. People's Medical Publishing House; 2005. [Google Scholar]
- 16.Raman P. Patino LC. Nair MG. Evaluation of metal and microbial contamination in botanical supplements. J Agric Food Chem. 2004;52:7822–7827. doi: 10.1021/jf049150+. [DOI] [PubMed] [Google Scholar]
- 17.Dolan SP. Nortrup DA. Bolger PM. Capar SG. Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. J Agric Food Chem. 2003;51:1307–1312. doi: 10.1021/jf026055x. [DOI] [PubMed] [Google Scholar]
- 18.Caldas ED. Machado LL. Cadmium, mercury and lead in medicinal herbs in Brazil. Food Chem Toxicol. 2004;42:599–603. doi: 10.1016/j.fct.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Franklin HR. Simonetti GP. Dubbelman AC, et al. Toxicity grading systems: A comparison between the WHO scoring system and the common toxicity criteria when used for nausea and vomiting. Ann Oncol. 1994;5:113–117. doi: 10.1093/oxfordjournals.annonc.a058760. [DOI] [PubMed] [Google Scholar]
- 20.Aickin M. The importance of early phase research. J Altern Complement Med. 2007;13:447–450. doi: 10.1089/acm.2007.6370. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GE. Early phase research and the process of scientific discovery. J Altern Complement Med. 2007;13:399. doi: 10.1089/acm.2007.7016. [DOI] [PubMed] [Google Scholar]
- 22.Fonnebo V. Early phase methodology is needed in CAM and conventional research endeavors. J Altern Complement Med. 2007;13:397–398. doi: 10.1089/acm.2007.7051. [DOI] [PubMed] [Google Scholar]
- 23.Mahony J. Chong S. Merante F, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol. 2007;45:2965–2970. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yurkovetsky ZR. Kirkwood JM. Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 25.Dehqanzada ZA. Storrer CE. Hueman MT, et al. Assessing serum cytokine profiles in breast cancer patients receiving a HER2/neu vaccine using luminex technology. Oncol Rep. 2007;17:687–694. [PubMed] [Google Scholar]
- 26.Termanini SM. Potent and persistent effects of an antiasthma herbal formula (ASHMI) on severe allergic airway responses in chronic asthma model [abstr] J Allergy Clin Immunol. 2006;117:S276. [Google Scholar]