Abstract
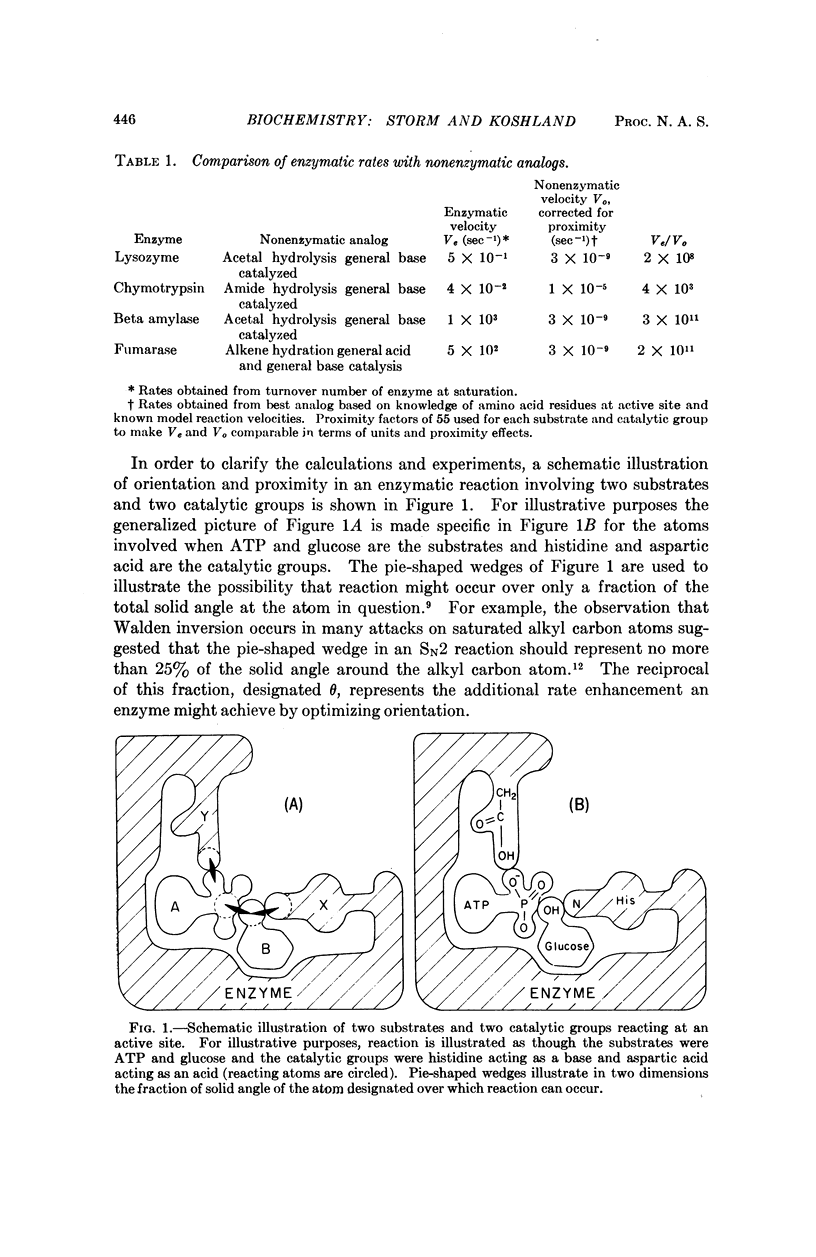
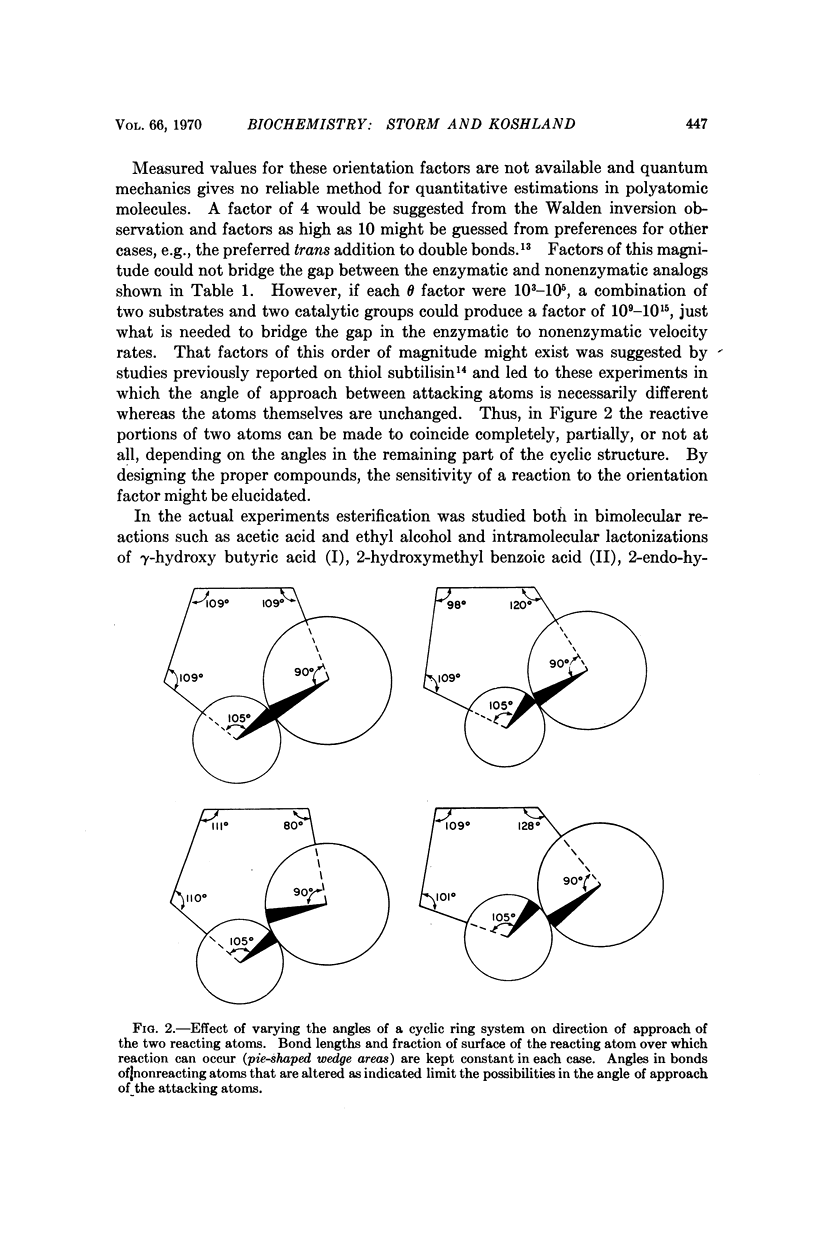
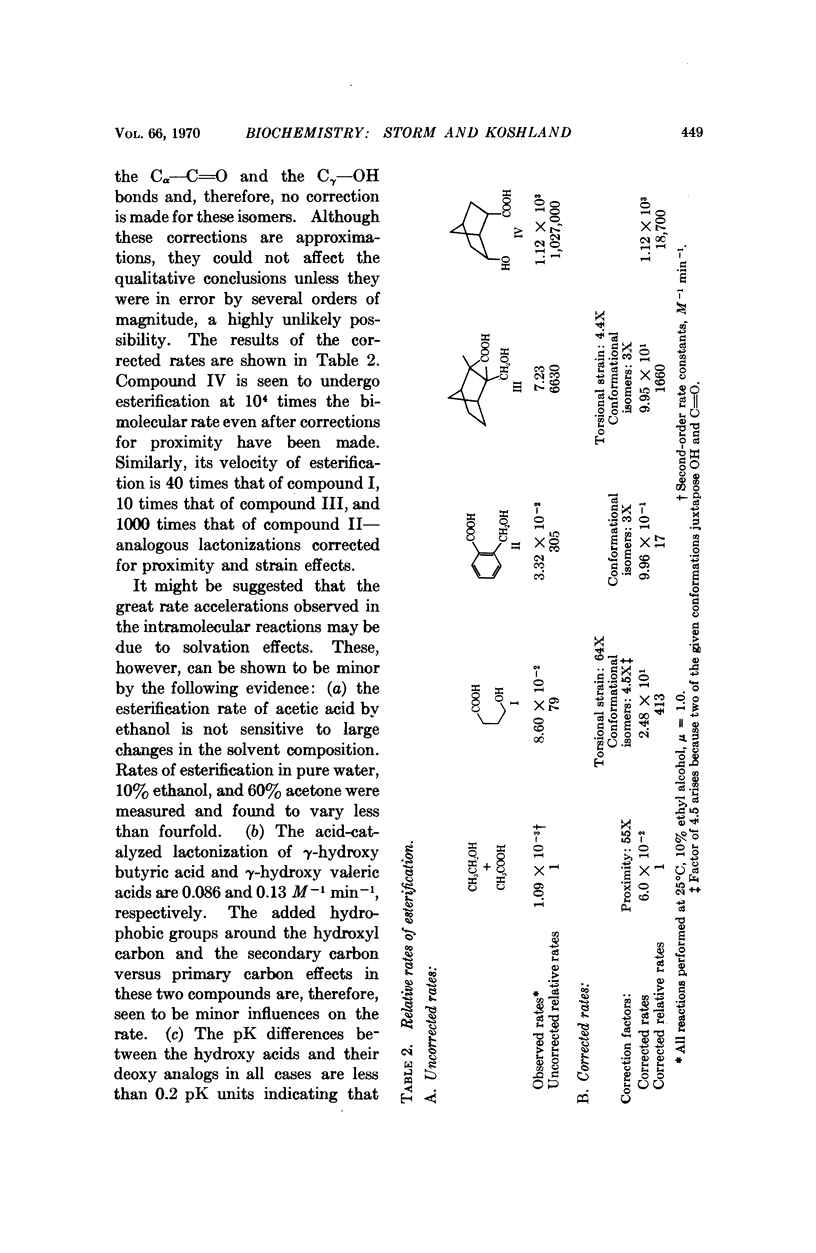
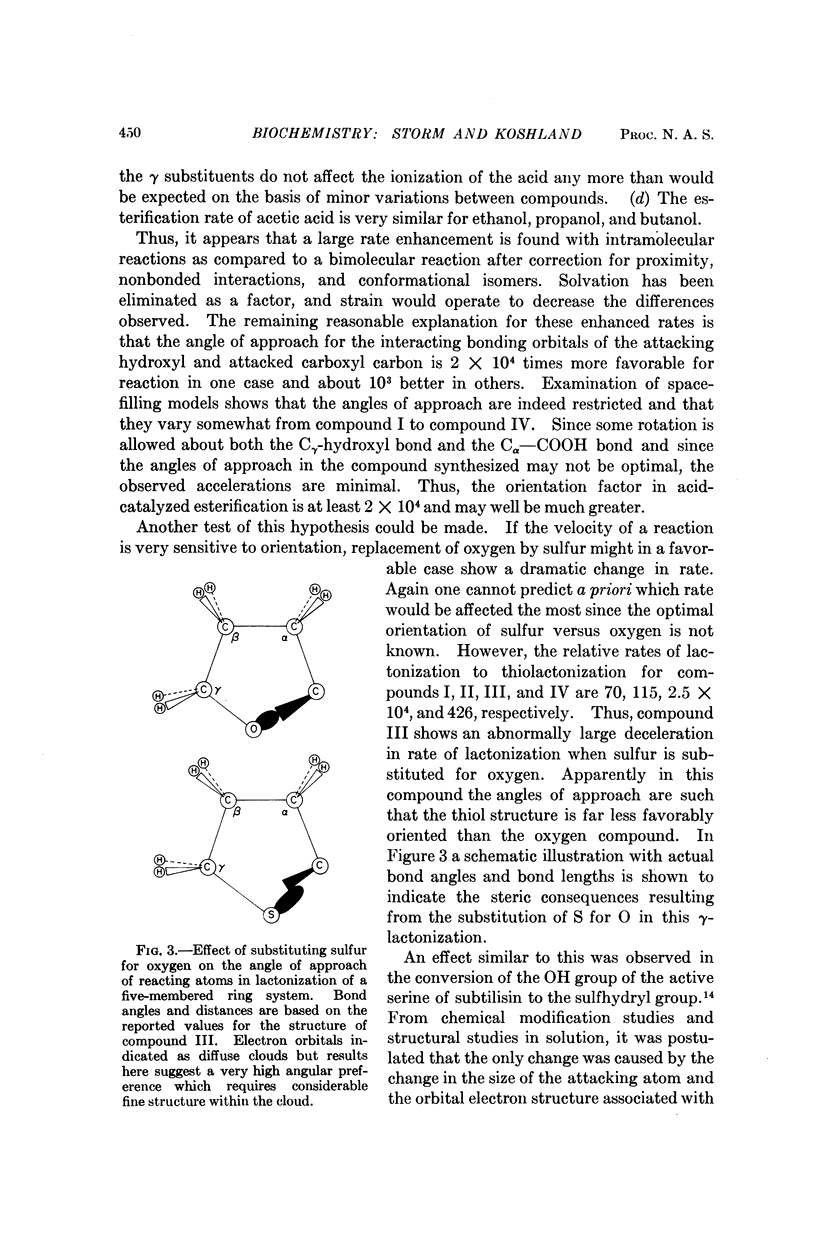
The velocities of acid catalyzed esterification and γ-lactonizations were studied to test the sensitivity of a chemical reaction to the orientation of the reacting atoms. Variation in orientation of the attacking oxygen atom relative to the carbon atom of the carboxylic acid was achieved by using bicyclic ring systems to limit the conformational mobility of the γ-hydroxy acids. Factors as high as 2 × 104 were observed for the acceleration of a reaction due to this „orientation factor” even after corrections for proximity and torsional strain have been made. The orientation factor is related to the shape of the electron orbitals and must have an angular preference far greater than previously estimated. Such sensitivity to orientation would provide factors large enough to explain the gap in our understanding of enzyme catalysis. It is suggested that the catalytic efficiency of enzymes depends on their ability not only to juxtapose the reacting atoms but also to „steer” their orbitals along a path which takes advantage of this strong directional preference.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREWSTER P., HUGHES E. D., INGOLD C. K., RAO P. A. D. S. Configuration of carbohydrates, hydroxy-acids and amino-acids; stereochemical standards of configuration. Nature. 1950 Jul 29;166(4213):178–179. doi: 10.1038/166178a0. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Nanci A., Koshland D. E., Jr Properties of thiol-subtilisin. The consequences of converting the active serine residue to cysteine in a serine protease. J Biol Chem. 1968 Dec 25;243(24):6392–6401. [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the properties of active sites of enzymes. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]



