Abstract
Infectious agents have long been implicated in the pathogenesis of systemic lupus erythematosus. Common viruses, such as the Epstein-Barr virus, transfusion transmitted virus, parvovirus and cytomegalovirus, have an increased prevalence in patients with systemic lupus erythematosus. They may contribute to disease pathogenesis through triggering autoimmunity via structural or functional molecular mimicry, encoding proteins that induce cross-reactive immune responses to self antigens or modulate antigen processing, activation, or apoptosis of B and T cells, macrophages or dendritic cells. Alternatively, some infectious agents, such as malaria, Toxoplasma gondii and Helicobacter pylori, may have a protective effect. Vaccinations may play dual roles by protecting against friend and foe alike.
Keywords: autoimmunity, infections, protective, systemic lupus erythematosus, vaccination
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease and the mechanisms of the aberrant immune responses remain unclear. Several environmental factors have been implicated in the etiology of SLE. There is a concordance rate of 25% in monozygotic twins for SLE, which indicates a discordance rate of 70% contributed to by environmental factors [1].
The possibility of a viral etiology was raised by the finding of virion-like tubuloreticular structures in endothelial cells and lymphocytes, and demonstration of increased concentration of type 1 interferon (IFN) in lupus patients [1]. Many viruses have been implicated in the etiology of SLE, which includes the Epstein–Barr virus (EBV), transfusion-transmitted virus (tissue transplant virus or Torque tenovirus), retroviruses, paramyxovirus, cytomegalovirus (CMV), parvovirus B19 and corona virus (Table 1). EBV, retroviruses and parvovirus B19 may play a role in the pathogenesis of SLE, when compared with other viruses such as CMV, transfusion transmitted virus, type C oncorna virus and measles virus which play a minor role. Immunodeficiency, such as C4 or C1q deficiency, may predispose to both lupus and infection without the two being directly linked. Infectious agents may induce autoimmune disease by several mechanisms.
Table 1.
Potential role of viruses in the pathogenesis of systemic lupus erythematosus.
| Virus |
Evidence |
Potential mechanism |
Ref. |
|---|---|---|---|
| Herpes viruses | |||
| EBV |
Increased prevalence; Increased viral load |
Molecular mimicry; Induction of lupus by EBV peptide; Bcl2-like protein – prevention of apoptosis |
[1,16,17,33] [28,29,38–40] [5] |
| Cytomegalovirus |
Increased prevalence |
Induction of lupus |
[14,20,21] |
| Parvovirus B19 |
– |
Molecular mimicry |
[19] |
| Transfusion-transmitted virus | Increased prevalence | Molecular mimicry | [16] |
| Retroviruses | |||
| HIV-1 |
– |
Molecular mimicry |
[44] |
| HTLV-1 | – | Regulation of CD4+ expression | [4,49,52] |
| RNA viruses | |||
| Type C oncorna virus |
Enhanced viral expression |
– |
[54,55] |
| Measles virus | Increased antibodies | Molecular mimicry | [56,57] |
EBV: Epstein–Barr virus; HTLV: Human T-lymphotropic virus.
Structural & functional molecular mimicry
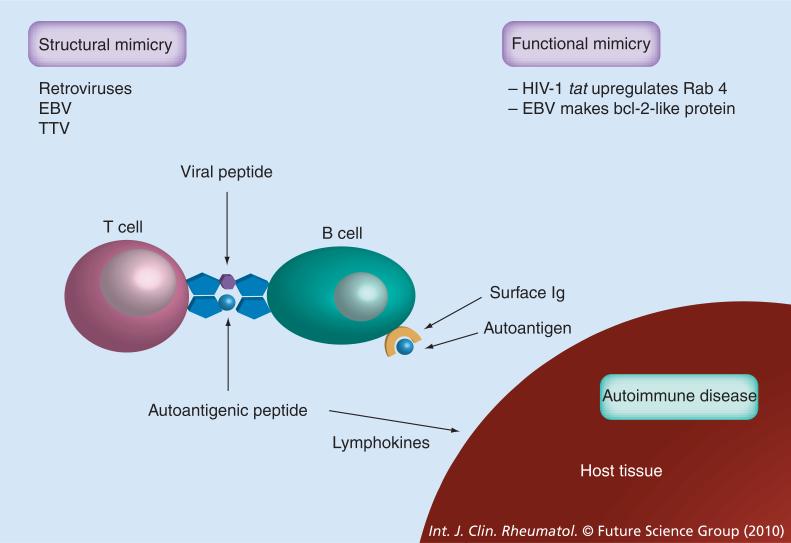
Molecular mimicry may be structural or functional. Structural molecular mimicry occurs when a viral peptide has an amino acid sequence similar or identical to an amino acid sequence of a self peptide, resulting in cross-reactive T-cell and B-cell responses. A potentially autoreactive T cell, possessing T-cell receptors that recognize both a foreign (viral) peptide and a self-peptide, is activated by a virus-derived peptide. Thus, in addition to mediating an antiviral response, the T cell is also capable of mediating self-directed responses.
Peptide sequences common to a virus (e.g., EBV protein) and a target lupus autoantigen (e.g., Smith antigen [Sm]) can also induce cross-reactive autoantibodies [2,3]. There is a wide range of structural, functional and immunological similarities between HIV-1 gp120 envelope protein and host proteins. HIV-1 tat gene upregulates Rab4, producing functional mimicry. HIV-1 tat gene stimulates transcriptional activity of the HRES-1/Rab4 promoter via transactivation of the HRES-1 long terminal repeat. There is coordinated upregulation of HRES-1/Rab4 and downregulation of CD4+ expression in HIV-infected peripheral blood mononuclear cells and CD4+ T cells. Enhanced expression of HRES-1/Rab4 may contribute to downregulation of CD4+ recycling to the cell surface, thus preventing infection by HIV-1 and protecting virus-infected cells against death by cytotoxic T cells. [4]. EBV protein regulates apoptosis by encoding a bcl-2-like protein (BHRF1-Bam HI fragment H rightward open reading frame 1), which is also an example of functional mimicry (Figure 1) [5].
Figure 1. Molecular mimicry in virus induced autoimmunity.
Viruses can influence adaptive immunity through molecular mimicry (i.e., homology between exogenous and endogenous epitopes). Peptide sequences common to a virus (e.g., EBV protein) and a target lupus autoantigen (e.g., Sm) can induce autoantibodies [2,3]. HIV tat upregulates Rab4 producing functional mimicry [4]. EBV protein regulates apoptosis by making a bcl-2-like protein, which is also an example of functional mimicry.
EBV: Epstein–Barr virus; TTV: Transfusion-transmitted virus.
Stimulation of pathogen recognition receptors
Exogenous stimuli are products of bacteria and viruses and have been termed pathogen- associated molecular patterns (PAMPs) [6]. Dendritic cells recognize PAMPs using pathogen recognition receptors, such as Toll-like receptors (TLRs). Necrotic debris from the cell death pathways, bacterial lipopolysaccharide, viral RNA and viral DNA act on TLRs [7]. A subset of dendritic cells, plasmacytoid dendritic cells (pDCs), is the body's major producer of type 1 IFNs. IFNs are important in host defense against viruses and there is overproduction of IFNs in SLE [8]. TLRs activate pDCs leading to the release of IFN-α/β. This in turn leads to the release of proinflammatory cytokines resulting in autoimmunity. IFN-α results in the maturation of antigen presenting cells and augmented T-cell activation including excessive helper activity. Natural killer cells produce significant amounts of cytokines, for example IFN-γ, that can influence the development of T cells [7].
Viruses may affect innate immunity by the release of proinflammatory cytokines. Proteins of commonly occurring viruses could produce profound effects on the cytokine milieu, antigen recognition and lymphocyte cell survival. PAMPs play a fundamental role in the early recognition of several infectious agents, such as Gram-positive and Gram-negative bacteria and RNA and DNA viruses. These molecules include bacterial cell surface lipopolysaccharides, lipoproteins, proteins such as flagellin from bacterial flagella, viral dsRNA, the unmethylated CpG islands of bacterial and viral DNA, and are sensed by a type of TLR pattern recognition receptor (Figure 2) [9].
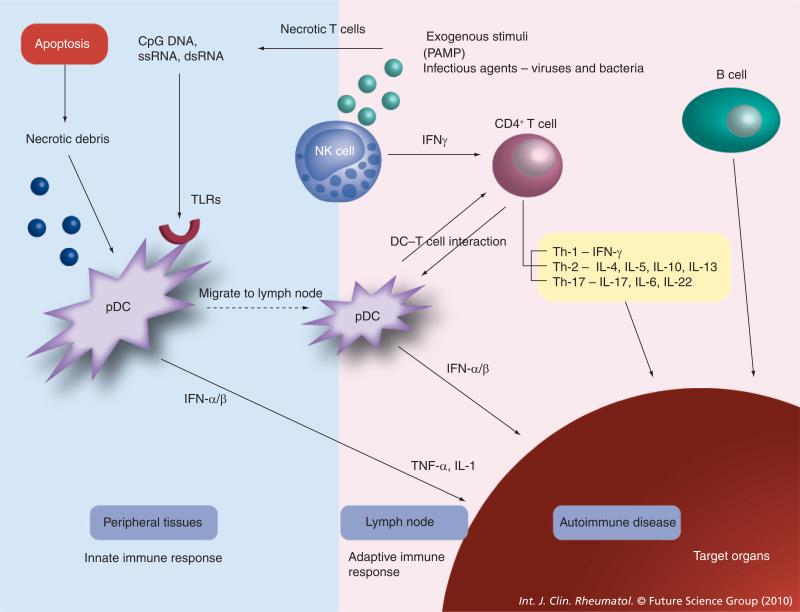
Figure 2. Role of interferons in virus-induced autoimmune disease.
Cells recognize PAMPs using pathogen recognition receptors such as TLRs. Necrotic debris from the apoptotic pathways, bacterial lipopolysaccharide, viral RNA and viral DNA act on TLRs. Plasmacytoid dendritic cells produces type 1 IFNs, which are important in host defense against viruses, and there is overproduction of IFNs in systemic lupus erythematosus. TLRs activate plasmacytoid dendritic cells leading to the release of IFN-α/β. IFN-α results in the maturation of antigen presenting cells and augmented T-cell activation, including excessive helper activity. Natural killer cells produce significant amounts of cytokines, for example IFN-γ, that can influence the development of T cells [7].
IFN: Interferon; PAMP: Pathogen-associated molecular patterns; TLR: Toll-like receptor.
There are at least 11 TLRs recognized in humans. TLR7 recognizes ssRNA and TLR9 binds dsDNA and CpG motif of bacterial DNA. TLR2 serves as a receptor for peptidoglycan and bacterial lipoproteins, TLR4 for Gram-negative lipopolysaccharide and TLR5 for flagellin. TLRs that bind to DNA and RNA lead to the production of large amounts of IFN-α, mostly derived from plasmacytoid dendritic cells [9]. In animal models of lupus nephritis, some TLRs (TLR3 and TLR9) are specifically immunolocalized in the kidneys, suggesting a possible pathogenetic role in the manifestation of the disease [10].
Microbial stimulation of TLRs leads to the initiation of the IL-1 signaling loop via the activation of IL-1 receptor-associated kinase (IRAK)1, IRAK4, TNF receptor-associated factor 6 (TRAF6), NF-κB kinase (IKK) complex and NF-κB activation. IL-1β activates its own receptor, which also signals via the same TLR pathway components to produce IL-1β [11]. Molecular mimicry and immunomodulation by viral proteins may account for both cross- reactivity with autoantigens and abnormal T- and B-cell functions in autoimmune disorders [1]. Necrotic and late apoptotic cells release material, which, when combined with immunoglobulins from SLE patients, induces the production of IFN-α from pDCs [12].
Infections can inhibit the development of autoimmune disease by several mechanisms. Some infections elicit responses to decrease the host response and thereby limit target tissue injury. The interactions of molecules derived from bacteria, fungi and parasites with TLRs and C-type lectin receptors on cells of the innate and adaptive immune system enable them to modulate the host immune responses [13]. Animal studies have demonstrated that exposure to infectious agents, or products derived from them, can inhibit the onset of autoimmune disorders through a variety of mechanisms, which includes reinforcement of regulatory networks and influencing trafficking of autoreactive T cells to sites of inflammation [13].
Meticulous exclusion of infections is essential in patients with SLE since infections may masquerade as flare-up of the disease, and immunosuppressive medications used to treat exacerbations of SLE may result in catastrophic consequences for the patient [14]. Patients with SLE appear to have an increased risk for infection due to dysregulation of the immune system. The usage of immunosuppressants, especially steroids and cyclophosphamide, are the strongest risk factors for infections in patients with SLE [15]. IFN-α is increased during a flare-up of SLE, which may reflect a viral infection or viral reactivation [8]. EBV and transfusion-transmitted virus (TTV) have an increased prevalence in SLE patients [16,17]. In SLE patients, sometimes it may be difficult to distinguish between a flare and superimposed infection. Further complicating matters, superimposed infections can lead to a flare in SLE. Parvovirus B19 [18,19] and CMV [14,20,21] have been reported to cause both induction of SLE and flare. Infections such as parvovirus B19 can mimic the symptoms of SLE [18,19].
Bacterial infections are the most common cause of infection in SLE and accounts for almost 80% of infections [22]. Most commonly identified bacteria include Staphylococcus aureus, Streptococus pyogenes, Streptococcus pneumoniae and Escherichia coli, which account for more than 50% of infections. Less commonly reported bacteria include Enterococcus, Klebsiella, Pseudomonas, Salmonella, Listeria, Mycobacterium tuberculosis and Nocardia [22]. Under these critical circumstances, it is vitally important to distinguish between lupus flare and infection. Elevated dsDNA and low C3 and C4 point towards a flare-up of lupus, whereas leukocytosis and a positive culture indicates infection requiring treatment with antibiotics.
Epstein–Barr virus
Epstein–Barr virus is a ubiquitous human DNA virus that infects B cells and causes their polyclonal activation and produces polyclonal antibodies. Polyclonal B-cell activation may be an early step in the pathogenesis of SLE. Serologic association, cross-reactivity of select EBV-specific antibodies with SLE autoantigens, SLE-like autoimmunity after immunization with EBV peptides, increased EB viral load in SLE patients and SLE-specific alterations in EBV humoral and cellular immunity implicate EBV in the development of SLE [23–25].
Increased prevalence of EBV infection in SLE patients
Interestingly, prevalence of EBV infection was reported to be as high as 99% in young SLE patients compared with 70% prevalence in controls [16,17]. Similar results were reported from a large study in adult patients. This study demonstrated that 99.5% of adults with SLE had seroconverted to EBV, compared with 95% of normal subjects, giving an odds ratio of 9.35 (p = 0.014) [26].
Peripheral blood mononuclear cells from SLE patients showed greater expression of both latent and lytic genes after infection, suggesting that EBV may participate in the etiology of SLE through several different mechanisms. Such altered infection patterns may contribute to the increased levels of EBV and the molecular mimicry seen in sera from SLE patients [27].
Increased viral load of EBV in patients with SLE
Several studies have demonstrated that EBV load is 10–15-fold higher in the peripheral blood of SLE patients compared with controls [28,29]. Real-time quantitative PCR showed a significant increase in the amount of EBV DNA in peripheral blood mononuclear cells from lupus patients compared with controls [30]. Studies have also demonstrated that the abnormally high frequency of EBV-infected cells in patients with SLE is associated with the occurrence of SLE disease flares [29]. Aberrant expression of viral lytic (BZLF1) and latency (latency membrane proteins 1 and 2a) genes was also detected in the blood of SLE patients. The abnormal regulation of EBV infection in SLE patients reflects the sensitivity of the virus to perturbation of the immune system [29].
Molecular mimicry between EBV & lupus autoantigens
Infection with EBV results in the production of the viral protein EBV nuclear antigen (EBNA)-1, antibodies against which cross-react with lupus-associated autoantigens, including Ro, Sm B/B′ and SmD1, in lupus patients (Table 2) [31,32]. A region of considerable homology, comprised of 11 highly charged residues (GRGRGRGRGRGRG), was identified as a site of cross-reactivity between the D component of Sm and EBNA-1 (Table 3) [1,33]. Antibodies directed against SmD1 are capable of binding the EBNA-1 peptide. Immunization of animals with the 35–38 EBNA-1 peptide induces the production of antibodies that can react with EBNA-1 and Sm D1 95–119.
Table 2.
Cross-reactivity of viral antigens with self antigens.
| Virus | Viral protein | Autoantigen |
|---|---|---|
| Epstein–Barr virus |
EBNA-1 |
Sm B/B′, Sm D1, Ro |
| |
EBNA-2 |
Sm B/B′, Sm D |
| Retrovirus |
gagp24 |
Sm B/B′, HRES-1/p28 |
| HRES-1 endogenous retrovirus |
p28 |
U1 70K |
| Cytomegalovirus | gB | U1 70K |
EBNA: Epstein–Barr virus nuclear antigen.
Table 3.
Molecular mimicry between lupus-associated autoantigens and viral antigens.
| Viral/auto antigen |
Amino acid sequence |
|
|---|---|---|
| EBV | ||
| Sm B/B′ | PPPGMRPP | |
| EBNA-1 |
PPPGRRP |
|
| Sm D1 (95–119) | RRPGGRGRGRGR | GRGRGRGRGRGA |
| EBNA-1 (35-58) | GPAGPRGGGRGRGR | GRGRGHNDGG |
| TTV (homologies between HRES-1 p28 epitopes and viral sequences) | ||
| HRES1 | PRHRHPQDPRSPGPA | |
| TTVORF2A |
AVLRAPQNPPPPGPQ |
|
| HRES1 | PRHRHPQDPRSPGPA | |
| TTVORF2b |
PRSRHPGGPGTPQIR |
|
| HRES1 | ATARRKRRWATRGPA | |
| TTVORF1a |
APWRRRRRWRTRRTR |
|
| HRES1 | A T ARRKRRWATRGPA | |
| TTVORF1b | WWARRRRRWRRWKRR | |
| CMV | ||
| HRES-1 | PRHRHPQDPRSPGPA | |
| Human herpes virus 5 | ||
| (CMV J1S) | VASRPL F P PRSPGPS | |
Position of identical residues (|) and position of functionally similar amino acids (+) are indicated [52].
CMV: Cytomegalovirus; EBNA-1: Epstein–Barr virus nuclear antigen-1; TTV: Transfusion-transmitted virus.
In patients where anti-Ro antibodies develop, the first sequence recognized usually contains the amino acids 169–180 (TKYKQRRNGWSHK). This sequence is cross-reactive with a sequence on EBNA-1 that is bound by antibodies from lupus patients [34]. Studies carried out in pediatric SLE patients have demonstrated that pediatric SLE patients and matched normal individuals make distinct antibody responses against EBNA-1, and that anti-EBNA-1 antibodies are associated with SLE.
Autoantibodies directed against the spliceosomal proteins, anti-Sm and antinuclear RNP are found in 30–50% of lupus patients’ sera. The possible molecular mimicry of the EBV peptide PPPGRRP by the peptide PPPGMRPP from Sm B′/B of the human spliceosome is consistent with the possibility that EBV infection is related to the origin of SLE in some patients [35–37].
Induction of lupus with EBV peptide
Autoantibodies from many patients with SLE bind the Sm autoantigen B/B′ polypeptide. The binding of serial serum specimens to the 233 overlapping octapeptides of Sm B/B′ has demonstrated that of the B/B′-derived octapeptides, PPPGMRPP and PPPGIRGP, are early targets of the autoimmune response in some lupus patients. Studies have demonstrated that rabbits immunized with PPPGMRPP and PPPGIRGP develop antibodies that not only bind these octapeptides, but also subsequently bind many other octapeptides of Sm B/B′. These peptide-immunized animals developed features of SLE, such as antinuclear antibodies, anti-Sm antibodies, antidouble-stranded DNA, thrombocytopenia, seizures and proteinuria. The immunogen used in these experiments, PPPGMRPP, was most similar to PPPGRRP from EBNA-1 of EBV, which is also bound by human sera containing anti-Sm autoantibodies [38–40].
Prevention of apoptosis
The persistence of EBV infection in patients with SLE may be related to the antiapoptotic potential of B cells due to their infection with EBV. During primary infection, autoreactive B cells are infected by EBV; they in turn proliferate and become latently infected memory B cells that are resistant to apoptosis that occurs during normal B-cell homeostasis because they express virus-encoded antiapoptotic molecules [14].
Cytomegalovirus
There have been several case reports of lupus associated with human CMV (HCMV) infection. In these cases, active infection with CMV determined by either the presence of anti-HCMV IgM or viral DNA has been detected at the time of flare-up of symptoms of lupus, implicating HCMV as a possible etiologic agent in lupus [20,21]. The development of SLE may be triggered by a CMV infection. Existing SLE may undergo an exacerbation following a CMV infection [14].
Parvovirus B19
There are striking similarities between the clinical features and hematological findings of SLE and those of parvovirus B19 infection, including anemia, thrombocytopenia and arthritis. There have been several case reports of parvovirus B19 infection causing a flare-up of SLE [19]. Parvovirus B19 may be accompanied by a transient subclinical state of autoimmunity and may mimic or exacerbate SLE [18]. Elevation of rheumatoid factor, antiphospholipid, antilymphocyte and antinuclear antibodies are frequently observed during the late phase of B19 infection occurring after the peak of viremia. The concentration of these antibodies usually decline rapidly within a few days, but, occasionally, the autoimmune response persists and may be associated with manifestations, such as arthritis, vasculitis and fibromyalgia [41].
Parvovirus B19 may have the property of inducing production of autoimmune antibodies by mimicking autoantigens [19]. This, in turn, may contribute to or exacerbate the symptoms in SLE.
It depletes erythroid progenitor cells by apoptosis. Chronic parvovirus B19 infection is associated with production of a wide array of autoantibodies [1,42].
Transfusion-transmitted virus
Transfusion-transmitted virus is a recently discovered virus of extremely high genetic diversity that commonly infects humans. SLE patients produce autoantibodies to HRES-1/p28, a human endogenous retrovirus encoded nuclear protein [16,43]. There is possible molecular mimicry between TTV and HRES-1/p28. The highest prevalence of cross-reactivity has been shown between HRES-1/p28 residues 41–55 and 156–170 and TTV peptide ORF2a. Prevalence of TTV was noted to be increased in SLE patients with respect to healthy controls [16,43].
Retroviruses
Exogenous retroviruses Dysregulation of apoptosis
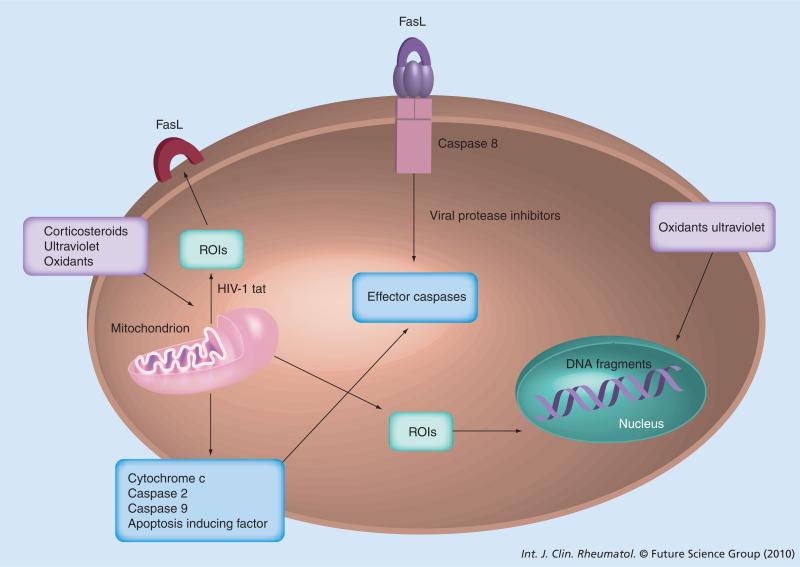
Dysregulation of apoptosis can occur both in HIV and SLE patients. HIV infection causes a shift from a T helper (Th)1 to Th2-type cytokine profile, which is similar to those found in patients with SLE [44–46]. The nef and tat genes of HIV-1 are thought to mediate a Th1 to Th2 shift in cytokine production. CD4+ T-cell decline is mediated by an increased rate of apoptosis or programmed cell death. Th1 cytokines protect against apoptosis, whereas Th2 cells increase the rate of apoptosis. The shift of Th1 to Th2 cells causes the accelerated apoptosis in SLE patients. Anemia, leucopenia, thrombocytopenia, polymyositis and vasculitis can occur in both SLE and HIV patients. These observations suggest that there is a common mechanism mediating the increased apoptosis and autoantibody production in SLE and AIDS [44–46]. This may indicate that SLE is probably caused by a virus that has not yet been identified. In turn, SLE, which is thought to be mediated by CD4+ T cells, remits in some patients after infection with HIV-1 [1,47]. The tat protein of HIV induces oxidative stress and increases surface expression of the Fas ligand, resulting in accelerated signaling through the Fas pathway (Figure 3). In addition, cleavage of bcl-2 by HIV protease may expose the cell to a variety of apoptotic signals (Figure 4) [1,48].
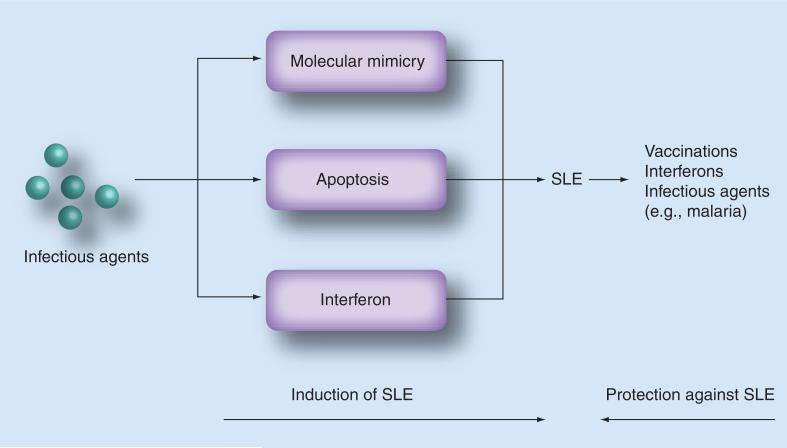
Figure 3. Mechanisms by which infectious agents induce or protect against systemic lupus erythematosus.
Infectious agents lead to the pathogenesis of SLE through several mechanisms, including molecular mimicry, apoptosis (programmed cell death) and IFN-α. On the other hand, some infections, such as malaria, can act as protective agents. Vaccinations may act as protective agents by preventing some of the infections that can induce SLE. IFN-β and IFN-γ may also have a protective effect.
SLE: Systemic lupus erythematosus.
Figure 4. Regulation of apoptosis pathways by viral proteins.
Oxidants, ultraviolet light and corticosteroids trigger apoptosis by damage of mitochondria, which in turn leads to caspase activating factors. This process is inhibited by viral bcl-2 and its homologs. Release of ROIs causes increased production of Fas ligand and DNA fragmentation. HIV-1 tat increases mitochondrial ROI production, thereby increasing apoptosis. The tat protein induces oxidative stress and increases surface expression of Fas ligand resulting in accelerated signaling through the Fas pathway.
ROI: Reactive oxygen intermediate.
Endogenous retroviruses Genetic factors
Endogenous retroviral sequences represent a link between viral and genetic factors that may influence the development of SLE [49]. HRES-1 is an endogenous retroviral element encoding a 28 kDa nuclear autoantigen, HRES-1/p28, which is expressed in a tissue-specific manner. Antibodies to HRES-1/p28 were detected in 21–50% of patients with SLE and overlap syndromes in several laboratories compared with normal donors or HIV-infected patients [50].
HRES-1 is represented as a single copy element per haploid genome that has been mapped to a common fragile site of chromosome 1 at q42 [51]. The 1q42 region is one of at least ten chromosomal loci shown in independent studies to be linked to the development of SLE [49]. The presence or absence of a polymorphic HindIII site defines two different allelic forms of the HRES-1 genomic locus. In comparison with normal blood donors, a differential segregation of polymorphic genotypes of the HRES-1 locus (i.e., a relative decrease of genotype I and increase of genotype III) were noted among patients with SLE. The q41→q42 region of chromosome 1 was found to contain susceptibility genes that confer risk for SLE in multiple ethnic groups, further supporting the notion that HRES-1 or another gene closely linked to HRES-1 may influence susceptibility to SLE [50].
Molecular mimicry between endogenous retroviruses & lupus autoantigens
The 70 kD protein of U1snRNP was the first lupus autoantigen shown to contain a region of homology and immunologic cross-reactivity with a p30 gag protein of most mammalian type C retroviruses. A mimicking epitope between another lupus autoantigen, Sm and HIV-1 p24 gag was defined based on cross-reactivity with monoclonal antibody 4B4. A proline-rich domain present in both the B/B′ subunit of Sm and HIV p24 gag was suggested to be the core of cross-reactive epitopes. Antibodies binding to HIV-1 p24 gag were found in 22 out of 61 patients with SLE [44].
HRES-1/p28 shows similarity to TTV proteins. Lupus sera had strong binding affinity to a peptide showing similarity to the retroviral gag-like region of 70 kD U1 snRNP lupus autoantigen, suggesting that cross-reactivity of HRES-1 p/28 with TTV and 70 kD U1 snRNP may lead to epitope spreading and contribute to generation of antinuclear antibodies.
Regulation of CD4+ expression
HRES1/Rab4 belongs to the family of small GTPases that regulate receptor endosome recycling. Regulation of HRES-1/Rab4 expression may play an active role in the lifecycle of HIV-1. This is supported by the fact that there is coordinated upregulation of HRES-1/Rab4 and down-regulation of CD4+ expression in HIV-infected CD4+ T cells and PBMC. HRES-1/Rab4 promoter activities and protein levels are increased in cells infected by HIV-1 or transfected by HIV-tat. In turn, enhanced expression of HRES-1/Rab4 may contribute to downregulation of CD4+ recycling to the cell surface, thus preventing reinfection by HIV-1, allowing for increased virion production and protecting virus-infected cells against killing by cytotoxic T cells. Thus, stimulation of HRES-1/Rab4 expression by HIV-1 and regulation of HIV coreceptor CD4+ recycling by HRES-1/Rab4 represent novel mechanisms of coordinate interaction between infectious viral particles and endogenous retroviruses of the human genome [4].
CD4+ expression is low in CD4+ lupus T cells, while HRES/Rab4 expression is high raising a possible role for a virus similar to HIV in SLE [52]. Alternatively, genetic factors may contribute to increased expression of HRES-1 in SLE [49].
Role of 3′ repair exonuclease 1 in autoimmunity
Detection of nucleic acids and induction of type 1 IFNs are principal elements of antiviral defence, but can result in autoimmunity if misregulated. Cytosolic DNA detection activates a potent, cell intrinsic antiviral response through a poorly defined pathway called the IFN-stimulatory DNA (ISD) pathway. Trex1 is an essential regulator of the ISD response and delineate the genetic pathway linking Trex1 deficiency to lethal autoimmunity [53].
Trex1 has been identified as an ISD inducible but negative regulator of the ISD response. Mutations in the human trex1 gene cause Aicardi-Goutieres syndrome and chilblain lupus. Stetson et al. have demonstrated that single-stranded DNA derived from endogenous retro elements accumulates in Trex1-deficient cells and that Trex1 can metabolize reverse-transcribed DNA. This is a new mechanism of autoimmunity where defective metabolism of intracellular nucleic acids triggers a cell-intrinsic autoimmune response and suggests the contribution of endogenous retro elements to autoimmunity.
RNA viruses
Type C oncorna virus
Type C oncorna viruses have been postulated to have an association with SLE. Lymphoblastoid cell lines derived from patients with active SLE by allowing spontaneous transformation of peripheral B lymphocytes (B cells) harboring endogenous EBV or by super infecting peripheral lymphocytes with exogenous EBV. Extensive studies were conducted searching for type C oncorna viruses using electron microscopy, DNA–DNA hybridization, reverse transcriptase assays and cocultivation experiments, which revealed negative results [54]. The results of experimental studies on the role of type C viruses in SLE were conflicting. There were several reports of increased viral expression, but the attempts at type C virus isolation from the tissues of SLE patients were unsuccessful [55].
Paramyxoviruses
Increased antibodies to measles and parainfluenza type 1 have been detected in patients with SLE. There are three possible explanations for this observation. First, they could reflect persistent viral infection. Second, the increased antibody levels might be a nonspecific result of immuno-logic hyper-reactivity. Finally, the increases in antibody may be due to antigens shared by host cell and virus (molecular mimicry) [56,57].
Vaccinations as a trigger for SLE
Vaccines have been associated with the onset or flare-up of SLE [58]. A total of five healthy patients who received immunizations by a combination of vaccines, including typhoid, influenza, meningococcal vaccines, tetanus toxoid, measles, mumps, rubella vaccine and anthrax vaccine, developed SLE 2–3 weeks after secondary immunization [58,59]. The trigger for the onset of SLE may be either the bacterial or viral components of the vaccine or the chemical components of the solvent (adjuvant).
Hepatitis B vaccine
There have been several case reports suggesting a temporal relation of SLE to hepatitis B vaccine, but a causal relationship has not yet been proven [58,60]. There are no studies on the safety and efficacy of HBV for patients with well-established lupus [61–64].
Influenza vaccine
Some reports suggest a link between influenza vaccination and lupus, but these are isolated and rare associations. The efficacy and safety of influenza vaccination in lupus patients have been proven by several studies [65–67].
Pneumococcal vaccine
In an Israeli cohort study, 24 patients with SLE who were vaccinated with pneumococcal vaccine showed no change in biological or clinical markers of disease activity 1 month after the vaccination [68,69]. A Hungarian cohort study of 18 SLE patients showed good tolerability of the pneumococcal vaccine [69,70].
Ethnicity
The prevalence and incidence of lupus is high in patients from certain ethnic groups. SLE is more common in those of Hispanic origin, those of African descent in North America, or with a Caribbean background in the UK, those of Asian descent (from India, Pakistan and China), as well as those from countries around the Pacific, including North American Indians, Australian Aborigines and New Zealand Maoris [71,72]. Hispanics, African–Americans, African–Caribbean, South Asians and Chinese tend to present with SLE at a younger age, have more renal involvement, earlier damage and often higher mortality than Caucasians [72].
Systemic lupus erythematosus is very rare in West Africa, more common in Central and Southern Africa and increases in frequency towards the west, including the Caribbean, Europe and America. One possible explanation is that malaria is very common in West Africa and the immune responses against malaria may act as a protective factor in the prevention of SLE. Most of the African–Americans in the USA originate from West Africa and the increased incidence of SLE in this group could be explained by the fact that they do not have the protective effect of malaria [73,74].
Genetic factors predisposing to infections in SLE
Recent studies have indicated that genetic factors may increase the risk of serious infections in SLE. Mannose-binding lectin (MBL) is one of the molecules in the lectin complement activation pathway that shares many features of C1q. Human MBL is derived from a single gene located on chromosome 10 (MBL2) [75]. Patients who had MBL deficiency associated with homozygous MBL variant alleles have been reported to be at increased risk of infections. Patients with SLE who were homozygous for MBL variant alleles had a fourfold increase in the incidence of infections [14,15]. It has been reported that low serum MBL predisposes Chinese SLE patients to infections, especially bacterial infections [76]. The presence of anti-MBL autoantibodies in sera of SLE patients can influence MBL plasma levels and its functional activity.
At least a third of the SLE patients with C1q deficiency also suffered from recurrent bacterial infections, including otitis media, meningitides and pneumonia [77]. An increased susceptibility to bacterial infections may be present in up to 30% of C2 deficient individuals, which occurs mainly in infancy and childhood [78].
IRF-5 is a transcription factor that is expressed in B cells and dendritic cells that regulates the expression of a variety of genes and leads to the induction of IFN and proinflammatory cytokines. In mice deficient in the IRF5 gene, the production of type 1 IFN against viral infections was decreased and there was impaired induction of proinflammatory cytokines by TLR stimulation [79,80]. In humans, overexpression of IRF5 stimulates the expression of type 1 IFN genes after viral infection, and knockdown of IRF5 by short interfering RNA reduces the induction of type 1 IFN in response to TLR7 ligand [81–83].
Variants of the gene encoding signal transducer and activator of transcription 4 (STAT4) have also been discovered to be risk factors for SLE. The identification of these risk genes supports the hypothesis that the type 1 IFN pathway plays a key role in the pathogenesis of SLE [84,85]. After 24 h infection with Pseudomonas aeruginosa, STAT4-deficient mice showed impaired production of the proinflammatory cytokines, TNF-α, IL-1β and macrophage inflammatory protein-2 [86]. Thus, STAT4 appears to contribute to Pseudomonas aeruginosa-induced inflammation, but it is not necessary for bacterial clearance [86].
Two new susceptibility loci for SLE have been identified: BLK-C8orf13 on chromosome 8 and ITGAM–ITGAX (genes encoding integrin-α M and integrin-α X) on chromosome 16 [84]. The genes most likely involved within these two loci are BLK (B lymphoid tyrosine kinase) and ITGAM (Table 4).
Table 4.
Genetic factors predisposing to infections in systemic lupus erythematosus.
| Gene | Chromosome | SNP | Infection | Ref. |
|---|---|---|---|---|
| Mannose-binding lectin deficiency |
10 |
Alleles B,C,D Codons 54,57,52 |
Staphylococcus aureus Pseudomonas aeruginosa Community-acquired pneumonia (Streptococcus pneumoniae, Haemophilus influenzae, Legionella, Mycoplasma pneumoniae) Invasive aspergillosis Influenza A and B Herpes simplex virus 1 and 2 |
[14,15,75,76] |
| C1q deficiency |
– |
rs631090 rs292001 rs294183 |
Recurrent bacterial infections Otitis media Meningitis Pneumonia |
[77] |
|
IRF5 |
7q32 |
rs2004640 |
Paramyxovirus (Newcastle disease virus) Other viral infections |
[79,80] |
|
STAT4 |
2 |
rs7574865 |
Pseudomonas |
[86] |
| ITGAM | 16 | rs1143679 | – | [84] |
Protective effect of infections in autoimmune diseases
The ‘hygiene hypothesis’ postulates that infection may protect from autoimmune diseases. Epidemiological evidence indicates that there is an alarming increase in autoimmune diseases over the last three decades in North America and Europe, which is thought to be linked to the improved socioeconomic status, better hygiene, and more specifically to the reduced microbial exposure of children, as a result of Westernized lifestyles [87–92].
However, the immunological changes induced by higher standards of hygiene during childhood are still under debate. The two possible explanations include: missing immune deviation from Th2 to Th1, caused by reduced production of Th1 polarizing cytokines by cells of the innate immunity in response to stimulation of their TLRs by microbial components; and reduced activation of T-regulatory cells caused by reduced stimulation of the immune system [92].
Studies have demonstrated that there is a north–south gradient for the distribution of autoimmune diseases – the incidence of autoimmune disease decreases from north to south in the northern hemisphere and reciprocally from south to north in the southern hemisphere. Some infections in European countries may be distributed according to a south–north gradient that is a mirror image of the gradient for autoimmune diseases [91].
It has been consistently observed that autoimmune diseases in susceptible strains of mice or rats develop earlier and at a higher rate among animals bred in a specific pathogen-free environment than among animals bred in a conventional environment. A similar effect of the use of pathogen-free conditions has been reported in rats with collagen-induced arthritis, which is an experimental model for rheumatoid arthritis (Figure 3).
Bacteria and viruses could protect against autoimmune diseases through their effect on TLRs. TLRs are receptors for various bacterial components – TLR2 serves as a receptor for peptidoglycan and bacterial lipoproteins, TLR4 for Gram-negative lipopolysaccharide, TLR5 for flagellin and TLR9 for CpG motif of bacterial DNA. When TLRs bind bacterial ligands they stimulate mononuclear cells to produce cytokines, which in turn could downregulate autoimmune responses [91].
Toxoplasma gondii
Animal studies have demonstrated that Toxoplasma gondii infection may prevent the development of a lupus-like syndrome in autoimmune New Zealand black and white (NZBW) F1 mice. The pathogenic isotypes (IgG2a and IgG3) of the anti-DNA antibodies in the serum of T. gondii-infected mice were significantly reduced [93,94].
The level of antiself heat-shock protein 70 (anti-HSP70) IgG autoantibody in the sera of NZBW F1 mice was significantly higher than that in control mice at 9 weeks after T. gondii infection. Furthermore, NZBW F1 mice treated with HSP70 monoclonal antibody were substantially protected against the onset of glomerulonephritis. In addition, downregulation of intracellular expression of IFN-γ and IL-10 was shown in spleen cells of T. gondii-infected NZBW F1 mice. These results indicate that T. gondii infection is capable of preventing the development of autoimmune renal disorder in NZBW F1 mice [94].
Helicobacter pylori
Helicobacter pylori infection may play a protective role against the development of SLE. Immunoregulatory events leading to H. pylori seropositivity correlate inversely with the risk of developing SLE. This has been suggested by the fact that there was an association between SLE and H.pylori seronegativity [95,96].
Malaria
As discussed previously, SLE is very rare in West Africa, more common in Central and Southern Africa and increases in frequency towards the west, including the Caribbean, Europe and America. One possible explanation is that malaria is very common in West Africa and acts as a protective factor in the development of SLE. Most of the African–Americans originate from West Africa and the increase in incidence in SLE in this population could also be explained by the fact that there has been considerable genetic admixture since their arrival in America.
There are several mechanisms that have been postulated by which malaria protects against the development of autoimmune disease such as SLE. Malaria is a potent inducer of tumor necrosis factor (TNF) and it is now known that the pathology in malaria results from the initial induction of this cytokine. Patients who are infected with Plasmodium falciparum and have high levels of TNF are at higher risk of developing the more severe form of the disease – cerebral malaria. TNF-induced activation of macrophages and neutrophils controls parasite replication in the early stages of malaria, but when there is uncontrolled overproduction of this cytokine it leads to severe disease. As a consequence to reduced ability to generate TNF in low endemic areas for malaria there is a greater risk of developing autoimmune disease, such as SLE. This is a likely explanation for the higher rate of SLE in African–Americans [74].
Another mechanism that has been described is a defunctioning, SLE-associated polymorphism of the inhibitory receptor FcγRIIb in African and Asian populations, which corresponds to the areas where malaria is endemic [97]. FcγRIIb-deficient mice have increased clearance of malarial parasites and develop less severe disease. FcγRIIb deficiency or dysfunction is associated with the development of autoimmunity, especially SLE, in mice and humans [97].
Protective effect of vaccinations
Vaccinations may have two roles in the development of autoimmune disease. They may induce autoimmunity by preventing the onset of infections, which may have a protective role in the development of autoimmune diseases. This is probably unlikely since there are very few infections implicated in the development of autoimmune diseases that are protected by vaccinations. The second postulation is that vaccination may contribute to the development of autoimmune disease by stimulation of the immune system by the repeated injections of the vaccine along with their adjuvant [98].
Bacillus Calmette Guerin (BCG) vaccine and Q fever vaccine have been evaluated in children for the prevention of diabetes in a population of children at risk of developing the disease. The difficulty encountered was that these vaccinations were not given at repeated intervals, and isolated immune stimulations were not sufficient to produce protective mechanisms, which were obtained in experimental models [98–103].
Protective effect of genetic factors in autoimmune disease
Susceptibility to SLE depends on cumulative interaction of many genetic loci containing polymorphic variants, and the likelihood of developing the disease is a function of the number of susceptibility genes present in the genome of the individual [104,105]. A locus on chromosome 1, Sle1, breaches immunological tolerance to chromatin, and Sle2, another locus on chromosome 4, triggers generalized B-cell hyperactivity [104]. Sle3, a locus on chromosome 7, is associated with a number of T-cell aberrations, which are mediated through myeloid cells. Genetic polymorphisms or deletions within CCR5 diminish or abrogate viral binding to the receptor, which leads to a lower susceptibility to infection and slower disease progression in persons carrying these mutations [4].
Mice with the Sle3 susceptibility locus have been demonstrated to have enhanced antibacterial responses, especially pneumonia and intra-abdominal sepsis, compared with wild-type mice. This was associated with markedly increased accumulation of neutrophils in infected tissues. Neutrophils from lupus-susceptible mice displayed markedly reduced rate of apoptosis, associated with altered expression of bcl-2 family proteins, leading to their greater accumulation. On the other hand, inhibition of apoptosis in wild-type mice increased the accumulation of neutrophils at the site of infection and resulted in an enhanced antibacterial response. These observations suggest that some of the genetic loci that mediate autoimmunity may also produce enhanced antibacterial immunity [104].
Role of interferon in the development of autoimmune disease
Viral infections induce the production of large amounts of IFN-α and -β, which in turn modulate immune responses. IFN-α produces a positive effect by stimulating Th1 cell mediated autoimmunity. However, IFN-β produces a negative response and its immunoregulatory properties have been utilized in the treatment of multiple sclerosis.
IFN-γ may also have a protective role in the development of autoimmune diseases, which has been demonstrated in some animal models of experimental allergic encephalomyelitis and to a lesser extent in human autoimmune diseases, such as rheumatoid arthritis [98].
Future perspective
In summary, infections play a major role in modulating the development of autoimmune diseases by performing a dual role of protection and also induction of the disease process. The underlying mechanisms are multiple and extremely complex and varies widely according to the specific pathogen involved [106].
The study of the role of infections in the development or protection of autoimmune diseases has been extremely difficult. There are several possible explanations for the difficulties in identifying infections as etiological agents in autoimmune diseases. One hypothesis is that there is a long lag time between the initial causal infection and the onset of clinical symptoms in SLE. The causative infection may resolve quickly and the serological evidence may have disappeared by the time of clinical onset of the autoimmune disease [106].
Evidence has emerged from studies of human SLE of a role of TLR7 and TLR9 in autoimmunity. TLR signaling in T cells leads to the production of Th1 cytokines and in B cells to cell proliferation, differentiation and immunoglobulin switching. The genetic deficiency of TLR-7 may confer protection from autoimmunity in murine SLE and the deletion of TLR9 may enhance disease activity [107]. Pharmacologic modulation of TLR signaling may offer new therapeutic targets for the treatment of SLE.
Several studies are in progress that may in the future identify viruses or bacteria as etiological agents in lupus. This will expand the therapeutic perspectives for SLE in the form of anti-infectious agents in the treatment of SLE and vaccinations, which could be used for the prevention of disease onset in SLE [106].
Executive summary.
Several viruses have been identified as possible etiological agents in systemic lupus erythematosus
■ The implicated viruses with a major role include Epstein–Barr virus, retroviruses and parvovirus B19, and those playing a less significant role include transfusion-transmitted virus, cytomegalovirus and paramyxovirus.
Infections may have a protective role in the development of autoimmune disease
■ Animal studies have suggested that Toxoplasma gondii may have a protective role in the development of autoimmune disease. In the serum of T. gondii-infected NZBW F1 mice, the pathogenic isotypes (IgG2a and IgG3) of the anti-DNA antibodies were significantly reduced. The level of antiself heat-shock protein 70 IgG autoantibody was significantly higher in the sera and there was downregulation of intracellular expression of IFN-γ and IL-10 in spleen cells of NZBW F1 mice infected with T. gondii. Helicobacter pylori may have a protective role in the development of autoimmune disease since seropositivity for H. pylori has been shown to decrease the risk for developing systemic lupus erythematosus (SLE). Epidemiological studies have suggested that infection with malaria can act as a protective factor in the development of SLE.
Vaccinations
■ Vaccinations may have a dual role in the development of autoimmune diseases, providing protection by preventing infections, but contributing to autoimmune disease by repeated injections of vaccine with their adjuvant.
■ There have been isolated case reports of flare-up of SLE secondary to the administration of Hepatitis B vaccine and influenza vaccine, but no change in serological markers with pneumococcal vaccine.
Genetics in autoimmune diseases
■ Sle1 on chromosome 1 and Sle 3/5 on chromosome 7 are perhaps the most critical lupus susceptibility loci identified in mouse models of lupus. Some of the genetic loci that mediate autoimmunity, such as Sle3, may also produce enhanced antibacterial immunity.
Interferons in autoimmune disease
■ Increased IFN-α production coincides with disease flare and may be proinflammatory, but IFN-β and IFN-γ have a protective effect.
■ The production of IFN-α may reflect viral infection or viral reactivation.
Conclusion
■ Infection can predispose to pathogenesis of SLE, can induce a flare and can also mimic a flare of SLE.
■ Meticulous exclusion of infections by improved detection of infectious agents is important in patients with SLE since they can masquerade as exacerbations of SLE.
■ The use of immunosuppressants in the presence of infection can lead to disastrous consequences.
■ Innate immunity-related receptors and signaling molecules could become therapeutic targets for treatment of SLE.
■ We need to identify an infection that can induce lupus in animals, which will prove infection to be an etiological factor in pathogenesis of SLE.
■ Future studies are required for evaluation of anti-infectious agents in treatment of SLE and vaccinations as a preventive measure for the onset of SLE.
Financial & competing interests disclosure
This work was supported in part by grants AI 048079, AI 072648, and AT004332 from the National Institutes of Health, the Alliance for Lupus Research, and the Central New York Community Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1 ■■.Perl A. Mechanisms of viral pathogenesis in rheumatic disease. Ann. Rheum. Dis. 1999;58:454–461. doi: 10.1136/ard.58.8.454. [Comprehensive review of viral pathogenesis in autoimmune diseases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 ■.Ronnblom L, Alm GV. A pivotal role for the natural interferon α producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001;94:59–63. doi: 10.1084/jem.194.12.f59. [Describes the important role of IFN-α-producing cells in the pathogenesis of systemic lupus erythematosus (SLE).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crow MK, Kirou KA. Interferon α in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2004;16:541–547. doi: 10.1097/01.bor.0000135453.70424.1b. [DOI] [PubMed] [Google Scholar]
- 4.Nagy G, Ward J, Perl A, et al. Regulation of CD4 expression via recycling by HRES- 1/Rab4 controls susceptibility to HIV infection. J. Biol. Chem. 2006;281(45):34574–34591. doi: 10.1074/jbc.M606301200. [DOI] [PubMed] [Google Scholar]
- 5.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl Acad. Sci. USA. 1993;90(18):8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisetsky DS. The role of innate immunity in the induction of autoimmunity. Autoimmun. Rev. 2008;8:69–72. doi: 10.1016/j.autrev.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25(7):360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengtsson AA, Sturfelt G, Truedsson L, et al. Activation of type 1 interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9(9):664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 9.Ferraccioli G. Infections, rheumatisms and autoimmunity. Future Rheumatol. 2008;3(3):223–225. [Google Scholar]
- 10.Patole PS, Pawar RD, Lech M, et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 11.Baccala R, Gonzalez QR, Lawson BR, et al. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5(8):448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 12.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferonα production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 13 ■■.Gaisford W, Cooke A. Can infections protect against autoimmunity? Curr. Opin. Rheumatol. 2009;21:391–396. doi: 10.1097/BOR.0b013e32832c2dee. [Describes the possible protective role of infections.] [DOI] [PubMed] [Google Scholar]
- 14.Goddard GZ, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 15.Kang I, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr. Opin. Rheumatol. 2003;15:528–534. doi: 10.1097/00002281-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Gergely P, Jr, Pullmann R, Stancato C, et al. Increased prevalence of transfusion transmitted virus and cross reactivity with immunodominant epitopes of the HRES-1/p28 endogenous retroviral autoantigen in patients with systemic lupus erythematosus. Clin. Immunol. 2005;116:124–134. doi: 10.1016/j.clim.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17 ■■.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJA, Harley JB. An increased prevalence of Epstein Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J. Clin. Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [Early paper that describes the increased prevalence of Epstein-Barr virus in SLE.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu TC, Tsay GJ. Human parvovirus B19 infection in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:152–157. doi: 10.1093/rheumatology/40.2.152. [DOI] [PubMed] [Google Scholar]
- 19.Hemauer A, Beckenlehner, Wolf H, Lang B, Modrow S. Acute parvovirus B19 infection in connection with a flare of systemic lupus erythematodes in a female patient. J. Clin. Virol. 1999;14:73–77. doi: 10.1016/s1386-6532(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Lee S, Ogasawara H, et al. Exacerbation of systemic lupus erythematosus related to cytomegalovirus infection. Lupus. 1998;7(8):561–564. doi: 10.1191/096120398678920596. [DOI] [PubMed] [Google Scholar]
- 21.Nawata M, Seta N, Yamada M, et al. Possible triggering effect of cytomegalovirus infection on systemic lupus erythematosus. Scand. J. Rheumatol. 2001;30(6):360–362. doi: 10.1080/030097401317148570. [DOI] [PubMed] [Google Scholar]
- 22.Gladmann DD, Hussain F, Ibanez D, et al. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11:234–239. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 23.Harley JB, Harley ITW, Guthridge JM, et al. The curiously suspicious: a role for Epstein Barr virus in lupus. Lupus. 2006;15:768–777. doi: 10.1177/0961203306070009. [DOI] [PubMed] [Google Scholar]
- 24.James JA, Harley JB, Scofield RH. Epstein Barr virus and systemic lupus erythematosus. Curr. Opin. Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 25 ■.Toussirot E. Epstein Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2008;22(5):883–896. doi: 10.1016/j.berh.2008.09.007. [Possible role of Epstein-Barr virus is described.] [DOI] [PubMed] [Google Scholar]
- 26.James JA, Neas BN, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44:1122–1146. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 27.Poole BD, Templeton AK, Guthridge JM, Brown EJ, Harley JB, James JA. Aberrant Epstein Barr viral infection in systemic lupus erythematosus. Autoimmun. Rev. 2009;8(4):337–342. doi: 10.1016/j.autrev.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon UY, Park SJ, Oh ST, et al. Patients with systemic lupus erythematosus have abnormally elevated Epstein Barr virus load in blood. Arthritis Res. Ther. 2004;6:295–302. doi: 10.1186/ar1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross AJ, Hochberg D, Rand W, et al. EBV and systemic lupus erythematosus: a new perspective. J. Immunol. 2005;174:6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 30.Yu SF, Wu HC, Tsai WC, et al. Detecting Epstein Barr virus DNA from peripheral blood mononuclear cells in adult patients with systemic lupus erythematosus in Taiwan. Med. Microbiol. Immunol. 2005;194(3):115–120. doi: 10.1007/s00430-004-0230-5. [DOI] [PubMed] [Google Scholar]
- 31.Petersen J, Rhodes G, Roudier J, et al. Altered immune response to glycine-rich sequences of Epstein-Barr nuclear antigen-1 in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1990;33:993–1000. doi: 10.1002/art.1780330711. [DOI] [PubMed] [Google Scholar]
- 32.Poole BD, Scofield RH, Harley JB, et al. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63–67. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 33.Sabbatini A, Bombardieri S, Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein-Barr virus-encoded nuclear antigen EBNA-1. Eur. J. Immunol. 1993;23:1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- 34.McClain MT, Heinlen LD, Dennis GJ, et al. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 2005;11(1):85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 35.Sabbatini A, Bombardieri S, Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein Barr virus-encoded nuclear antigen EBNA 1. Eur. J. Immunol. 1993;23:1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- 36.Chen CJ, Lin KH, Lin SC, et al. High prevalence of immunoglobulin A antibody against Epstein-Barr virus capsid antigen in adult patients with disease flare: case control studies. J. Rheumatol. 2005;32:44–47. [PubMed] [Google Scholar]
- 37.McClain M, Poole B, Bruner B, et al. An altered immune response to Epstein Barr virus nuclear antigen-1(EBNA-1) in pedriatic systemic lupus erythematosus. Arthritis Rheum. 2006;54(1):360–368. doi: 10.1002/art.21682. [DOI] [PubMed] [Google Scholar]
- 38.James JA, Gross T, Schofield RH, et al. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J. Exp. Med. 1995;181(2):453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James JA, Harley JB. A model of peptide-induced lupus autoimmune B cell epitope spreading is strain specific and is not H-2 restricted in mice. J. Immunol. 1998;160(1):502–508. [PubMed] [Google Scholar]
- 40.Arbuckle MR, Gross T, Scofield RH, et al. Lupus humoral autoimmunity induced in a primate model by short peptide immunization. J. Invest. Med. 1998;46(2):58–65. [PubMed] [Google Scholar]
- 41.Soloninka CA, Anderson MJ, Laskin CA. Anti-DNA and antilymphocyte antibodies during acute infection with human parvovirus B19. J. Rheumatol. 1989;16:777–781. [PubMed] [Google Scholar]
- 42.Morey AL, Ferguson DJ, Fleming KA. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: evidence for cell death by apoptosis. J. Pathol. 1993;169:213–220. doi: 10.1002/path.1711690207. [DOI] [PubMed] [Google Scholar]
- 43.Gergely P, Perl A, Poor G. Possible pathogenic nature of the recently discovered TT virus: does it play a role in autoimmune rheumatic diseases? Autoimmun. Rev. 2006;6:5–9. doi: 10.1016/j.autrev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Perl A. Role of endogenous retroviruses in autoimmune diseases. Rheum. Dis. Clin. North Am. 2003;29:123–143. doi: 10.1016/s0889-857x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- 45.Meyaard L, Otto SA, Jonker RR, et al. Programmed death of T cells in HIV infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 46.Emlen W, Niebur JA, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J. Immunol. 1994;152:3685–3692. [PubMed] [Google Scholar]
- 47.Espinoza LR, Cuellar ML. Arthritis and Allied Conditions Baltimore. Vol. 2361. Williams and Wilkins; PA, USA: 1998. Retrovirus associated rheumatic syndromes. [Google Scholar]
- 48.Strack PR, Frey MW, Rizzo CJ, et al. Apoptosis mediated by HIV protease is preceded by cleavage of bcl-2. Proc. Natl Acad. Sci. USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pullmann R, Bonilla E, Phillips PE, Middleton FA, Perl A. Haplotypes of the HRES-1 endogenous retrovirus are associated with development and disease manifestations of systemic lupus erythematosus. Arthritis Rheum. 2008;58(8):532–540. doi: 10.1002/art.23161. [DOI] [PubMed] [Google Scholar]
- 50.Perl A, Colombo E, Dai H, et al. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes. Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995;38:1660–1671. doi: 10.1002/art.1780381119. [DOI] [PubMed] [Google Scholar]
- 51.Perl A, Isaacs CM, Eddy RI, et al. The human T-cell leukemia virus-related endogenous sequence (HRES1) is located on chromosome 1 at q42. Genomics. 1991;11:1172–1173. doi: 10.1016/0888-7543(91)90052-g. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez DR, Telarico T, Bonilla E, et al. Activation of mammalian target of rapamycin controls the loss of TCRζin lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J. Immunol. 2009;182(4):2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hicks JT, Aulakh GS, McGrath PP, Washington GC, Kim E, Alepa FP. Search for Epstein Barr and type C oncorna viruses in systemic lupus erythematosus. Arthritis Rheum. 1979;22(8):845–857. doi: 10.1002/art.1780220807. [DOI] [PubMed] [Google Scholar]
- 55.Markenson JA, Phillips PE. Type C viruses and systemic lupus erythematosus. Arthritis Rheum. 1978;21:2. doi: 10.1002/art.1780210217. [DOI] [PubMed] [Google Scholar]
- 56.Phillips PE, Christian CL. Myxovirus antibody increases in human connective tissue disease. Science. 1970;168(934):982–984. doi: 10.1126/science.168.3934.982. [DOI] [PubMed] [Google Scholar]
- 57 ■.Phillips PE, Christian CL. Virus antibodies in systemic lupus erythematosus and other connective tissue diseases. Ann. Rheum. Dis. 1973;32(5):450–456. doi: 10.1136/ard.32.5.450. [Early paper that suggested the role of viral infections in SLE.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aron-Maor A, Shoenfeld Y. Vaccination and systemic lupus erythematosus: the bidirectional dilemmas. Lupus. 2001;10:237–240. doi: 10.1191/096120301673085478. [DOI] [PubMed] [Google Scholar]
- 59.Older SA, Battafarano DF, Enzenauer RJ, Krieg AM. Can immunization precipitate connective tissue disease? Report of five cases of systemic lupus erythematosus and review of literature. Semin. Arthritis Rheum. 1999;30(3):131–139. doi: 10.1016/s0049-0172(99)80024-9. [DOI] [PubMed] [Google Scholar]
- 60.Finielz P, Lam Kang Sang LF, Guiserix J. Systemic lupus erythematosus and thrombocytopenic purpura in two members of the same family following hepatitis B vaccine. Nephrol. Dial. Transplant. 1998;13(9):2420–2421. doi: 10.1093/ndt/13.9.2420. [DOI] [PubMed] [Google Scholar]
- 61.Tudela P, Marti S, Bonal J. Systemic lupus erythematosus and vaccination against hepatitis B. Nephron. 1992;62:236. doi: 10.1159/000187043. [DOI] [PubMed] [Google Scholar]
- 62.Guiserix J. Systemic lupus erythematosus following Hepatitis B vaccine. Nephron. 1996;74:441. doi: 10.1159/000189357. [DOI] [PubMed] [Google Scholar]
- 63.O Neill SG, Isenberg DA. Immunizing patients with systemic lupus erythematosus: a review of effectiveness and safety. Lupus. 2006;15:778–783. doi: 10.1177/0961203306069355. [DOI] [PubMed] [Google Scholar]
- 64.Kuruma KA, Borda EF, Lopez MH, de Carvalho JF, Bonfa E. Safety and efficacy of hepatitis B vaccine in systemic lupus erythematosus. Lupus. 2007;16:350–354. doi: 10.1177/0961203307078225. [DOI] [PubMed] [Google Scholar]
- 65.Brown MA, Bertouch JV. Rheumatic complications of influenza vaccination. Aust. N Z J Med. 1994;24:572–573. doi: 10.1111/j.1445-5994.1994.tb01760.x. [DOI] [PubMed] [Google Scholar]
- 66.Del Porto F, Lagana B, Biselli R, et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24:3217–3223. doi: 10.1016/j.vaccine.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 67.Holvast A, Huckriede A, Wilschut J, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann. Rheum. Dis. 2006;65:913–918. doi: 10.1136/ard.2005.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elkayam O, Paran D, Caspi D, et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin. Infect. Dis. 2002;34:147–153. doi: 10.1086/338043. [DOI] [PubMed] [Google Scholar]
- 69.Millet A, Decaux O, Perlat A, Grosbois B, Jego P. Systemic lupus erythematosus and vaccination. Eur. J. Intern. Med. 2009;20:236–241. doi: 10.1016/j.ejim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Tarjan P, Sipka S, Marodi L, et al. No short term immunological effects of pneumococcus vaccination in patients with systemic lupus erythematosus. Scand. J. Rheumatol. 2002;31:211–215. doi: 10.1080/030097402320318396. [DOI] [PubMed] [Google Scholar]
- 71.Symmons DP. Frequency of lupus in people of African origin. Lupus. 1995;4(3):176–178. doi: 10.1177/096120339500400303. [DOI] [PubMed] [Google Scholar]
- 72.Kumar K, Chambers S, Gordon C. Challenges of ethnicity in SLE. Best Pract. Res. Clin. Rheumatol. 2009;23(4):549–561. doi: 10.1016/j.berh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Butcher G. Autoimmunity and malaria. Trends Parasitol. 2008;24(7):291–292. doi: 10.1016/j.pt.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Butcher GA. Does malaria select for predisposition to autoimmune disease? JR Soc. Med. 1991;84(8):451–453. doi: 10.1177/014107689108400802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garred P, Voss A, Madsen HO, Junker P. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes Immun. 2001;2:442–450. doi: 10.1038/sj.gene.6363804. [DOI] [PubMed] [Google Scholar]
- 76.Mok MY, Ip WK, Lau CS, Lo Y, Wong WH, Lau YL. Mannose binding lectin and susceptibility to infection in Chinese patients with systemic lupus erythematosus. J. Rheumatol. 2007;34(6):1270–1276. [PubMed] [Google Scholar]
- 77.Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;99:265. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 78.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Ann. Rev. Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 79.Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll- like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 80.Yanai H, Chen HM, Inuzuka T, et al. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc. Natl Acad. Sci. USA. 2007;104:3402–3407. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng TF, Brzostek S, Ando O, Scoy SV, Kumar KP, Reich NC. Differential activation of IFN regulatory factor (IRF)-3 and IRF-5 transcription factors during viral infection. J. Immunol. 2006;176:7462–7470. doi: 10.4049/jimmunol.176.12.7462. [DOI] [PubMed] [Google Scholar]
- 82.Barnes BJ, Kellum MJ, Field AE, Pitha PM. Multiple regulatory domains of IRF-5 control activation, cellular localization and induction of chemokines that mediate recruitment of T lymphocytes. Mol. Cell Biol. 2002;22:5721–5740. doi: 10.1128/MCB.22.16.5721-5740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schoenmeyer A, Barnes BJ, Mancl ME, et al. The interferon regulatory factor, IRF-5, is a central mediator of Toll-like receptor 7 signaling. J. Biol. Chem. 2005;280:17005–17012. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 84.Hom G, Graham RR, Modrek B, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX N. Engl. J. Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 85.Remmers EF, Plenge RM, Lee AT, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Sullivan R, Carrigan SO, Marshall JS, Lin TJ. Signal transducer and activator of transcription 4 (STAT4), but not IL-12 contributes to Pseudomonas aeruginosa-induced lung inflammation in mice. Immunobiology. 2008;213(6):469–479. doi: 10.1016/j.imbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Severin MC, Levy L, Shoenfeld Y. Systemic lupus erythematosus and parvovirus B-19: causal coincidence or causative culprit? Clin. Rev. Allergy Immunol. 2003;25:41–48. doi: 10.1385/CRIAI:25:1:41. [DOI] [PubMed] [Google Scholar]
- 88.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 89.Vercelli D. Mechanisms of the hygiene hypothesis-molecular or otherwise. Curr. Opin. Immunol. 2006;18:733–737. doi: 10.1016/j.coi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Liu AH, Leung DYM. Renaissance of the hygiene hypothesis. J. Allergy Clin. Immunol. 2006;117:1063–1066. doi: 10.1016/j.jaci.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 91.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 92.Romagnoni S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Praprotnik S, Sodin-Semrl S, Tomsic M, et al. The curiously suspicious: Infectious disease may ameliorate an ongoing autoimmune destruction in systemic lupus erythematosus. J. Autoimmun. 2008;30:37–41. doi: 10.1016/j.jaut.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Chen M, Aosai F, Norose K, et al. Toxoplasma gondii infection inhibits the development of lupus-like syndrome in autoimmune (New Zealand Black × New Zealand white) F1 mice. Int. Immunol. 2004;16:937–946. doi: 10.1093/intimm/dxh095. [DOI] [PubMed] [Google Scholar]
- 95.Sawalha AH, Schmid WR, Binder SR, Bacino DK, Harley JB. Association between systemic lupus erythematosus and Helicobacter pylori seronegativity. J. Rheumatol. 2004;31:1546–1550. [PubMed] [Google Scholar]
- 96.Amital H, Govoni M, Maya R, et al. Role of infectious agents in systemic rheumatic diseases. Clin. Exp. Rheumatol. 2008;26(1 Suppl 48):S27–S32. [PubMed] [Google Scholar]
- 97.Clatworthy MR, Willcocks L, Urban B, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcγRIIb reduce susceptibility to malaria. Proc. Natl Acad. Sci. USA. 2007;104(17):7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98 ■■.Bach JF. Protective role of infections and vaccinations on autoimmune diseases. J. Autoimmun. 2001;16:347–353. doi: 10.1006/jaut.2000.0478. [Excellent description of the role of infections in the pathogenesis of autoimmune diseases.] [DOI] [PubMed] [Google Scholar]
- 99.Pozzilli P. BCG vaccine in insulin-dependent diabetes mellitus IMDIAB Group. Lancet. 1997;349:1520–1521. doi: 10.1016/s0140-6736(05)62100-9. [DOI] [PubMed] [Google Scholar]
- 100.Elliott JF, Marlin KL, Couch RM. Effect of Cacille Calmette-Guerin vaccination on C peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21:1691–1693. doi: 10.2337/diacare.21.10.1691. [DOI] [PubMed] [Google Scholar]
- 101.Allen HF, Klingensmith GJ, Jensen P, et al. Effect on Bacillus Calmette-Guerin vaccination on new-onset Type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22:1703–1707. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 102.Bonn D. Q fever vaccine on trial for Type 1 diabetes. Mol. Med. Today. 1999;5:143. doi: 10.1016/s1357-4310(99)01450-1. [DOI] [PubMed] [Google Scholar]
- 103.Ristori G, Buzzi MG, Sabatini U, et al. Use of Bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology. 1999;53:1588–1589. doi: 10.1212/wnl.53.7.1588. [DOI] [PubMed] [Google Scholar]
- 104.Mehrad B, Park SJ, Akangire G, et al. The lupus-susceptibility locus, Sle3, mediates enhanced resistance to bacterial infections. J. Immunol. 2006;176:3233–3239. doi: 10.4049/jimmunol.176.5.3233. [DOI] [PubMed] [Google Scholar]
- 105.Wakui M, Morel L, Butfiloski EJ, Kim C, Sobel ES. Genetic dissection of systemic lupus erythematosus pathogenesis: partial functional complementation between Sle1 and Sle3/5 demonstrates requirement for intracellular coexpression for full phenotypic expression of lupus. J. Immunol. 2005;175:1337–1345. doi: 10.4049/jimmunol.175.2.1337. [DOI] [PubMed] [Google Scholar]
- 106.Bach JF. Infections and autoimmune diseases. J. Autoimmun. 2005;25:74–80. doi: 10.1016/j.jaut.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Kim WU, Sreih A, Bucala R. Toll-like receptors in systemic lupus erythematosus; prospects for therapeutic intervention. Autoimmun. Rev. 2009;8:204–208. doi: 10.1016/j.autrev.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]