Abstract
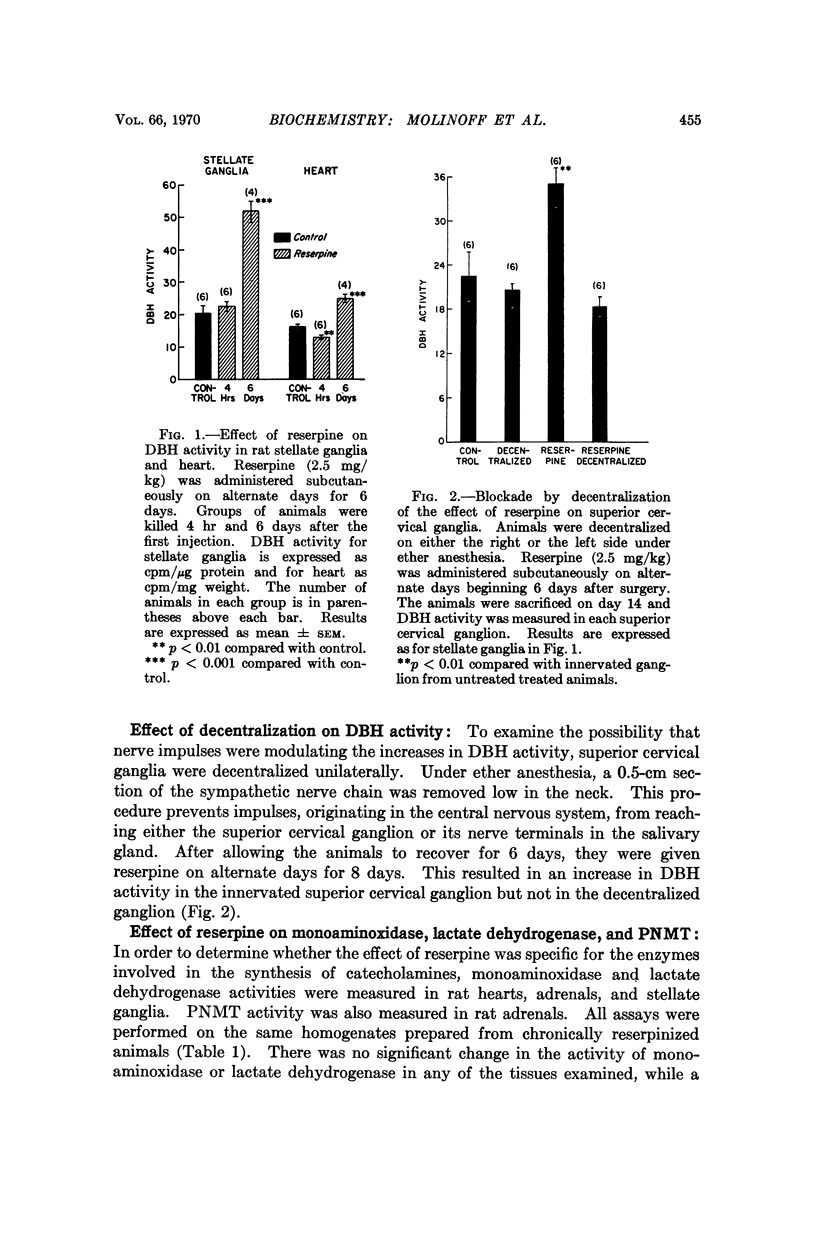
The development of a sensitive and specific enzymatic assay for dopamine-β-hydroxylase has enabled us to measure the activity of this enzyme in several tissues where it has previously been measured. The administration of reserpine leads to an increase in dopamine-β-hydroxylase activity in the rat adrenal, heart, salivary gland, and in sympathetic ganglia. The increase in the heart is preceded by a small but significant fall. We have confirmed the increase in tyrosine hydroxylase which follows the administration of reserpine and have found that the activity of phenylethanolamine-N-methyltransferase also increases after administration of this drug. The activities of two enzymes not involved in the synthesis of catecholamines, monoamine oxidase and lactate dehydrogenase, are not affected by reserpine treatment. The rise of dopamine-β-hydroxylase activity in the sympathetic ganglia is blocked by surgical decentralization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962 May;237:1657–1660. [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Heptinstall S. The distribution of the glucosyl substituents along the chain of the teichoic acid in walls of Lactobacillus buchneri N.C.I.B. 8007. Biochem J. 1969 Jan;111(2):245–246. doi: 10.1042/bj1110245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin L., Livett B. G., Chubb I. W. Increased synthesis and release of noradrenaline and dopamine during nerve stimulation. Life Sci. 1967 Jan 1;6(1):97–104. doi: 10.1016/0024-3205(67)90366-9. [DOI] [PubMed] [Google Scholar]
- Ciaranello R. D., Barchas R. E., Byers G. S., Stemmle D. W., Barchas J. D. Enzymatic synthesis of adrenaline in mammalian brain. Nature. 1969 Jan 25;221(5178):368–369. doi: 10.1038/221368a0. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S., FRIEDMAN S. DOPAMINE-BETA-HYDROXYLASE. Pharmacol Rev. 1965 Jun;17:71–100. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitt M., Gibb J. W., Daly J. W., Lipton M., Udenfriend S. A new class of tyrosine hydroxylase inhibitors and a simple assay of inhibition in vivo. Biochem Pharmacol. 1967 Jul 7;16(7):1313–1321. doi: 10.1016/0006-2952(67)90162-1. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Landsberg L., Axelrod J. An enzymatic assay for octopamine and other beta-hydroxylated phenylethylamines. J Pharmacol Exp Ther. 1969 Dec;170(2):253–261. [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Inhibition of trans-synaptically increased tyrosine hydroxylase activity by cycloheximide and actinomycin D. Mol Pharmacol. 1969 Sep;5(5):463–469. [PubMed] [Google Scholar]
- POTTER L. T., AXELROD J. PROPERTIES OF NOREPINEPHRINE STORAGE PARTICLES OF THE RAT HEART. J Pharmacol Exp Ther. 1963 Dec;142:299–305. [PubMed] [Google Scholar]
- Pohorecky L. A., Zigmond M., Karten H., Wurtman R. J. Enzymatic conversion of norepinephrine to epinephrine by the brain. J Pharmacol Exp Ther. 1969 Feb;165(2):190–195. [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1969 Oct;169(2):249–254. [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Connett R. J., Kirshner N. Mechanism of secretion from the adrenal medulla. 3. Studies of dopamine beta-hydroxylase as a marker for catecholamine storage vesicle membranes in rabbit adrenal glands. Mol Pharmacol. 1969 Jan;5(1):60–68. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Weiner N., Rabadjija M. The effect of nerve stimulation on the synthesis and metabolism of norepinephrine in the isolated guinea-pig hypogastric nerve-vas deferens preparation. J Pharmacol Exp Ther. 1968 Mar;160(1):61–71. [PubMed] [Google Scholar]



