Abstract
Activation of AMPK (AMP-activated protein kinase) by phosphorylation at Thr172 is catalysed by at least two distinct upstream kinases, i.e. the tumour suppressor LKB1, and CaMKKβ (Ca2+/calmodulin-dependent protein kinase kinase-β). The sequence around Thr172 is highly conserved between the two catalytic subunit isoforms of AMPK and the 12 AMPK-related kinases, and LKB1 has been shown to act upstream of all of them. In the present paper we report that none of the AMPK-related kinases tested could be phosphorylated or activated in intact cells or cell-free assays by CaMKKβ, although we did observe a slow phosphorylation and activation of BRSK1 (brain-specific kinase 1) by CaMKKα. Despite recent reports, we could not find any evidence that the α and/or β subunits of AMPK formed a stable complex with CaMKKβ. We also showed that increasing AMP concentrations in HeLa cells (which lack LKB1) had no effect on basal AMPK phosphorylation, but enhanced the ability of agents that increase intracellular Ca2+ to activate AMPK. This is consistent with the effect of AMP on phosphorylation of Thr172 being due to inhibition of dephosphorylation, and confirms that the effect of AMP is independent of the upstream kinase utilized.
Keywords: AMP-activated protein kinase (AMPK), AMPactivated protein kinase-related kinase (ARK), Ca2+/calmodulindependent protein kinase (CaMK), Ca2+/calmodulin-dependent protein kinase kinase (CaMKK)
Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-amino-4-imidazolecarboxamide riboside; AMPK, AMP-activated protein kinase; ARK, AMPK-related kinase; BRSK, brain-specific kinase; CaMK, Ca2+/calmodulin-dependent protein kinase; CaMKK, Ca2+/calmodulin-dependent protein kinase kinase; FBS, foetal bovine serum; GFP, green fluorescent protein; GST, glutathione transferase; HEK, human embryonic kidney; MARK, microtubule affinity-regulating kinase; MO25, mouse protein 25; NUAK, SNF1 (sucrose-non-fermenting kinase-1)-like kinase; SIK, salt-inducible kinase; STRAD, Ste20-related adaptor; TBS, Tris-buffered saline; UBA, ubiquitin-associated
INTRODUCTION
AMPK (AMP-activated protein kinase) is an energy-sensing system involved in regulating energy balance at both the cellular and the whole-body levels [1,2]. The kinase occurs as heterotrimeric complexes composed of a catalytic α subunit and regulatory β and γ subunits, with each subunit existing in mammals as isoforms encoded by multiple genes (α1, α2; β1, β2; γ1, γ2, γ3). Metabolic stresses that inhibit ATP synthesis (e.g. hypoxia, hypoglycaemia) or that stimulate ATP consumption (e.g. muscle contraction) cause an increase in the cellular ADP/ATP ratio, which is amplified by adenylate kinase into an even larger increase in the AMP/ATP ratio. AMP and ATP bind antagonistically to two sites formed by the four tandem CBS (cystathionine β-synthase) motifs on the γ subunit [3,4]. The kinase is only active after phosphorylation of a critical threonine residue within the activation loop of the kinase domain (Thr172 in rat α1/α2) by upstream kinases. The major upstream kinase in most cells was identified to be a complex between the tumour suppressor LKB1 and two accessory subunits, STRAD (Ste20-related adaptor) and MO25 (mouse protein 25) [5,6]. LKB1 appears to be constitutively active [7,8] and may therefore phosphorylate AMPK continually, but under basal conditions the phosphate appears to be immediately removed by protein phosphatases. However, binding of AMP to the AMPK γ subunits inhibits dephosphorylation of Thr172, an effect that is antagonized by high concentrations of ATP [9–11]. In addition, binding of AMP (but not ATP) triggers a further allosteric activation of the phosphorylated kinase by up to 10-fold, with the combination of these two effects producing >1000-fold activation [11]. Both stimulatory effects appear to occur because AMP binding relieves the inhibitory effects of an auto-inhibitory domain on the α subunit, which binds to the kinase domain on the opposite side to the substrate-binding site [12]. This represents a sensitive switch mechanism that produces a large activation of AMPK in response to a small increase in the cellular AMP/ATP ratio.
Some human tumour cells (e.g. HeLa cells) do not express LKB1, but AMPK can still be phosphorylated at Thr172 and activated in such cells using Ca2+ ionophores. This led to the discovery that the CaMKKs [CaMK (Ca2+/calmodulin-dependent protein kinase) kinases], especially CaMKKβ, could act as alternative upstream kinases that can phosphorylate Thr172 on AMPK [13–15]. CaMKKα and CaMKKβ [16] were originally discovered as CaMKs that acted upstream of CaMKI and CaMKIV. The Ca2+→CaMKK→AMPK pathway is triggered by a rise in cytosolic Ca2+ without any requirement for an increase in AMP, and is responsible for AMPK activation in response to K+-induced depolarization in neurons [13], muscarinic activation in neuroblastoma cells [17], thrombin activation of endothelial cells [18], treatment of smooth muscle cells with vasoconstrictors [19] and stimulation of antigen receptors in T-cells [20].
The sequence around Thr172 on the AMPK α subunits is highly conserved (see Figure 1) in the kinase domain sequences of kinases [termed ARKs (AMPK-related kinases)], that lie on the same branch of the human kinome [21]. By studying phosphorylation in cell-free assays and in cells lacking LKB1, including LKB1−/− mouse embryo fibroblasts and HeLa cells, it was shown that at least 12 of these were also dependent on LKB1 for basal phosphorylation of the threonine residue equivalent to Thr172, and hence for basal activity. These include the brain-specific kinases BRSK1 and BRSK2 (also known as SAD-B and SAD-A), SIK (salt-inducible kinase) 1, SIK2 and SIK3 (also known as SIK, QIK and QSK respectively), NUAK [SNF1 (sucrose-non-fermenting kinase-1)-like kinase] 1 and NUAK2 (also known as ARK5 and SNARK), MARK (microtubule affinity-regulating kinase) 1, MARK2, MARK3 and MARK4, and the testis-specific kinase SNRK (SNF1-related kinase) [7,22].
Figure 1. Alignment of activation loops of the ARKs and CaMKs.
Sequences between the conserved DFG and YAAPE motifs were manually aligned, with gaps indicated by dots. The arrow shows the threonine residue (P) whose phosphorylation is required for kinase activity. Residues identical between all sequences are boxed, while the conserved hydrophobic residue at P−2 is in bold type.
In the present study we have addressed three questions regarding the regulation of AMPK and the ARKs by CaMKKs. First, it had not been established whether the ARKs are substrates for CaMKKs, as is AMPK itself. Secondly, it has recently been claimed that CaMKKβ can form a complex with the α and β subunits of AMPK in the absence of a γ subunit [23], a hypothesis that we wished to confirm. Thirdly, if the effect of AMP on phosphorylation of Thr172 on AMPK is due to inhibition of dephosphorylation we were puzzled as to why, in cells such as HeLa cells that express CaMKKβ but lack LKB1, AMPK is not activated by treatments that increase intracellular AMP [5].
EXPERIMENTAL
Materials and proteins
STO-609 was from Tocris Bioscience and calmodulin was from CN Biosciences. A23187, phenformin and 2-deoxyglucose were from Sigma. Zeocin and blasticidin were from Invitrogen. Expression and purification of GST (glutathione transferase)–AMPK-α1, GST–BRSK1, GST–BRSK2, GST–LKB1:STRAD:MO25 and GST–CaMKKβ were as described previously [24]. For GST–CaMKKα, a pGEX plasmid encoding CaMKKα (residues 1–505) was used to transform Escherichia coli [BL21 (DE3) pLys], which were grown in LB (Luria–Bertani) ampicillin at 37 °C until the A600 was 0.4–0.6. Expression was induced with 50 μM IPTG (isopropyl β-D-thiogalactoside) and cells harvested after 18 h at 15 °C. The harvested cells were lysed and GST–CaMKKα affinity-purified using glutathione–Sepharose chromatography.
Plasmids
The GFP (green fluorescent protein)–AMPK-α1, –AMPK-β1 and –AMPK-γ1 plasmids were as described previously [25]. To make FLAG-tagged CaMKKβ, a pGEX construct encoding human CaMKKβ was digested with BamHI and XhoI, and the insert cloned into the vector pCMV-Tag2B using standard methods.
Antibodies
The antibody against the phosphorylated form of Thr172 on the AMPK α subunits was from Cell Signaling Technology. Antibodies against actin and the FLAG epitope, and sheep immunoglobulin were from Sigma, antibodies against GFP were from Roche, antibodies against MARK3 (C-TAK1) were from Millipore, and antibodies against AMPK-γ1 were from Abcam. Antibodies against AMPK-α1 and -α2 [26], LKB1 (N-terminal dephospho-Ser31 [24]), CaMKKβ [13], AMPK-pan β [25], GST [27], and SIK1, SIK2, SIK3, NUAK2, MARK1 and MARK4 [7] were as described previously.
Cell culture
HEK (human embryonic kidney)-293 cells and the HeLa cells used in Figures 6 and 7 were cultured in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) FBS (foetal bovine serum), 100 i.u./ml penicillin and 100 μg/ml streptomycin. HeLa cells stably expressing wild-type LKB1 and derived from T-REx™ HeLa cells (Invitrogen) were as previously described [28]. The parental T-REx™ line was used in all experiments involving HeLa cells except Figures 6 and 7, and was cultured in Eagle's minimal essential medium, 10% (v/v) FBS and 5 μg/ml blasticidin, but without tetracycline. Cells stably expressing LKB1 were grown in this medium with the addition of 100 μg/ml zeocin. The parental cell line was transfected with 10 μg (per 100 mm diameter dish) of FLAG–CaMKKβ using the polyethylenimine method [29]. Cells were transfected with combinations (3 μg each) of GFP–AMPK-α1, β1, γ1 and FLAG–CaMKKβ as indicated in the text using the polyethylenimine method [29]. The cells were cultured for a further 36 h and were left untreated, or were treated with 10 μM A23187 for 1 h. Cells were lysed in 0.5 ml of ice-cold lysis buffer [50 mM Tris/HCl (pH 7.2), 1 mM EGTA, 1 mM EDTA, 50 mM NaF, 1 mM sodium pyrophosphate, 1% (w/v) Triton X-100, 0.1 mM PMSF, 1 mM DTT (dithiothreitol), 0.1 mM benzamidine and 5 μg/ml soybean trypsin inhibitor] after rapid rinsing in ice-cold PBS. The lysates were centrifuged (21000 g for 10 min at 4 °C) and the supernatants frozen for later analysis.
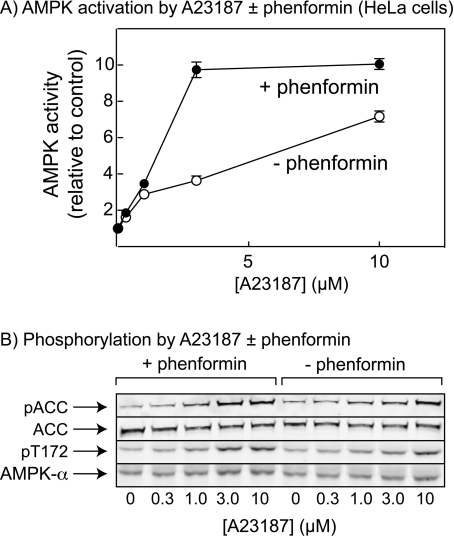
Figure 6. In cells lacking LKB1, phenformin increases activation and phosphorylation by the Ca2+ ionophore A23187.
(A) HeLa cells were treated with increasing concentrations of A23187 in the presence and absence of 10 mM phenformin. AMPK was immunoprecipitated and assayed at 200 μM AMP. Results are means±range for duplicate cell incubations. (B) Cell lysates treated as in (A) were analysed by Western blotting using antibodies against the phosphorylated form of ACC (pACC), streptavidin to detect total ACC, phosphospecific antibodies against the Thr172 site on AMPK-α (pT172) and antibodies against total AMPK-α1/α2 (AMPK-α).
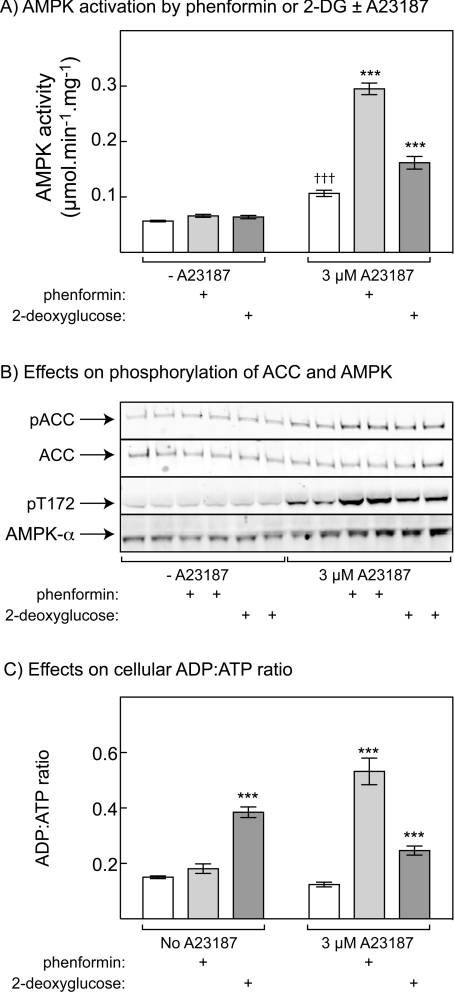
Figure 7. Phenformin and 2-deoxyglucose cause increased phosphorylation and activation of AMPK in HeLa cells treated with low concentrations of Ca2+ ionophore.
(A) HeLa cells were treated with or without 3 μM A23187 in the presence and absence of 10 mM phenformin or 100 μM 2-deoxyglucose (2DG) for 1 h. AMPK activity was determined in immunoprecipitates, and results are means±S.D. (n=3). (B) Samples from the experiment in (A) were analysed in duplicate by Western blotting using antibodies as described in Figure 6(C). (C) ADP/ATP ratios in an experiment identical with that shown in (A). Cells were extracted with perchloric acid and acid-free extracts analysed by capillary electrophoresis. ADP/ATP ratios were determined and results are expressed as means±S.D. (n=3). ***P<0.001, significantly different from relevant control without phenformin or 2DG, as measured by one-way ANOVA; †††P<0.001, significantly different from control without A23187, as measured by one-way ANOVA.
Immunoprecipitation of AMPK and CaMKKβ
HEK-293 cell lysates (600 μg of protein) were incubated at 4 °C for 2 h on a roller mixer with 5 μl of Protein G–Sepharose non-covalently conjugated to 5 μg of AMPK-α1/α2 or CaMKKβ antibodies. After extensive washing, the immunoprecipitates were heated in SDS sample buffer, resolved by SDS/PAGE and blots were probed with the antibodies indicated.
Immunoprecipitate kinase assays
Endogenous AMPK and the ARKs from HeLa cells were assayed in immunoprecipitates as described in [24,30] using the AMARA peptide [31] as a substrate. GST–AMPK-α1, GST–BRSK1 and GST–BRSK2 were assayed as described previously [24]. For CaMKKα/β, assays were performed with or without 1 μM calmodulin and 1 mM CaCl2 as indicated in the text.
Estimation of the cellular ADP/ATP ratio
Culture medium was quickly aspirated from cells grown on 10 cm diameter culture dishes and cells were washed with 1 ml of ice-cold PBS. After rapid aspiration of PBS, a minimal volume of icecold 5% perchloric acid was added and the samples vortex-mixed to ensure complete lysis. After centrifugation (14000 g for 3 min at 4 °C) to remove acid-insoluble material, the supernatant was extracted with two washes of an equal volume of 1:1 tri-n-octylamine and 1,1,2-trichlorotrifluoroethane. The nucleotides remaining in the aqueous phase were then separated by capillary electrophoresis with on-column isotachophoretic concentration, using run buffers consisting of 50 mM sodium phosphate and 50 mM NaCl (pH 5.2; leading buffer) and 100 mM Mes/Tris (pH 5.2; tailing buffer). To each buffer was added 0.2% hydroxyethylcellulose to decrease electro-osmotic flow. Nucleotide peaks were detected by UV absorbance at 260 nM, and integrated using System Gold software (Beckman). Nucleotide ratios were calculated from peak areas after correction for retention times. Identification of peaks as ATP, ADP and AMP were confirmed by additional runs spiked with internal standards and analysis of absorbance spectra of individual peaks.
Statistics
Where multiple treatments were compared, one- or two-way ANOVA was used as indicated, with Bonferroni's post-hoc test of selected treatments, using GraphPad Prism software.
Other analytical procedures
SDS/PAGE was performed using precast BisTris 4–12% gradient polyacrylamide gels in the Mops buffer system (Invitrogen). Proteins were transferred on to nitrocellulose membranes (Bio-Rad) using the Xcell II blot module (Invitrogen). Membranes were blocked for 1 h in TBS (Tris-buffered saline; 20 mm Tris/HCl and 133 mM NaCl, pH 7.5) containing 5% (w/v) non-fat dried skimmed milk. The membranes were probed with appropriate antibody (0.1–1 μg/ml) in TBS-Tween 20 (0.1%) and 2% (w/v) non-fat dried skimmed milk. Detection was performed using secondary antibody (1 μg/ml) coupled to IR 680 or IR 800 dye, and the membranes scanned using the Li-Cor Odyssey IR imager. Protein concentrations were determined by Coomassie Blue binding [32] with BSA as a standard.
RESULTS
Alignment of sequences around activation loop phosphorylation sites
Figure 1 shows an alignment of the activation loops (lying between the conserved DFG and APE motifs) of the ARKs, as well as the four isoforms of CaMKI (CaMKI-α, -β, -δ and -γ) and the single form of CaMKIV. All contain a threonine residue in the position equivalent to Thr172 in AMPK-α2, and a conserved hydrophobic residue (usually leucine) two residues N-terminal to this. Many of the other residues conserved between these sequences are also found in other protein kinases not downstream of LKB1 and/or CaMKKs, such as the DFG motif prior to the threonine, and the CG and YAAPE motifs following it. Using a peptide library approach, it has been shown that LKB1 only phosphorylates threonine residues, and strongly prefers a leucine two residues N-terminal to the threonine (i.e. at P−2) [33]. Figure 1 shows that leucine is present at P−2 in all ARKs and in CaMK1-α and -β, although in CaMK1-δ, CaMKI-γ and CaMKIV, the residue at P−2 is methionine. The features of primary sequence recognized by CaMKKα and CaMKKβ, if any, are not known.
CaMKKs activate AMPK, but not ARKs, in HeLa cells
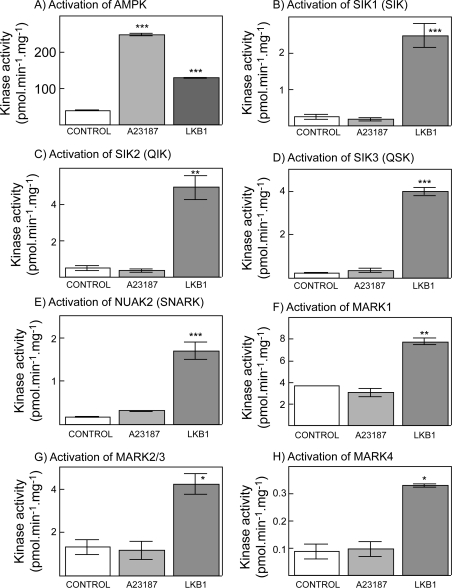
As an initial test of whether CaMKKs would activate any of the ARKs we utilized HeLa cells, which express both CaMKKα and CaMKKβ [13], but do not express LKB1 due to a biallelic deletion of the gene in the cervical cancer from which the cells were originally derived in 1951 [34]. We have shown previously that the Ca2+ ionophore A23187 caused activation of endogenous AMPK in HeLa cells, and that this effect was blocked by the CaMKK inhibitor, STO-609 [13]. Activation of AMPK (Figure 2A), and phosphorylation of Thr172 (results not shown), occurred in T-Rex™ HeLa cells not only in response to A23187, but also in response to stable expression of wild-type active LKB1. However, while stable expression of LKB1 in T-Rex™ HeLa cells increased the basal activity of the endogenous ARKs SIK1, SIK2, SIK3, NUAK2, MARK1, MARK2/MARK3 and MARK4, unlike AMPK none was significantly activated by A23187 in the parental cells (Figures 2B–2H) (for these experiments, MARK2 and MARK3 were assayed together because we do not have an antibody that distinguishes between these two closely related kinases [7]). These results show that the CaMKKs do not activate any of the endogenous ARKs that are expressed in HeLa cells.
Figure 2. Effect of treatment of T-Rex™ HeLa cells with A23187, or stable expression of LKB1 in the same cells, on the activity of endogenous AMPK and ARKs.
The indicated kinase was immunoprecipitated from cell lysates and assayed using the AMARA peptide as the substrate. Note that we do not have an antibody that distinguishes between MARK2 and MARK3 so the activity measured with this antibody could represent either MARK2 or MARK3, or a combination of the two. Values are means±S.D. for duplicate cell incubations. *P<0.05, **P<0.01 and ***P<0.001, significantly different from control without A23187 or LKB1.
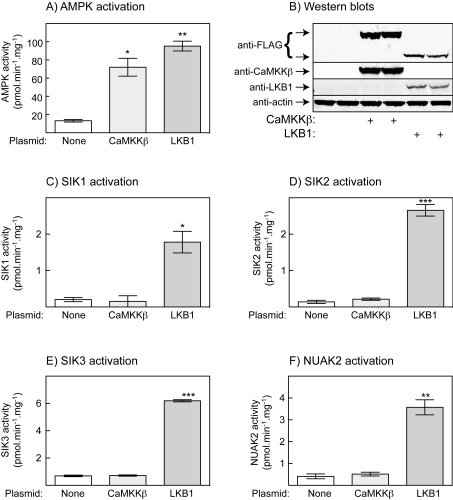
CaMKKβ is activated by Ca2+ and calmodulin, but also has a substantial basal activity [13]. We therefore examined the effect of overexpressing CaMKKβ in HeLa cells in the absence of a Ca2+ ionophore. Figure 3(A) shows that transient expression of CaMKKβ in the parental T-Rex™ HeLa cells caused almost as much activation of AMPK as stable expression of LKB1 in the same cell line, even without addition of A23187. Based on the signal obtained in a Western blot using an anti-FLAG antibody (both overexpressed proteins being FLAG-tagged), the expression of CaMKKβ in these experiments was somewhat higher than that of LKB1 (Figure 3B). Despite the large activation of AMPK by overexpression of CaMKKβ, none of the ARKs examined (SIK1, SIK2, SIK3 or NUAK2) was activated compared with the untransfected cells, although they were activated by stable expression of LKB1 (Figures 3C–3F).
Figure 3. Effect of transient expression in T-Rex™ HeLa cells of CaMKKβ, or stable expression of LKB1, on the phosphorylation and activity of AMPK and ARKs.
(A) Endogenous AMPK activity; (B) Western blot analysis of duplicate cell lysates shown in (A); (C–F) activities of endogenous SIK1, SIK2, SIK3 and NUAK2. Values are means±S.D. for duplicate cell incubations. *P<0.05, **P<0.01 and ***P<0.001, significantly different from control without CaMKKβ or LKB1.
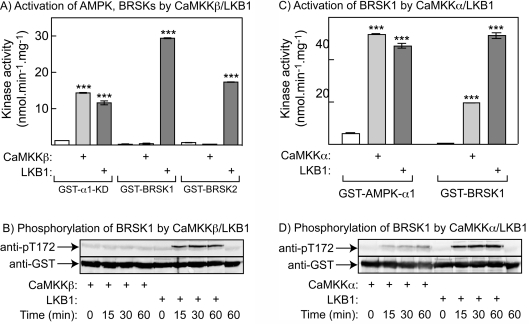
CaMKKβ activates the AMPK kinase domain, but not BRSK1/BRSK2, in cell-free assays
The brain-specific ARKs, BRSK1 and BRSK2, are not expressed in HeLa cells. In order to address whether they could be phosphorylated and activated by CaMKKβ, we expressed their combined kinase and UBA (ubiquitin-associated)-like domains in bacteria as GST-fusion proteins, as well as the kinase domain of the α1 isoform of the AMPK catalytic subunit (GST–α1-KD). We also expressed GST–CaMKKβ in bacteria and a GST–LKB1:FLAG-STRADα:myc–MO25α complex in HEK-293 cells. All fusion proteins/complexes were purified on glutathione–Sepharose and the upstream kinases were tested for their ability to phosphorylate and activate GST–α1-KD, GST–BRSK1 and GST-BRSK2 in cell-free assays. Figure 4(A) shows that, although the LKB1 complex and CaMKKβ were both able to activate GST–α1-KD, only the LKB1 complex was able to activate GST–BRSK1 and GST–BRSK2. There was also no phosphorylation of BRSK1 or BRSK2 by CaMKKβ under conditions where there was a robust phosphorylation of AMPK (results not shown). These experiments were performed in the absence of Ca2+ and calmodulin, since in cell-free assays CaMKKβ appears to be at least partially independent of Ca2+ and calmodulin [13]. However, Figure 4(B) confirms that BRSK1 was not phosphorylated by CaMKKβ, even in the presence of Ca2+ and calmodulin.
Figure 4. Phosphorylation and activation of GST–AMPK-α1 (kinase domain only), GST–BRSK1 or GST–BRSK2 (kinase domains plus UBA-like domains) by CaMKKs.
(A) Activity of GST–AMPK-α1, –BRSK1 or –BRSK2 after incubation in the presence of MgATP for 1 h with or without CaMKKβ (minus Ca2+/calmodulin), or LKB1:STRADα:MO25α. (B) Time course of phosphorylation of GST–BRSK1 by CaMKKβ plus Ca2+/calmodulin, or LKB1:STRADα:MO25α. (C) Activity of AMPK-α1 and BRSK1 after incubation in the presence of MgATP for 1 h with or without CaMKKα plus Ca2+/calmodulin, or LKB1:STRADα:MO25α. (D) Time course of phosphorylation of GST–BRSK1 in the experiment shown in (C). Values are means±S.D. for duplicate cell incubations. ***P<0.001, significantly different from control without CaMKKβ or LKB1.
Interestingly, although CaMKKβ was ineffective, CaMKKα was able to activate (Figure 4C) and phosphorylate (Figure 4D) GST–BRSK1 in the presence of Ca2+ and calmodulin. However, the concentration of CaMKKα used in these experiments was approx. 20-fold higher than that required to obtain a comparable level of phosphorylation of AMPK-α1 (results not shown).
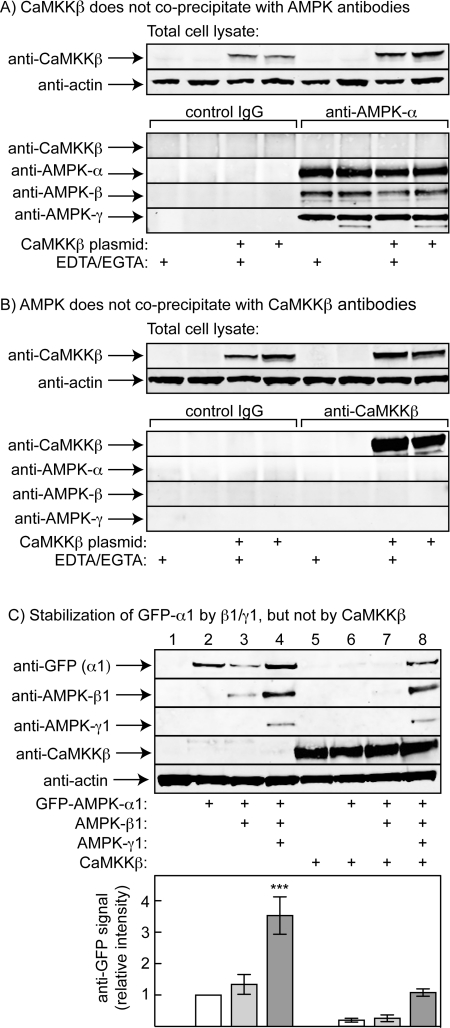
AMPK and CaMKKβ do not form a stable complex
Anderson et al. [23] have recently reported evidence suggesting that CaMKKβ can form a stable complex with the α and β subunits of AMPK in the absence of a γ subunit. To test this hypothesis, we initially studied whether overexpressed CaMKKβ would co-precipitate with endogenous AMPK from extracts of HEK-293 cells. Figure 5(A) shows that, although CaMKKβ was readily detectable by Western blot analysis in the crude cell lysates, none was detected by Western blot analysis in immunoprecipitates made using anti-AMPK-α1/α2 antibodies (although we could readily detect the α, β and γ subunits of AMPK). Anderson et al. [23] claimed that the interaction between CaMKKβ and AMPK was Ca2+-dependent, but the same results were obtained regardless of whether EDTA and EGTA were present in the lysis and immunoprecipitation buffers. When the converse experiment was performed, i.e. by immunoprecipitating using an anti-CaMKKβ antibody, we could detect CaMKKβ, but not the α, β or γ subunits of AMPK in the precipitate, irrespective of the presence of EDTA and EGTA in the buffers (Figure 5B).
Figure 5. AMPK and CaMKKβ do not form a stable complex.
(A) CaMKKβ was overexpressed in HEK-293 cells; the top panels show Western blot analysis of CaMKKβ and actin in the cell lysates, whereas the bottom panels show Western blot analysis performed on immunoprecipitates made with control immunoglobulin or anti-AMPK-α1/α2 antibodies in the presence and absence of EDTA plus EGTA. (B) As (A), except that Western blot analysis was performed on immunoprecipitates made with control immunoglobulin or anti-CaMKKβ antibodies in the presence and absence of EDTA plus EGTA. (C) HeLa cells were transfected with various combinations of plasmids encoding GFP-tagged AMPK-α1, AMPK-β1, AMPK-γ1 or FLAG–CaMKKβ. Lysates were analysed by Western blotting using antibodies against GFP, pan-β, γ1, FLAG or actin. The top panel shows one representative experiment, whereas the bottom panel shows quantification of the anti-GFP signal from five experiments; the results are significantly different from the expression level of GFP-α1 alone (***P<0.001).
Figure 5(C) shows results of expressing GFP-tagged α1 with or without various combinations of β1, γ1 and FLAG-tagged CaMKKβ in HeLa cells. It has been previously shown that co-expression with β and γ subunits greatly increases expression of GST-tagged α1 in COS7 cells, most likely because the heterotrimeric complex is more stable and is degraded less rapidly [35]. Consistent with this, co-expression of GFP–α1 with β1 and γ1, but not β1 alone, led to a 3–4-fold increase in its expression; co-expression with γ1 together with GFP–α1 also increased the expression of β1. When CaMKKβ was expressed, the protein was expressed at equal levels irrespective of whether or not particular AMPK subunits had been co-expressed. If, as suggested by Anderson et al. [23], CaMKKβ forms a complex with AMPK α and β subunits in the absence of γ, one might have expected that co-expression of CaMKKβ would have stabilized and increased the expression of GFP–α1 and β1 in the absence of γ1. In fact, the opposite was observed: when GFP–α1 and β1 were co-expressed with CaMKKβ, their expression level was reduced (compare lane 7 with lane 3 in Figure 5C). Only when GFP–α1 and β1 were co-expressed with γ1 was there a marked increase in their expression.
Increasing Ca2+ and AMP causes a synergistic activation of AMPK in HeLa cells
Although it had been proposed that AMP promoted phosphorylation of AMPK by LKB1 [5], previous studies suggest that this may have been caused by trace contamination of the kinase preparations used with protein phosphatase, and that the effect of AMP on Thr172 phosphorylation is entirely via inhibition of dephosphorylation [10]. This raised the question as to why drugs such as AICAR (5-amino-4-imidazolecarboxamide riboside), which is converted into the AMP mimetic ZMP [36], or phenformin, which increases cellular AMP [5], do not activate AMPK in HeLa cells [5], even though CaMKKβ is expressed and is capable of phosphorylating Thr172 in those cells [13]. One explanation was that the activity of CaMKKβ in cells with basal Ca2+ is so low that the endogenous protein phosphatase activity is sufficient to cause net dephosphorylation of Thr172, even though dephosphorylation is being inhibited by AMP. To test this idea, we incubated HeLa cells with increasing concentrations of the Ca2+ ionophore A23187 in the presence and absence of phenformin. As found previously [5], in the absence of A23187, phenformin did not give a significant stimulation of AMPK activity, phosphorylation of Thr172 on the AMPK α subunits, or phosphorylation of the AMPK site on ACC (acetyl-CoA carboxylase). As expected [13], in the absence of phenformin, concentrations of A23187 up to 10 μM increasingly stimulated AMPK activity (Figure 6A), as well as phosphorylation of AMPK and ACC (Figure 6B). However, Figure 6 reveals the new result that the presence of phenformin sensitized the cells to A23187. Thus the presence of phenformin shifted the curve of AMPK phosphorylation and activation against A23187 concentration to the left. A stimulation of the activation of AMPK, and the phosphorylation of AMPK and ACC by phenformin was particularly evident at 3 μM A23187. These results show that, under appropriate conditions, phenformin can activate AMPK even in cells lacking LKB1.
We repeated the experiments using 3 μM A23187 in the presence and absence of phenformin or 2-deoxyglucose, another agent that can deplete cellular ATP, and measured AMPK activity, phosphorylation of ACC and AMPK, and levels of cellular nucleotides (Figure 7). The level of AMP in HeLa cells under basal conditions was too low to measure accurately, although it could be detected in cells treated with phenformin and A23187 (results not shown). We therefore used ADP/ATP as a surrogate for AMP/ATP, since the latter is expected to vary as the square of ADP/ATP [37]. Phenformin and 2-deoxyglucose did not cause phosphorylation of ACC or AMPK, or activation of AMPK, in HeLa cells that had not been treated with a Ca2+ ionophore, despite the fact that 2-deoxyglucose produced a large rise in ADP/ATP ratio (any small effect of phenformin on the ADP/ATP ratio was not significant). However, in cells treated with 3 μM A23187, both phenformin and 2-deoxyglucose significantly stimulated AMPK activity, and increased phosphorylation of ACC and AMPK, over and above the effect of A23187 alone, with the effect of phenformin being larger than that of 2-deoxyglucose (Figures 7A and 7B). The increase in ADP/ATP induced by 2-deoxyglucose was not affected by A23187 but, surprisingly, a much larger increase in ADP/ATP occurred in the presence of A23187 in cells treated with phenformin (Figure 7C). Despite the contrasting effects of phenformin and 2-deoxyglucose on cellular ADP/ATP ratios, the results in Figure 7 confirm that, under appropriate conditions, both agents are capable of activating AMPK, even in cells completely lacking LKB1.
DISCUSSION
Despite the close similarity in the sequences surrounding Thr172 on AMPK and the equivalent threonine residue on the ARKs (Figure 1), it is clear that the latter are not phosphorylated significantly by CaMKKs either in intact cells (Figures 2 and 3) or in cell-free assays (Figure 4). We have shown previously that HeLa cells lack LKB1 but express both CaMKKα and CaMKKβ, although it was only when CaMKKβ was knocked down using siRNA (small interfering RNA) that the phosphorylation and activation of AMPK in response to Ca2+ ionophores was reduced [13]. Thus it seems that neither CaMKKα nor CaMKKβ are capable of activating any of the ARKs that are expressed in HeLa cells, i.e. SIK1 (SIK), SIK2 (QIK), SIK3 (QSK), NUAK2 (SNARK) or any of the MARKs. Similarly, overexpression of CaMKKβ caused activation of AMPK in HeLa cells, but failed to activate any of the ARKs tested (Figure 3).
BRSK1 (SAD-B) and BRSK2 (SAD-A) are brain-specific kinases that are not expressed in HeLa cells. Fujimoto et al. [38] recently reported a search for proteins from rat brain extracts that interacted with the kinase domain of CaMKKα, and detected both the α1 subunit of AMPK and BRSK1 (SAD-B). They also reported that BRSK1 could be phosphorylated (at Thr189, equivalent to Thr172 on AMPK-α1) and activated by both CaMKKα and CaMKKβ, although the rate of phosphorylation was much lower with CaMKKβ than CaMKKα. The results in Figure 4 confirm that CaMKKα is capable of phosphorylating and activating BRSK1 in cell-free assays. However, in the present study neither BRSK1 nor BRSK2 was significantly phosphorylated by CaMKKβ, although the concentration used was sufficient to fully activate AMPK. It should also be pointed out that the concentration of CaMKKα used to phosphorylate BRSK1 in Figure 4 was approx. 20-fold higher than that required to obtain a comparable level of phosphorylation of AMPK-α1 (results not shown). In addition, AMPK is itself a relatively poor substrate for CaMKKα, with CaMKKα being approx. 7-fold less active against AMPK than CaMKKβ when the activities of the two CaMKKs were normalized using phosphorylation of CaMKI [13]. Thus BRSK1 appears to be a poor substrate for CaMKKα and an even poorer subtrate for CaMKKβ, and we propose that LKB1 is the major upstream kinase for BRSK1 and BRSK2 in vivo. In support of this are results of Barnes et al. [39], who knocked-out LKB1 in pyramidal neurons of the cerebral cortex by crossing mice harbouring a conditional ‘floxed’ allele with mice expressing Cre recombinase from the Emx1 promoter. This almost abolished phosphorylation of BRSK2 (SAD-A) and BRSK1 (SAD-B) at the threonine residues equivalent to Thr172, while not affecting phosphorylation of AMPK at Thr172. This finding can be explained if CaMKKβ (or CaMKKα) provides an alternative upstream pathway for activation of AMPK, but not BRSK1 and BRSK2, in the cerebral cortex. Also in agreement with our results, Bright et al. [40] reported that neither BRSK1 nor BRSK2 was activated in CCL13 cells overexpressing CaMKKβ, while Uboha et al. [41] reported that CaMKK partially purified from mouse cortex (isoform not specified) failed to phosphorylate MARK2 in cell-free assays.
Given that the sequences around the threonine residue in the activation loop are highly conserved between AMPK and the ARKs, it remains unclear why the latter should be phosphorylated and activated by LKB1 complexes, but not by CaMKK-α or -β. Our findings suggest that some feature of the structure of the ARKs, other than the primary sequence of the activation loop, may be required to determine this specificity. This is supported by previous studies showing that a peptide derived from CaMKIV was a very poor substrate for CaMKKα compared with CaMKIV itself [42], and that thermal denaturation of CaMKI or CaMKIV abolished their phosphorylation by CaMKKα [42,43]. Thus the CaMKKs appear to recognize determinants on CaMKI and CaMKIV other than just the primary sequence of the activation loop.
Our findings have important implications regarding the regulation of the ARKs. None of them appears to be interacting with the regulatory β or γ subunits of the AMPK complex [44] and, consistent with this, those that are expressed in skeletal muscle (SIK2, SIK3, MARK2/3 and MARK4) are not phosphorylated or activated when AMPK is activated in muscle by contraction, AICAR or phenformin [8]. The results of the present study show that the ARKs, unlike AMPK itself, are not regulated by increases in cytosolic Ca2+ via activation of CaMKK. That being the case, what are the upstream signals that regulate the ARKs? In most cases this remains unclear, although there is some information available in the case of SIK1. SIK1 was discovered as a protein kinase induced in the adrenal glands of rats fed a high-salt diet [45], and is also induced at the transcriptional level by glucagon and forskolin in primary rat hepatocytes [46]. In Y1 mouse adrenocortical tumour cells treated with adrenocorticotrophic hormone, SIK1 is phosphorylated at a C-terminal site by PKA (protein kinase A), resulting in its translocation to the cytoplasm [47]. Thus SIK1 may be regulated by altered transcription and/or by altered subcellular localization rather than by altered phosphorylation by LKB1.
Anderson et al. [23] recently provided evidence suggesting that CaMKKβ (also known as CaMKK2) can form a stable complex with the AMPK α and β subunits. They expressed FLAG-tagged CaMKKβ in HEK-293 cells and, using anti-FLAG antibodies, showed co-precipitation not only of calmodulin, but also of AMPK α subunits and ACC, the latter two detected using phosphospecific antibodies against Thr172 (AMPK) and Ser79 (ACC). These co-precipitation events appeared to be dependent on Ca2+ because they were not observed in the presence of EDTA and EGTA. Anderson et al. [23] also detected in the anti-FLAG immunoprecipitates the α1, α2, β1 and β2 subunits of AMPK, but not γ1, although they could detect the latter in cell extracts. They therefore proposed that CaMKKβ could replace the γ subunit within the AMPK complex. The results of the present study do not support this concept. When CaMKKβ was overexpressed in HEK-293 cells, we could not detect any AMPK-α, -β or -γ subunits in an immunoprecipitate made using anti-CaMKKβ antibodies, either with or without EDTA and EGTA in the lysis and immunoprecipitation buffers (Figure 5B). In the converse experiment (which was not reported by Anderson et al. [23]) we found that no CaMKKβ was detected in an anti-AMPK-α1/α2 immunoprecipitate, although we could readily detect the α, β and γ subunits of AMPK (Figure 5A). This suggests to us that the AMPK subunits recovered by Anderson et al. [23] in their anti-FLAG immunoprecipitates may have been an artefact caused by the high level of FLAG–CaMKKβ expression in the cells.
We also addressed the same question in a different way, making use of previous findings that the α subunits of AMPK are unstable unless co-expressed with β and γ subunits [35]. This phenomenon can be explained by recent structural studies showing that the C-terminal region of the β subunit forms extensive interactions with the C-terminal domain of α and with the N-terminal region of γ [4]. If these subunits do not find their interaction partners, they may be recognized as misfolded proteins and become degraded. We argued that, if the AMPK α and β subunits can form a complex with CaMKKβ in the absence of the γ subunit, then co-expression with CaMKKβ should stabilize the α subunit in the presence of a β subunit. However, Figure 5(C) shows that this is not the case: in the presence of β1 the γ1 subunit stabilizes the α1 subunit, but CaMKKβ does not substitute for γ1 in this respect. Rather than stabilizing the AMPK α and β subunits, overexpression of CaMKKβ decreased the level of expression of all three AMPK subunits (Figure 5C), perhaps because the transfected DNA was competing for the available biosynthetic machinery.
Finally, the results shown in Figures 6 and 7 show that the two known mechanisms by which phosphorylation of AMPK is activated, i.e. inhibition of dephosphorylation by AMP and increased phosphorylation via activation of CaMKKβ by Ca2+ can, under the right circumstances, be synergistic. They also provide an explanation for our previous findings [5] that agents that cause increases in AMP (e.g. phenformin) or that are converted into an AMP mimetic (AICAR) do not increase phosphorylation and activation of AMPK in HeLa cells. The basal rate of phosphorylation of Thr172 in these cells, which lack LKB1 and would only have the Ca2+-independent activity of CaMKKβ, appears to be too low for any effect of increased AMP on dephosphorylation to become evident. However, if CaMKKβ is activated using the Ca2+ ionophore A23187 at sub-optimal concentration (3 μM), the effect of a rise in AMP to inhibit dephosphorylation now becomes apparent (Figures 6 and 7). In agreement with our findings, Yu et al. [48] have recently reported that A23187 and AICAR act in an additive manner to inhibit cell proliferation and/or survival in HeLa cells.
A somewhat unexpected finding shown in Figure 7 was that, although treatment with 3 μM A23187 alone did not significantly increase the ADP/ATP ratio, when combined with 10 mM phenformin it caused a much larger increase in this ratio than that caused by phenformin alone. By contrast, 2-deoxyglucose treatment increased ADP/ATP, but there was no additional effect when it was added together with A23187. Phenformin is an inhibitor of complex I of the respiratory chain [49,50], thus potentially causing inhibition of mitochondrial ATP synthesis, whereas A23187 is a Ca2+ ionophore that causes release of cytosolic Ca2+, potentially increasing ATP turnover due to activation of Ca2+ pumps. Although 2-deoxyglucose is often described as an inhibitor of glycolysis, its major effect may be to accelerate ATP consumption, because it is rapidly phosphorylated within the cell to 2-deoxyglucose 6-phosphate. We propose that a combination of treatments that both inhibit ATP synthesis and accelerate ATP turnover (phenformin and A23187), as opposed to two different treatments that accelerate ATP turnover (2-deoxyglucose and A23187) may make it more difficult for the cells to maintain their energy status. The very large increase in AMPK activity in response to addition of both A23187 and phenformin (Figure 7A) could be explained by an increase in cytosolic Ca2+ coupled with a larger increase in AMP than that caused by 2-deoxyglucose. Whatever the explanation, the results in Figure 7 show that treatments which elevate cellular AMP (phenformin and 2-deoxyglucose) can, under the right circumstances (elevated cytosolic Ca2+), cause increased phosphorylation and activation of AMPK even in cells lacking LKB1. Thus the effect of AMP on phosphorylation of AMPK is independent of the upstream kinase utilized.
Increased cytosolic Ca2+ tends to switch on many processes that consume ATP, such as operation of ion pumps or channels, interaction of motor proteins, and membrane trafficking and secretion. The ability of Ca2+ and AMP to additively activate AMPK to restore cellular energy balance may therefore have physiological relevance.
AUTHOR CONTRIBUTION
Sarah Fogarty designed and performed the experiments in Figures 1–5. Simon Hawley designed and performed the experiments in Figures 6, 7(A) and 7(B). Kavin Green designed and performed the experiments in Figure 7(C). Nazan Saner and Kirsty Mustard made the FLAG-tagged CaMKKβ construct and performed pilot experiments for Figure 5. Grahame Hardie directed the project and wrote the first draft of the manuscript.
ACKNOWLEDGEMENTS
We are very grateful for antibodies against SIK1, SIK2, SIK3, NUAK2, MARK1 and MARK4, as well as the GST–CaMKKα protein, which were supplied by the antibody and protein production teams of the DSTT (Division of Signal Transduction Therapy) co-ordinated by Hilary McLauchlan and James Hastie.
FUNDING
This work was supported by the Wellcome Trust [grant number 080982 (Programme Grant)]; the EXGENESIS Integrated Project of the European Commission [grant number LSHM-CT-2004-005272]; and by the pharmaceutical companies (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KGaA and Pfizer) which support the DSTT. S.F. was supported by a Wellcome Trust 4-year Studentship.
References
- 1.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Hardie D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 3.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao B., Heath R., Saiu P., Leiper F. C., Leone P., Jing C., Walker P. A., Haire L., Eccleston J. F., Davis C. T., et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 5.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto K., Goransson O., Hardie D. G., Alessi D. R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 9.Davies S. P., Helps N. R., Cohen P. T. W., Hardie D. G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 10.Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Jiao Z. H., Zheng L. S., Zhang Y. Y., Xie S. T., Wang Z. X., Wu J. W. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- 13.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 16.Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- 17.Thornton C., Sardini A., Carling D. Muscarinic receptor activation of AMP-activated protein kinase inhibits orexigenic neuropeptide mRNA expression. J. Biol. Chem. 2008;283:17116–17122. doi: 10.1074/jbc.M708987200. [DOI] [PubMed] [Google Scholar]
- 18.Stahmann N., Woods A., Carling D., Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol. Cell. Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horman S., Morel N., Vertommen D., Hussain N., Neumann D., Beauloye C., El Najjar N., Forcet C., Viollet B., Walsh M. P., et al. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J. Biol. Chem. 2008;283:18505–18512. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 20.Tamas P., Hawley S. A., Clarke R. G., Mustard K. J., Green K., Hardie D. G., Cantrell D. A. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 22.Jaleel M., McBride A., Lizcano J. M., Deak M., Toth R., Morrice N. A., Alessi D. R. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Anderson K. A., Ribar T. J., Lin F., Noeldner P. K., Green M. F., Muehlbauer M. J., Witters L. A., Kemp B. E., Means A. R. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Towler M. C., Fogarty S., Hawley S. A., Pan D. A., Martin D., Morrice N. A., McCarthy A., Galardo M. N., Meroni S. B., Cigorraga S. B., et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem. J. 2008;416:1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 25.Salt I. P., Celler J. W., Hawley S. A., Prescott A., Woods A., Carling D., Hardie D. G. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem. J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods A., Salt I., Scott J., Hardie D. G., Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 27.Goransson O., McBride A., Hawley S. A., Ross F. A., Shpiro N., Foretz M., Viollet B., Hardie D. G., Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J. Biol. Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapkota G. P., Deak M., Kieloch A., Morrice N., Goodarzi A. A., Smythe C., Shiloh Y., Lees-Miller S. P., Alessi D. R. Ionizing radiation induces ataxia telangiectasia mutated kinase (ATM)-mediated phosphorylation of LKB1/STK11 at Thr-366. Biochem. J. 2002;368:507–516. doi: 10.1042/BJ20021284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durocher Y., Perret S., Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie D. G., Salt I. P., Davies S. P. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol. Biol. 2000;99:63–75. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- 31.Dale S., Wilson W. A., Edelman A. M., Hardie D. G. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 1995;361:191–195. doi: 10.1016/0014-5793(95)00172-6. [DOI] [PubMed] [Google Scholar]
- 32.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingo S. N., Gallardo T. D., Akbay E. A., Liang M. C., Contreras C. M., Boren T., Shimamura T., Miller D. S., Sharpless N. E., Bardeesy N., et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyck J. R. B., Gao G., Widmer J., Stapleton D., Fernandez C. S., Kemp B. E., Witters L. A. Regulation of the 5′-activated protein kinase activity by the noncatalytic β and γ subunits. J. Biol. Chem. 1996;271:17798–17803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- 36.Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 37.Hardie D. G., Hawley S. A. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto T., Yurimoto S., Hatano N., Nozaki N., Sueyoshi N., Kameshita I., Mizutani A., Mikoshiba K., Kobayashi R., Tokumitsu H. Activation of SAD kinase by Ca2+/calmodulin-dependent protein kinase kinase. Biochemistry. 2008;47:4151–4159. doi: 10.1021/bi702528r. [DOI] [PubMed] [Google Scholar]
- 39.Barnes A. P., Lilley B. N., Pan Y. A., Plummer L. J., Powell A. W., Raines A. N., Sanes J. R., Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Bright N. J., Carling D., Thornton C. Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J. Biol. Chem. 2008;283:14946–14954. doi: 10.1074/jbc.M710381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uboha N. V., Flajolet M., Nairn A. C., Picciotto M. R. A calcium- and calmodulin-dependent kinase Ialpha/microtubule affinity regulating kinase 2 signaling cascade mediates calcium-dependent neurite outgrowth. J. Neurosci. 2007;27:4413–4423. doi: 10.1523/JNEUROSCI.0725-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuno S., Kitani T., Fujisawa H. Studies on the substrate specificity of Ca2+/calmodulin-dependent protein kinase kinase α. J. Biochem. (Tokyo) 1997;122:337–343. doi: 10.1093/oxfordjournals.jbchem.a021758. [DOI] [PubMed] [Google Scholar]
- 43.Hook S. S., Means A. R. Ca2+/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- 44.Al-Hakim A. K., Goransson O., Deak M., Toth R., Campbell D. G., Morrice N. A., Prescott A. R., Alessi D. R. 14-3-3 cooperates with LKB1 to regulate the activity and localisation of QSK and SIK. J. Cell Sci. 2005;118:5561–5573. doi: 10.1242/jcs.02670. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z., Takemori H., Halder S. K., Nonaka Y., Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Lett. 1999;453:135–139. doi: 10.1016/s0014-5793(99)00708-5. [DOI] [PubMed] [Google Scholar]
- 46.Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1114. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 47.Takemori H., Katoh Y., Horike N., Doi J., Okamoto M. ACTH-induced nucleocytoplasmic translocation of salt-inducible kinase. Implication in the protein kinase A-activated gene transcription in mouse adrenocortical tumor cells. J. Biol. Chem. 2002;277:42334–42343. doi: 10.1074/jbc.M204602200. [DOI] [PubMed] [Google Scholar]
- 48.Yu S. Y., Chan D. W., Liu V. W., Ngan H. Y. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP-activated protein kinase. Tumour Biol. 2009;30:80–85. doi: 10.1159/000216843. [DOI] [PubMed] [Google Scholar]
- 49.Owen M. R., Doran E., Halestrap A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 50.El-Mir M. Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]