Abstract
Background/Objective:
To evaluate an implantable guidance channel (GC) seeded with autologous Schwann cells to promote regeneration of transected spinal nerve root axons in a primate model.
Methods:
Schwann cells were obtained from sural nerve segments of monkeys (Macaca fascicularis; cynomolgus). Cells were cultured, purified, and seeded into a PAN/PVC GC. Approximately 3 weeks later, monkeys underwent laminectomy and dural opening. Nerve roots of the L4 through L7 segments were identified visually. The threshold voltage needed to elicit hindlimb muscle electromyography (EMG) after stimulation of intact nerve roots was determined. Segments of 2 or 3 nerve roots (each ∼8–15 mm in length) were excised. The GC containing Schwann cells was implanted between the proximal and distal stumps of these nerve roots and attached to the stumps with suture. Follow-up evaluation was conducted on 3 animals, with survival times of 9 to 14 months.
Results:
Upon reexposure of the implant site, subdural nerve root adhesions were noted in all 3 animals. Several of the implanted GC had collapsed and were characterized by thin strands of connective tissue attached to either end. In contrast, 3 of the 8 implanted GC were intact and had white, glossy cables entering and exiting the conduits. Electrical stimulation of the tissue cable in each of these 3 cases led to low-threshold evoked EMG responses, suggesting that muscles had been reinnervated by axons regenerating through the repair site and into the distal nerve stump. During harvesting of the GC implant, sharp transection led to spontaneous EMG in the same 3 roots showing a low threshold to electrical stimulation, whereas no EMG was seen when harvesting nerve roots with high thresholds to elicit EMG. Histology confirmed large numbers of myelinated axons at the midpoint of 2 GC judged to have reinnervated target muscles.
Conclusions:
We found a modest rate of successful regeneration and muscle reinnervation after treatment of nerve root transection with a Schwann cell–seeded, implanted synthetic GC. Newer treatments, which include the use of absorbable polymers, neurotrophins, and antiscar agents, may further improve spinal nerve regeneration for repair of cauda equina injury.
Keywords: Spinal cord injuries; Primates; Schwann cells; Guidance channels; Regeneration, axonal; Reinnervation, muscle; Cauda equina
INTRODUCTION
Traumatic injuries to the caudal thoracic and/or lumbar spine account for approximately 15% of admissions for spinal cord injury (1–4). Because the caudal margin of the spinal cord lies at approximately the first lumbar level (L1), these fractures often lead to compression and/or laceration of the conus medullaris and the nerve roots giving rise to the cauda equina. No effective method exists for repair of the disrupted spinal nerve roots (5,6).
In animals, attempts to repair axotomized nerve roots through surgical anastomosis date back more than a century (7–12), but the success rate of these repairs has been typically low. Attempts to surgically repair the injured cauda equina in humans have been reported (13–17). Although some authors suggested a surgically induced improvement in lower extremity and urethral sphincter function (16,18), these reports were hampered by limited details of the degree and time course of improvements and by the small sample size.
Recent advances in biomaterials and tissue culture techniques may lead to improved methods for nerve reconstruction (19–21). Guidance channels (GC) seeded with Schwann cells have been reported to support regeneration of central (22–25) and peripheral (26–31) axons in numerous rat studies and have supported regeneration of peripheral nerves in the primate (32). A summary of clinical trials using GC implants for repair of human peripheral nerve has recently appeared (33). There are no studies of spinal root regeneration using this approach in the primate.
This study was designed to test the feasibility of bridging gap lesions in spinal nerve roots in a primate species using a semipermeable GC seeded with autologous Schwann cells. Our intent was to determine whether such a device could sustain axonal regeneration and muscle reinnervation; detailed behavioral and labeling studies were not carried out.
METHODS
Schwann cells were harvested from 7 adult monkeys (Macaca fascicularis; cynomolgus) of between 5 and 7.5 kg presurgical weights. Animals were anesthetized (10 mg kg−1 ketamine; 0.5 mg kg−1 xylazine); the left hindlimb was shaved, draped, and prepped; and a 5-cm segment of sural nerve was harvested for purification and expansion of Schwann cells. The lumbosacral enlargement was also exposed on 4 animals, and nerve root testing was carried out, as described below. These 4 animals were then killed without further study. A primary reason for these primate studies was to ultimately develop a viable protocol for purification and expansion of human Schwann cells (34–36), based on results from these initial efforts with primate cells. The other 3 animals were allowed to survive, and nerve repair attempts were carried out, as reported herein.
Schwann cells were purified and expanded, which took at least 3 weeks from the time of nerve harvest. Guidance channels were loaded with Schwann cells suspended in a 2:1 mixture of Matrigel:Dulbecco's Modified Eagle Medium (DMEM) (26). Channels were formed from 60:40 acrylonitrile vinyl chloride copolymer and were characterized by a smooth semipermeable inner layer trabecular wall with a nominal molecular weight of 50,000 Da (37). The inner diameter of the GC was approximately 0.85 mm. The filled channels were incubated overnight in a culture dish with DMEM at 37°C. During this incubation, the Schwann cells within the implant elongated, producing a cellular cable consisting of the Schwann cells aligned longitudinally, in parallel with the long axis of the channel (26).
On the day after seeding of multiple GC, each of the 3 animals receiving implants of autologous Schwann cells was anesthetized, intubated, and artificially ventilated. A forelimb catheter was placed for intravenous anesthesia. A constant infusion of propofol (200–400 μg kg−1 min−1) was established, supplemented with intermittent bolus injections of fentanyl. The animal was positioned prone on top of a water-circulating heating pad for maintenance of core temperature. Oxygen saturation and heart rate were monitored with a pulse-oximeter probe placed on the tongue.
A dorsal midline incision was made, followed by soft-tissue stripping and a lumbar laminectomy, preserving the pars to minimize risk of bony deformity. The laminectomy extended from L2 to the lumbosacral junction (the junction was used to confirm root levels). The dura was incised along the midline and reflected.
Individual nerve roots were identified based upon their position within the thecal sac (dorsal vs ventral; medial vs lateral) and their foraminal exit points. Roots were gently teased apart with a pair of blunt nerve hooks. Upon identification of a targeted nerve root, it was transected within approximately 1 cm of its origin from the spinal cord. A second transection was made about 8 to 15 mm distally, leaving distinct gaps in the nerve root (the roots would retract slightly, increasing the gap space beyond the length of the excised nerve segment). A piece of the Schwann cell/GC construct was trimmed to a length approximately 2 mm longer than the excised nerve segment, and the proximal and distal ends of the transected root were coaxed into the GC with sharp forceps. Due to immediate swelling of the cut nerve root, it remained inserted within the GC in most cases. Nevertheless, a single 10–0 suture was tied through each end of the GC/nerve root stump. In several cases, the suture tore out of the root and was retied. Once all targeted roots had been grafted (2 to 3 per animal), an electrocautery was used to minimize intrathecal hemorrhage, and the dura was closed with a 6–0 suture. Tissues were closed in layers, propofol infusion was discontinued, and the monkey was extubated once spontaneous breathing had resumed. Standard postoperative care (analgesics; antibiotics) was administered in accordance with the approved animal care protocol.
The survival times of the 3 animals implanted varied from 9 to 14 months. In a terminal study, the dorsal incision was reopened, the original nerve grafts were exposed, nerve conduction was tested, and the GC constructs were resected for examination. The monkeys were then killed by lethal injection.
Electrophysiology
For intraoperative electrophysiology, pairs of half-inch noninsulated stainless steel needle electrodes were positioned percutaneously above or within lower limb muscles and connected to preamplifiers for recording electromyogram (EMG). For 2 of the 3 animals, the muscles recorded were gluteal, quadriceps, hip adductors, lateral hamstring, medial hamstring, tibialis anterior (TA), medial gastrocnemius (MG), and abductor hallucis (AbH, an intrinsic muscle of the hindpaw); in the other animal EMG was recorded only from the quadriceps, TA, MG, and AbH. Preamplifier outputs were amplified and filtered (2024F, Intronix Technologies Corp, Bolton, Ontario, Canada; gain = 1,000; filter = 100 Hz to 2.5 kHz) and stored on magnetic tape (PCM 4000, Vetter Pharma International GmbH, Rebersburg, Germany) for later analysis. At the same time, EMG was displayed both visually (RC Electronics Computerscope) and audibly. After completing nerve root implantation or harvesting on one side, EMG leads were shifted to the opposite hindlimb for testing of contralateral nerve root(s).
A Grass S88 stimulator (West Warwick, Rhode Island) was used to elicit stimulus-evoked EMG, using an insulated monopolar nerve hook (cathode). A needle electrode in the soft tissue at the proximal end of the incision served as the anode. Stimulus duration was fixed at 200 μs; stimulus intensity was varied from less than 0.1 volt (V) to a maximum of 20 V. Stimulus rate was between 2 and 3 pulses per second. Motor thresholds were established by slowly increasing the stimulus intensity until a response was seen from one or more muscles.
Histology
After their surgical removal, nerve roots were immersed in 4% paraformaldehyde 0.1M sodium phosphate buffer (pH 7.4) overnight at 4°C and then postfixed in 2% glutaraldehyde in 0.1M phosphate buffer (pH 7.4) for 7 days (also at 4°C). Next, the nerves were osmicated in 1% osmium tetroxide in phosphate buffer (pH 7.4) overnight at 4°C. The fixed nerves were dehydrated and embedded in Epon/Araldite resin for sectioning. One-micron transverse sections were prepared and stained with aqueous 1% toluidine blue.
RESULTS
Schwann cells were successfully isolated and expanded from all 7 monkeys. The purity of expanded Schwann cells in the first 2 animals studied was approximately 45%. Purity values in excess of 90% were seen in later animals as improved techniques for isolating and expanding primate Schwann cells were developed (32,35). Typical concentrations of Schwann cells in the latest implants were 1.2 × 108 cells/mL.
The minimum voltage needed to elicit EMG from one or more hindlimb muscles upon intradural stimulation of intact ventral roots ranged from 0.15 to 0.22 V (mean = 0.18 ± 0.02 V; n = 25). Thresholds did not differ significantly by root level. Minimum EMG-evoked response latencies increased for more distal muscles (quads = 4.4 ± 1.1 ms; TA = 6.4 ± 0.6 ms; MG = 7.1 ± 1.1 ms; AbH = 9.8 ms [n = 1]).
Surgical manipulation to isolate nerve roots often led to brief periods of spontaneous EMG, as reported for human surgery cases (38,39). Transection of a given ventral root for GC implantation caused a brief burst of EMG from multiple muscles. After graft implantation but before dural closure, the threshold voltage needed to elicit EMG for stimulation of the proximal end of the (now-grafted) nerve root was determined for 2 implants. For both roots, the pretransection threshold was 0.17 V; these thresholds increased to 4.4 V and 4.8 V immediately after implantation of the GC. Because each root had just been transected and its proximal stump was being stimulated, we interpreted the presence of an evoked response at this much higher stimulus intensity as reflecting current spread to nearby, intact ventral root axons.
Postoperative bowel and bladder function in each monkey was normal at all times, and no animal showed evidence of neuropathic pain or dysesthesia over the 9- to 14-month survival times. For voluntary movements, the hindlimbs in which ventral roots were lesioned appeared to be weakened in the first few days after surgery, but in all cases movement and activity levels were indistinguishable (by visual inspection) from those of intact animals by approximately 1 week after injury. This movement recovery was not surprising for 2 of the 3 animals because only one ventral root on either side was lesioned, and extensive overlap of myotomes for adjacent ventral roots has been described (40–42). The one animal with injury to 2 (nonadjacent) roots on the same side would probably have demonstrated hindlimb weakness if faced with more challenging movement requirements than allowed by the animal housing facility.
Follow-up examinations of 8 nerve root/GC implants (7 ventral roots and 1 dorsal root) are summarized in Table 1. Repair numbers 1 to 3 were from animal no. 3, repair numbers 4 and 5 were from animal no. 6, and repair numbers 6 to 8 were from animal no. 7. Note that the one dorsal root repair attempt (no. 3) was associated with a high (4.0 V) threshold for stimulus-evoked EMG. This high threshold is to be expected because any EMG would have been reflex in nature; stimulation of this dorsal root with a brief pulse train would likely have evoked EMG.
Table 1.
Follow-up Examinations of Eight Nerve Root/Guidance Channel Implants
A common observation upon surgical reexposure in all animals was that there was extensive subdural scar tissue, and nerve roots that had been grafted were adherent to adjacent, noninjured nerve roots. Not surprisingly, during dissection to isolate the grafted roots, there were frequent and prolonged bursts of EMG from hindlimb muscles being monitored. This EMG could not be considered as proof of reinnervation by grafted roots, because adherent but nongrafted (ie, intact) roots could have been the source of action potential activity.
Of the 8 GCs implanted, tissue on both ends of the channel appeared glossy (ie, healthy) in 3 cases; each of these implants maintained a cylindrical shape along the length of the GC. In contrast, 3 other GC appeared to have collapsed either along the entire length or at one end. The 2 remaining GC neither were collapsed nor did they have what appeared to be healthy root tissue exiting. Instead, these implants were ensheathed within dense connective tissue.
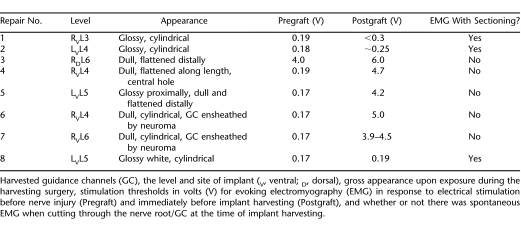
Examples of cylindrical and collapsed GC are shown in Figure 1 (Repair No. 1 to 3 listed in Table 1). These implants were harvested 9 months after grafting. Both grafted ventral roots appeared thick and shiny (Figure 1A and B), suggesting they contained myelinated axons. The right L6 GC (used on a dorsal root) was collapsed distally (Figure 1C; distal to the left).
Figure 1. Guidance channels (GC) (Repair No. 1 to 3 from Table 1) recovered after a 9-month period of implantation showing evidence of myelinated nerve fibers entering and emerging from the GC in the top 2 panels (A and B) but only thin connective tissue attached to the GC in the lower panel (C). The original implant lengths were 14, 17, and 12 mm for the roots shown in Figure 1A, B, and C, respectively.
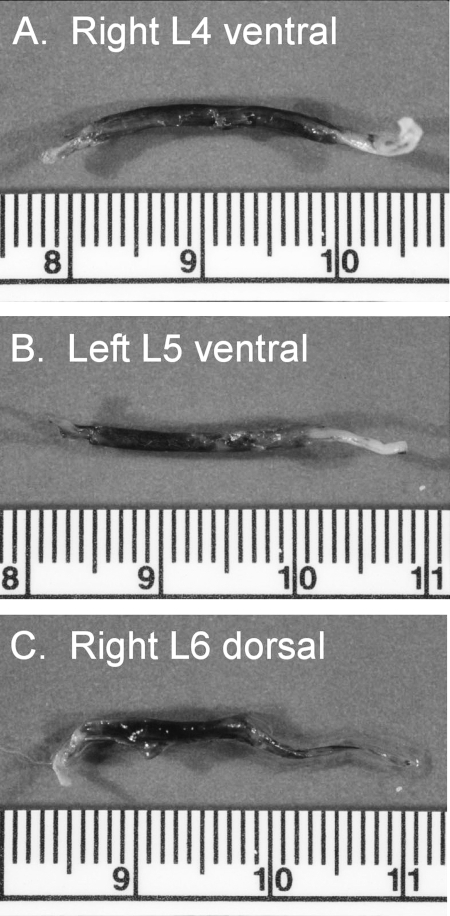
Figure 2 shows cross-sections made within the GC depicted in Figure 1. For Figure 2A and B, this section was made approximately 3 mm from the distal end of the GC. For Figure 2C, the section was at the midpoint of the GC. There were hundreds of myelinated axons near the distal end of each of the ventral root GC (Figure 2A and B). Conversely, Figure 2C shows that there were no myelinated nerve fibers near the midpoint of the GC in the grafted L6 dorsal root.
Figure 2. Cross-sections of the same 3 guidance channels illustrated in Figure 1. Many myelinated axons are evident in the 2 ventral roots (panels A and B), but only connective tissue can be seen in the lower panel (taken from a dorsal root, which had collapsed distally).
Although the GC/ventral root constructs shown in Figures 1 and 2 appeared to contain regenerated and myelinated axons, exact thresholds for eliciting EMG upon stimulation of these nerve constructs could not be established because of a failure in the voice record of the tape-recorded data. From notes made at the time of testing, the threshold for the R L4 implant was < 0.3 V and the left L5 implant threshold was approximately 0.25 V. These values are only slightly higher than pregraft thresholds, suggesting that motor axons regenerated through and beyond the implanted GC to reinnervate multiple muscles of the monkey's lower limb. In support of this conclusion, cutting through these nerve roots distal to the GC led to spontaneous EMG in hindlimb muscles (not shown).
In the second animal from which implanted GC were harvested (14-month survival), neither implant appeared to support successful regeneration of axons (Repair No. 4 and 5 in Table 1). Both implants were collapsed, one along its entire length and the other at its distal end. A hole through the GC was visible in the right L4 ventral root (Repair No. 4 in Table 1). For these implants, as well as for those from the final animal discussed in the following paragraph, tissue samples were damaged when freezer contents were moved between Miami Project buildings, precluding histologic examination. As shown in Table 1, EMG was evoked when stimulating these roots at the relatively high threshold values of 4.7 and 4.2 V, values consistent with current spread to neighboring, intact nerve roots. Finally, there was no EMG from any hindlimb muscles during nerve root transection and harvesting of these 2 implants, suggesting failure of any muscle reinnervation by motor axons.
In the final survival animal, 3 nerve roots were injured and repaired with GC implants. After a 10-month survival period, one of the 3 treated roots (Repair No. 8 in Table 1) appeared to have sustained regeneration of ventral root axons. Stimulation of this root elicited EMG at a threshold intensity of only 0.19 V. Examples of the evoked responses can be seen in Figure 3A, in which repeated stimuli (0.25 V) were delivered at times indicated by vertical arrows. All but the TA and AbH muscles demonstrated repeated M-waves in response to each stimulus.
Figure 3. Evoked electromyography (EMG) (A) from multiple lower limb muscles in response to repeated electrical stimulation of guidance channel (GC) no. 8 before resection. Stimuli at arrows at bottom of record; note that stimulus artifacts can just be made out in some of the traces and are best seen in records from the lateral hamstring group. All but the tibialis anterior and abductor hallucis muscles discharge at short latency in response to each of the weak stimuli (0.25 V) used. Panel B shows spontaneous EMG caused by surgical transection of the guidance channel. The initial cut (first arrow at bottom of record) failed to transect the GC, whereas the second cut (second arrow) transected the remainder of the soft tissue passing through the GC. Each “cut” was associated with a burst of EMG from the same hindlimb muscles that responded to electrical stimulation (ie, panel A of this figure). Note the difference in time base for panels A and B. The vertical line denotes 1 mV for all records in A and B. Quads, quadriceps; Hip add, hip adductors; Lat Hams, lateral hamstring; Med Hams, medial hamstring; Tib Ant, tibialis anterior; Med Gastroc, medial gastrocnemius; Abd Hallucis, abductor hallucis.
The most proximal muscle groups studied (gluteals and quadriceps) had identical response latencies of 8.7 ms, roughly 2 times those seen with stimulation of intact, uninjured nerve roots. The most distal muscle showing responses (MG) had a minimum onset latency of 10.4 ms, still prolonged from intact animals, but not by as large a factor as for the gluteal and quadriceps latencies.
An interesting rhythmic discharge pattern was noted in the hip adductors for some stimulus trials. This is best seen after the second and fourth stimuli of the record, in the third trace from the top of Figure 3A. For the second stimulus shown, there was an initial EMG burst (ie, M-wave) followed by repeated smaller-amplitude bursts, with interburst intervals of 72, 64, 66, 71, and 59 ms (as measured between cursors evident as thin vertical lines between the second and third stimuli of the record). With an average interburst interval of 66.4 ms, this corresponds to a mean frequency of approximately 15 Hz.
Figure 3B shows that when this nerve root was transected, there were bursts of EMG for each of the cuts made by the microscissors (arrows show the approximate timing of cuts, based on the voice record). Note also that the same 6 muscles showing EMG activity when cutting through the graft to harvest the implant were also those that had responded to low-intensity electrical stimulation (Figure 3A). Based on electrophysiology alone (because histology was not available for this nerve root), it appears that ventral root axons successfully regenerated through this implant and reinnervated multiple muscles of the hindlimb.
The 2 other implants in this animal (Repair No. 6 and 7 in Table 1) did not appear to sustain regeneration, based on implant appearance (ensheathed by thick connective tissue appearing as a neuroma, with thin, dull strands of tissue entering and exiting the GC), high stimulus thresholds to elicit EMG, and an absence of spontaneous EMG in any hindlimb muscles when either graft was transected for harvesting.
DISCUSSION
Fracture of the distal thoracic or lumbar spine can damage the caudal spinal cord (ie, the conus medularis), the spinal nerves (ie, the cauda equina), or some combination of the two. Loss of strength, sensation, and bowel/bladder function are common for more severe injuries, as is an early onset of neuropathic pain (43–45). If significant functional recovery has not emerged by 6 months after injury, the probability of major improvement beyond this time point diminishes considerably (46–49). Surgical treatment of the fracture is common (13,14,50–53), but at the present, attempts to repair damaged nerve roots (5,16,17) or the spinal cord (18) in humans are rare.
Unlike “pure” spinal cord injury, the paralysis associated with thoracolumbar injury is often of the flaccid variety, characterized by profound muscle atrophy and an absence of involuntary muscle spasms. Unable to reinnervate muscle fibers, many motoneurons degenerate after axotomy, but some survive (54–58). Therefore, it seems reasonable to attempt nerve root repair after this type of injury. Restoring innervation to target muscles (in the limbs or sphincters) would likely lead to behavioral recovery, because communication would be maintained between higher motor centers and the motoneurons whose axons had successfully regenerated.
Such a concept for repair dates back close to a century, but results from earlier studies have been disappointing (9,13–18,59). Only in the cases reported by Carlsson and Sundin (16) and Cheng et al (18) (for spinal cord) is there plausible evidence that recovery of function was due to nerve regeneration after intrathecal repair. After transection, spinal roots retract, making it nearly impossible to achieve surgical anastomosis, especially because the roots lack mechanical strength. In the few reports of surgical exploration of the injured human cauda equina, widespread subdural adhesions and arachnoiditis were found, even as early as 1 week after injury (17,50,60–63). This was true whether the dura was intact or lacerated. Nerve roots tended to bundle into a dense tangle, limiting the opportunity for regeneration. In addition to significant scar formation, an absence of neurotrophins to promote axonal elongation is now known to be another hindrance to regeneration and spontaneous recovery (9,17,64).
The present proof-of-concept study addressed several of these issues. Implantation of a biocompatible GC was designed to provide a protected environment for regenerating axons to bridge the nerve root gap. Autologous Schwann cells were added to provide a cellular substrate for axonal growth and to produce neurotrophins in higher concentrations than would otherwise be encountered by the regenerating axons (20,65–67). Evidence from this study suggests that this approach promoted successful regeneration in 3 of the 8 nerve roots (38%) in which repair was attempted.
Our data suggest reinnervation was successful, but absolute proof is lacking, reflecting the preliminary nature of this investigation. Injection of potential target muscles with a retrograde label, such as Fast Blue or Fluoro-Gold/Fluoro-Ruby, followed by demonstration that the label product was present within axons at the midpoint of the GC implant would provide such proof.
The rhythmic contraction pattern of the hip adductors shown in Figure 3B warrants further comment. We suggest this may be an example of clonus. The much higher frequency of this pattern in this animal preparation compared with the usual 6 to 7 Hz seen in humans probably reflects the much shorter conduction distances involved. If correct, this would suggest some ongoing cord abnormality, because clonus would not be expected after a pure nerve root lesion. Increased excitability of rat motoneurons has been demonstrated after avulsion of neighboring ventral roots (68).
The experiments described in this report were carried out in the early-to-mid 1990s. Since that time, there have been dramatic advances in biomaterials and delivery devices (19,69,70). A wider variety of neurotrophins has been identified and is now available to support motoneuron survival and axonal elongation (66,71,72). Fixation between nerve root and GC with fibrin glue (eg, Tisseel [73]) may ameliorate problems with root detachment encountered in this and other studies (9,17,64). Agents to minimize dural adhesions are being evaluated (74–76), as are methods to overcome myelin-derived and other growth-inhibitory molecules, such as chondroitin sulfate proteoglycans (eg, chondroitinase ABC) (77–81). Schwann cells derived from motor-specific axons (ie, ventral roots) appear to be better suited for sustaining motor axon elongation compared with those originating in the more traditional donor nerves (eg, sural) (82). In the dog, studies of bladder reinnervation after nerve root transection and repair using some of these newer strategies are promising (83,84). Finally, clinical trials of peripheral nerve repair with synthetic GC are under way, with encouraging results, provided that repair distances are short and resorbable GC are used (33). This latter point may be very important; GC collapse was seen in 3 of the 8 implants from this study, and it has been reported in other studies utilizing nonresorbable nerve guides (32,85).
CONCLUSIONS
There has been an explosion of animal-based research into the mechanisms of and recovery from spinal cord injury. Yet viable clinical trials are few, and the prospects for useful treatments emerging within the next 5 years are slim. Conversely, we believe that a subset of the spinal cord injury population (those with damage to the cauda equina) could benefit from the judicious application of existing technologies to promote ventral root regeneration and muscle reinnervation. Findings from the present study represent a small step towards this goal.
Acknowledgments
Comments on this manuscript were kindly provided by Dennis Stelzner. We thank William Puckett for photographing the histologic sections and Margaret Bates for performing the histologic preparation.
Footnotes
This work was supported by NINDS Grant NS 009923, National Institute of Neurological Disorders and Stroke, Bethesda, MD; The Miami Project to Cure Paralysis, Miami, FL; and the Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, FL.
REFERENCES
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24 suppl):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med. 2005;28(2):92–96. doi: 10.1080/10790268.2005.11753804. [DOI] [PubMed] [Google Scholar]
- Spinal cord injury. Facts and figures at a glance. J Spinal Cord Med. 2008;31(3):357–358. [PubMed] [Google Scholar]
- Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2007;35(4):529–531. doi: 10.1002/mus.20696. [DOI] [PubMed] [Google Scholar]
- Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol. 2004;12(3–4):343–358. doi: 10.1016/j.trim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Harrop JS, Hunt GE, Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus. 2004;16(6):e4. doi: 10.3171/foc.2004.16.6.4. [DOI] [PubMed] [Google Scholar]
- Moyer EK, Kimmel DL. The repair of severed motor and sensory spinal nerve roots by the arterial sleeve method of anastomosis. J Comp Neurol. 1948;88(2):285–317. doi: 10.1002/cne.900880205. [DOI] [PubMed] [Google Scholar]
- Tarlov IM, Berman D. Cauda equina nerve root regeneration through nerve grafts: an experimental study in monkeys. J Nerv Ment Dis. 1949;110(2):161–164. [PubMed] [Google Scholar]
- Freeman LW. Observations on spinal nerve root transplantation in the male guinea baboon. Ann Surg. 1952;136(2):206–210. doi: 10.1097/00000658-195208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C, Sollmann H. Regeneration of cauda equina fibres after transsection and end-to-end suture: light and electron microscopic study in the pig. J Neurol. 1977;215(2):81–90. [PubMed] [Google Scholar]
- Conzen MA, Sollmann H. Reinnervation of the urinary bladder after microsurgical reconstruction of transsected caudal fibres: an experimental study in pigs. Urol Res. 1982;10(3):141–144. doi: 10.1007/BF00255957. [DOI] [PubMed] [Google Scholar]
- Conzen MA, Sollmann H. Reinnervation after microsurgical repair of transected cauda equina fibres. Neurochir Stuttg. 1985;28(1):6–7. doi: 10.1055/s-2008-1054171. [DOI] [PubMed] [Google Scholar]
- Kaufer H, Hayes JT. Lumbar fracture-dislocation: a study of twenty-one cases. J Bone Joint Surg Am. 1966;48(4):712–730. [PubMed] [Google Scholar]
- Gertzbein SD, Court-Brown CM, Marks P, et al. The neurological outcome following surgery for spinal fractures. Spine. 1988;13(6):641–644. [PubMed] [Google Scholar]
- Frazier CH, Mills CK. Intradural root anastomosis for the relief of paralysis of the bladder and the application of the same method in other paralytic affections. JAMA. 1912;LIX(25):2202–2206. [Google Scholar]
- Carlsson CA, Sundin T. Reconstruction of efferent pathways to the urinary bladder in a paraplegic child. Rev Surg. 1967;24(1):73–76. [PubMed] [Google Scholar]
- LeBlanc HJ, Gray LW, Kline DG. Stab wounds of the cauda equina: case report. J Neurosurg. 1969;31(6):683–685. doi: 10.3171/jns.1969.31.6.0683. [DOI] [PubMed] [Google Scholar]
- Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine. 2004;29(14):E284–E288. doi: 10.1097/01.brs.0000131217.61390.2c. [DOI] [PubMed] [Google Scholar]
- Nomura H, Tator CH, Shoichet MS. Bioengineered strategies for spinal cord repair. J Neurotrauma. 2006;23(3–4):496–507. doi: 10.1089/neu.2006.23.496. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Ramer MS, Steeves JD. Setting the stage for functional repair of spinal cord injuries: a cast of thousands. Spinal Cord. 2005;43(3):134–161. doi: 10.1038/sj.sc.3101715. [DOI] [PubMed] [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23(3–4):453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, David S, Bray GM. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95(1):231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell–seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351(1):145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Oudega M, Gautier SE, Chapon P, et al. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22(10):1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Pearse DD. Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev. 2003;40(4):55–62. doi: 10.1682/jrrd.2003.08.0055. [DOI] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semi-permeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12(9):3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- Rodriguez FJ, Verdu E, Ceballos D, Navarro X. Nerve guides seeded with autologous Schwann cells improve nerve regeneration. Exp Neurol. 2000;161(2):571–584. doi: 10.1006/exnr.1999.7315. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B, Muller E, Schroder B, Planck H, Muller HW. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963(1–2):321–326. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- McCormack ML, Goddard M, Guenard V, Aebischer P. Comparison of dorsal and ventral spinal root regeneration through semipermeable guidance channels. J Comp Neurol. 1991;313(3):449–456. doi: 10.1002/cne.903130305. [DOI] [PubMed] [Google Scholar]
- Sinis N, Schaller HE, Schulte-Eversum C, et al. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J Neurosurg. 2005;103(6):1067–1076. doi: 10.3171/jns.2005.103.6.1067. [DOI] [PubMed] [Google Scholar]
- Levi AD, Sonntag VK, Dickman C, et al. The role of cultured Schwann cell grafts in the repair of gaps within the peripheral nervous system of primates. Exp Neurol. 1997;143(1):25–36. doi: 10.1006/exnr.1996.6344. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B, Dreesmann L, Schaller HE, et al. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59(4):740–747. 747–748. doi: 10.1227/01.NEU.0000235197.36789.42. discussion. [DOI] [PubMed] [Google Scholar]
- Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11(8):2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17(4):327–338. doi: 10.1002/(SICI)1098-1136(199608)17:4<327::AID-GLIA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Levi ADO, Bunge RP, Lofgren JA, et al. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15(2):1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebischer P, Guenard V, Valentini RF. The morphology of regenerating peripheral nerves is modulated by the surface microgeometry of polymeric guidance channels. Brain Res. 1990;531(1–2):211–218. doi: 10.1016/0006-8993(90)90776-8. [DOI] [PubMed] [Google Scholar]
- Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine. 2002;27(13):1444–1450. doi: 10.1097/00007632-200207010-00014. [DOI] [PubMed] [Google Scholar]
- Kothbauer K, Schmid UD, Seiler RW, Eisner W. Intraoperative motor and sensory monitoring of the cauda equina. Neurosurgery. 1994;34(4):702–707. 707. doi: 10.1227/00006123-199404000-00020. discussion. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Cornblath DR. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve. 1992;15(6):687–693. doi: 10.1002/mus.880150610. [DOI] [PubMed] [Google Scholar]
- Kothbauer KF, Novak K. Intraoperative monitoring for tethered cord surgery: an update. Neurosurg Focus. 2004;16(2):E8. doi: 10.3171/foc.2004.16.2.1. [DOI] [PubMed] [Google Scholar]
- Phillips LHII, Park TS. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve. 1991;14(12):1213–1218. doi: 10.1002/mus.880141213. [DOI] [PubMed] [Google Scholar]
- Stormer S, Gerner HJ, Gruninger W, et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997;35(7):446–455. doi: 10.1038/sj.sc.3100411. [DOI] [PubMed] [Google Scholar]
- Segatore M. Understanding chronic pain after spinal cord injury. J Neurosci Nurs. 1994;26(4):230–236. doi: 10.1097/01376517-199408000-00007. [DOI] [PubMed] [Google Scholar]
- Tasker RR, DeCarvalho TC, Dolan EJ. Intractable pain of spinal cord origin: clinical features and implications for surgery. J Neurosurg. 1992;77(3):373–378. doi: 10.3171/jns.1992.77.3.0373. [DOI] [PubMed] [Google Scholar]
- Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine. 2001;26(24 suppl):S137–S145. doi: 10.1097/00007632-200112151-00023. [DOI] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. EMG for assessing the recovery of voluntary movement after acute spinal cord injury in man. Clin Neurophysiol. 2004;115(8):1748–1759. doi: 10.1016/j.clinph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Graziani V, Crozier KC, Selby-Silverstein L. Lower extremity function following spinal cord injury. Top Spinal Cord Inj Rehabil. 1996;1(1):46–55. [Google Scholar]
- Rutz S, Dietz V, Curt A. Diagnostic and prognostic value of compound motor action potential of lower limbs in acute paraplegic patients. Spinal Cord. 2000;38(4):203–210. doi: 10.1038/sj.sc.3100979. [DOI] [PubMed] [Google Scholar]
- Landau B, Ransohoff J. Late surgery for incomplete traumatic lesions of the conus medullaris and cauda equina. J Neurosurg. 1968;28(3):257–261. doi: 10.3171/jns.1968.28.3.0257. [DOI] [PubMed] [Google Scholar]
- Campagnolo DI, Esquieres RE, Kopacz KJ. Effect of timing of stabilization on length of stay and medical complications following spinal cord injury. J Spinal Cord Med. 1997;20(3):331–334. doi: 10.1080/10790268.1997.11719484. [DOI] [PubMed] [Google Scholar]
- Fisher RG. Sacral fracture with compression of cauda equina: surgical treatment. J Trauma. 1988;28(12):1678–1680. doi: 10.1097/00005373-198812000-00013. [DOI] [PubMed] [Google Scholar]
- Kohles SS, Kohles DA, Karp AP, Erlich VM, Polissar NL. Time-dependent surgical outcomes following cauda equina syndrome diagnosis: comments on a meta-analysis. Spine. 2004;29(11):1281–1287. doi: 10.1097/00007632-200406010-00019. [DOI] [PubMed] [Google Scholar]
- Brannstrom T, Havton L, Kellerth JO. Changes in size and dendritic arborization patterns of adult cat spinal à-motoneurons following permanent axotomy. J Comp Neurol. 1992;318(4):439–451. doi: 10.1002/cne.903180408. [DOI] [PubMed] [Google Scholar]
- Vanden Noven S, Wallace N, Muccio D, Turtz A, Pinter MJ. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp Neurol. 1993;123(1):147–156. doi: 10.1006/exnr.1993.1147. [DOI] [PubMed] [Google Scholar]
- Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res. 2001;139(2):216–223. doi: 10.1007/s002210100769. [DOI] [PubMed] [Google Scholar]
- Blits B, Carlstedt TP, Ruitenberg MJ, et al. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp Neurol. 2004;189(2):303–316. doi: 10.1016/j.expneurol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McPhail LT, Oschipok LW, Liu J, Tetzlaff W. Both positive and negative factors regulate gene expression following chronic facial nerve resection. Exp Neurol. 2005;195(1):199–207. doi: 10.1016/j.expneurol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Petrov MA. Transplantations of nerves and roots of the spinal cord. Reconstr Surg Traumatol. 1971;12(0):250–262. [PubMed] [Google Scholar]
- Yashon D, Jane JA, White RJ. Prognosis and management of spinal cord and cauda equina bullet injuries in sixty-five civilians. J Neurosurg. 1970;32(2):163–170. doi: 10.3171/jns.1970.32.2.0163. [DOI] [PubMed] [Google Scholar]
- Six E, Alexander E, Jr, Kelly DL, Jr, Davis CH, Jr, McWhorter JM. Gunshot wounds to the spinal cord. South Med J. 1979;72(1):699–702. doi: 10.1097/00007611-197906000-00020. [DOI] [PubMed] [Google Scholar]
- Nakano M, Matsui H, Miaki K, Tsuiji H. Postlaminectomy adhesion of the cauda equina: inhibitory effects of anti-inflammatory drugs on cauda equina adhesion in rats. Spine. 1998;23(3):298–304. doi: 10.1097/00007632-199802010-00003. [DOI] [PubMed] [Google Scholar]
- Ramamurthi B, Ramamurthi R, Narayanan R. Late laminectomy in traumatic paraplegia. Surg Neurol. 1983;20(5):414–416. doi: 10.1016/0090-3019(83)90012-5. [DOI] [PubMed] [Google Scholar]
- Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina: presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine. 1995;20(4):2758–2764. doi: 10.1097/00007632-199512150-00019. [DOI] [PubMed] [Google Scholar]
- Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242(1):S19–S21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol (Lond). 2004;533(1):83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Tetzlaff W. Spinal cord regeneration: from gene to transplants. Spine. 2001;26(24):S13–S22. doi: 10.1097/00007632-200112151-00003. [DOI] [PubMed] [Google Scholar]
- Havton LA, Kellerth JO. Plasticity of lumbosacral monosynaptic reflexes after a ventral root transection injury in the adult cat. Exp Brain Res. 2004 Mar;155(1):111–114. doi: 10.1007/s00221-003-1802-0. epub 2004 Jan 15. [DOI] [PubMed] [Google Scholar]
- Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Stelzner DJ, Garma P, Hasenwinkel JM, et al. Timed release chondroitinase to treat spinal cord injury [abstract] Abstr Soc Neurosci. 2004;619:613. [Google Scholar]
- Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002;2(4):244–258. doi: 10.1124/mi.2.4.244. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24(28):6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am. 1988;19(1):187–199. [PubMed] [Google Scholar]
- Liu S, Boutrand JP, Bittoun J, Tadie M. A collagen-based sealant to prevent in vivo reformation of epidural scar adhesions in an adult rat laminectomy model. J Neurosurg. 2002;97(1 suppl):69–74. doi: 10.3171/spi.2002.97.1.0069. [DOI] [PubMed] [Google Scholar]
- Haq I, Cruz-Almeida Y, Siqueira EB, Norenberg M, Green BA, Levi AD. Postoperative fibrosis after surgical treatment of the porcine spinal cord: a comparison of dural substitutes. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2005(;2(1):50–54. doi: 10.3171/spi.2005.2.1.0050. [DOI] [PubMed] [Google Scholar]
- Akeson WH, Massie JB, Huang B, et al. Topical high-molecular-weight hyaluronan and a roofing barrier sheet equally inhibit postlaminectomy fibrosis. Spine J. 2005;5(2):180–190. doi: 10.1016/j.spinee.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25(5):1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg J, Brösamle C, Metz GA, Schwab ME. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp Neurol. 1998;154(2):583–594. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22(2):226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23(28):9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130(4):926–939. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D'Andrea L, et al. Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J Neurotrauma. 2006;23(7):1125–1136. doi: 10.1089/neu.2006.23.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D'Andrea L, Betz R, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. J Neurotrauma. 2008;25(4):401–409. doi: 10.1089/neu.2007.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Katayama Y, Shoichet MS, Tator CH. Complete spinal cord transection treated by implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery. 2006;59(1):183–192. doi: 10.1227/01.NEU.0000219859.35349.EF. [DOI] [PubMed] [Google Scholar]