Abstract
Background:
Some people with chronic spinal cord injury (SCI) have low vitamin D levels and secondary hyperparathyroidism.
Objective:
To determine whether, and to what extent, an acute calcium infusion decreased levels of N-telopeptide (NTx), a marker of osteoclastic activity, in individuals with chronic SCI.
Study Design:
Case series.
Subjects:
Eight men with chronic SCI. A relatively low serum 25 hydroxyvitamin D concentration (25[OH]D ≤20 ng/mL) and/or a high parathyroid hormone (PTH) (>55 pg/mL) was a prerequisite for study inclusion.
Methods:
Calcium gluconate bolus 0.025 mmol elemental calcium/kg over 20 minutes followed by a constant infusion of 0.025 mmol/kg per hour for 6 hours was infused; blood samples were collected every 2 hours for measurement of serum total calcium, creatinine, NTx, and PTH.
Results:
All results are expressed as means (± SDs). Baseline serum 25-hydroxyvitamin D level was 14.5 ± 3.5 ng/mL (range: 10.2–19.6 ng/mL); PTH, 70 ± 25 pg/mL (range: 37–100 pg/mL); and NTx, 21 ± 7 nM bone collagen equivalents (BCE) (range: 14–34 nM). At 2, 4, and 6 hours after the calcium infusion, serum calcium rose from 9.3 ± 0.2 to 10.8 ± 0.9, 10.5 ± 0.8, and 10.6 ± 0.6 mg/d; PTH was suppressed from 70 ± 25 pg/mL to 18 ± 12, 16 ± 9, and 15 ± 9 pg/mL, respectively; NTx fell from 21 ± 8 nM BCE to 17 ± 5, 12 ± 4, and 12 ± 3 nM BCE, respectively.
Conclusions:
Serum NTx is a marker for bone collagen catabolism, and its reduction suggests that bone turnover was decreased. A relative deficiency of vitamin D associated with chronically elevated levels of PTH would be expected to increase bone turnover and to worsen the bone loss associated with immobilization.
Keywords: Immobilization, Tetraplegia, Paraplegia, Vitamin D, Calcium, Parathyroid hormone, N-telopeptide, Bone resorption, Osteoporosis
INTRODUCTION
Acute immobilization is associated with prompt loss of muscle and, fairly soon thereafter, bone. According to several studies of persons after acute spinal cord injury (SCI), bone is rapidly and dramatically lost below the level of lesion (1–3). What has been less well recognized is that bone may continue to be lost at an accelerated rate over the lifetime of a person who is paralyzed (4). Osteoporosis is recognized as predisposing to fracture, and assuming that other factors predisposing to fracture are held constant (eg, age and level of activity), the greater the depletion of bone mass, the greater the risk of fracture. As appreciated, a significant loss of bone substance will inevitably result in an associated loss of bone quality, or “connectivity,” between trabecular structures. If a young person with SCI loses a significant amount of bone mass immediately after injury, and if bone loss related to disuse continues at an accelerated rate over decades, any additional loss of bone would be expected to increase the risk of fracture.
An extensive literature exists regarding the association between fracture risk and calcium intake in older persons (5–8). Although some controversy remains, there is a sizeable body of evidence that persons who are either on a low calcium intake and/or are vitamin D deficient, which would limit gastrointestinal calcium absorption, have an increased risk of fracture (9–11). Individuals with SCI have been reported to have a higher prevalence of vitamin D deficiency than the able-bodied population (12,13). A mild depression in gut absorption of calcium may result in a small, but clinically significant, depression in the ionized serum calcium concentration that may trigger a secondary increase in parathyroid hormone (PTH) concentration, which would result in increased bone turnover (14,15). Because persons with SCI are activity-limited for life, the metabolic milieu may determine, in part, their ability to maintain bone mass. With these considerations in mind, we sought to determine whether individuals with a relative vitamin D deficiency could be shown to have a bone turnover rate that could be suppressed by calcium infusion. In principle, by decreasing bone turnover in those with SCI, it may be assumed that bone integrity would be better maintained.
METHODS
Subjects
Eight men with SCI and low 25 hydroxyvitamin D (25[OH]D ≤20 ng/mL) and/or elevated serum parathyroid hormone (PTH >55 pg/mL) concentrations were identified by screening a population sample, and eligible persons were recruited for study. The exclusion criteria were medications that affect calcium homeostasis, history of cardiac disease, arrhythmia, renal insufficiency, or nephrolithiasis. The average age of the participants was 34 ± 7 years (range: 23–43 years), with an average duration of injury of 12 ± 8 years (range: 3–27 years). Six participants had paraplegia and 2 had tetraplegia. The ethnic distribution was 5 Latinos, 2 African Americans, and 1 Caucasian. Participants were recruited from the SCI Service at the VA Medical Center, Bronx, NY. Institutional Review Board approval and informed consent were obtained prior to study participation.
Calcium Infusion
The infusion consisted of a calcium gluconate bolus (0.025 mmol elemental calcium/kg) over 20 minutes; the bolus infusion was followed by a constant infusion for 6 hours of calcium gluconate (0.025 mmol/kg/h) (16). The calcium bolus infusion was begun between 10:00 and 11:00 am and completed between 4:00 and 5:00 pm.
Blood Collection and Assay
Blood was collected at baseline and at 2, 4, and 6 hours and immediately placed on ice prior to separation by centrifugation at 4°C. The serum or plasma samples were kept at −30°C until assayed. Samples for vitamin D and PTH were run in batches in their respective assays. Serum calcium determinations were performed in the general chemistry laboratory on an autoanalyzer (Technicon Instruments, Tarrytown, NY). A commercial 25(OH)D radioimmunoassay was performed (DiaSorin, Stillwater, MN); 25(OH)D2 (ergocalciferol) and 25(OH)D3 (cholecalciferol) are measured with identical cross-reactivity for both calciferols, with levels expressed as 25(OH)D. The sensitivity of the 25(OH)D assay was 5 ng/mL; serum within-assay coefficients of variation were 11.7%, 10.5%, 8.6%, and 12.5% at 8.6, 22.7, 33, and 49 ng/mL; the normal range for 25(OH)D is 9 to 38 ng/mL. Immunoreactivity of PTH (Nichols Institute Diagnostics, San Juan Capistrano, CA) was determined using a kit assay. The sensitivity of the PTH assay was 10 pg/mL; serum intra-assay coefficients of variation were 3.1%, 3.5%, and 1.9% at 13, 23, and 476 pg/mL; the normal range for PTH is 9 to 55 pg/mL. Levels of N-telopeptide (NTx), a marker of osteoclastic activity, were determined by a competitive-inhibition enzyme-linked immunosorbent assay (NTx Osteomark, Wampolo Laboratory, Princeton, NJ). The sensitivity of the serum NTx assay was 5 nM bone collagen equivalents (BCE); serum intra-assay variability is 4.6%; normal range for men is 8.1 to 24.8 nM BCE.
Statistical Analyses
Results are expressed as means plus or minus standard deviations of the mean. After calcium infusion, changes in serum calcium, PTH, and NTx were determined with one-way repeated-measures ANOVA with Fisher protected least square difference post hoc analysis.
RESULTS
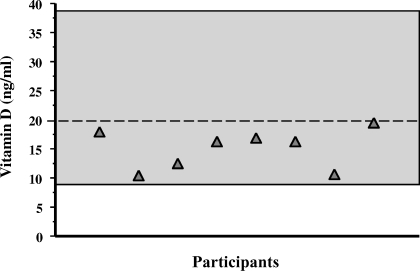
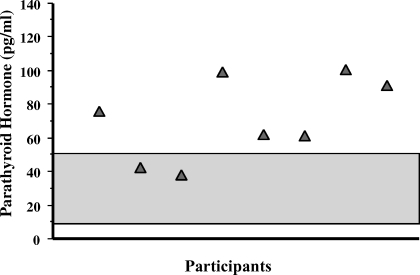
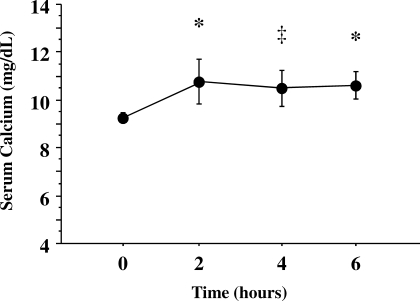
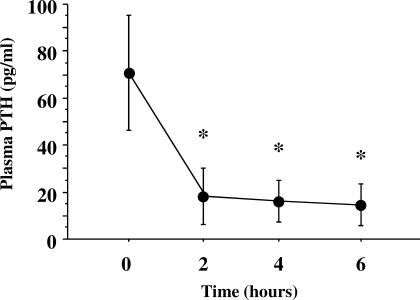
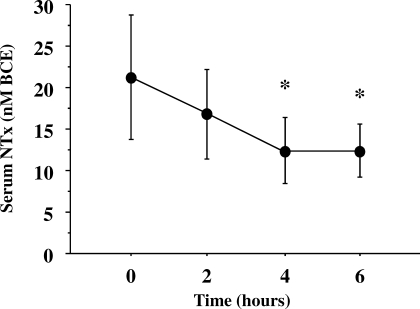
Mean baseline serum 25(OH)D level was relatively low (14.5 ± 3.5 ng/mL), but no study participants had values below the lower limit of normal (<9 ng/mL) (Figure 1). The mean PTH concentration at baseline was elevated (71 ± 25 pg/mL) (Figure 2). The urinary NTx at baseline was 21 ± 7 nM BCE; all participants, except one, had values ≥17 nM BCE, and 2 participants had values ≥30 nM BCE. At 2, 4, and 6 hours during the calcium infusion, the serum calcium concentration rose from 9.3 ± 0.2 mg/dL to 10.8 ± 0.9, 10.5 ± 0.8, and 10.6 ± 0.6 mg/dL, respectively (Figure 3). PTH concentration was suppressed to 73 ± 13% (18 ± 12 pg/mL), 76 ± 11% (16 ± 9 pg/mL), and 78 ± 11% (15 ± 9 pg/mL) at 2, 4, and 6 hours, respectively (Figure 4). Serum NTx concentration was reduced by 17 ± 18% (17 ± 5 nMBCE), 38 ± 16% (12 ± 4 nMBCE), and 38 ± 22% (12 ± 3 nMBCE) at 2, 4, and 6 hours, respectively (Figure 5). No adverse effects were associated with the calcium infusion.
Figure 1.
Distribution of baseline 25 hydroxyvitamin D (25[OH]D) levels. Individual participants are represented by shaded triangles. Note that all participants had levels <20 ng/mL, but none were absolutely vitamin D deficient. The normal range (9 to 38 ng/mL) is represented by the shaded area.
Figure 2.
Distribution of baseline plasma parathyroid hormone (PTH) concentrations. Individual participants are represented by shaded triangles. Note that 6 participants had levels above the normal range, and 2 participants had levels just at the upper limit of normal. The normal range (9 to 55 pg/mL) is represented by the shaded area.
Figure 3.
Elevation of serum calcium after bolus infusion of calcium gluconate. *P < 0.005 and ‡P < 0.01 between the baseline value and that observed after calcium infusion. The normal range (9 to 55 pg/ml) is represented by the shaded area.
Figure 4.
Suppression of plasma parathyroid hormone (PTH) after bolus infusion of calcium gluconate. *P < 0.001 between the baseline value and that seen after calcium infusion. The normal range (9 to 55 pg/ml) is represented by the shaded area.
Figure 5.
Suppression of serum N-telopeptide (NTx) after bolus infusion of calcium gluconate. *P < 0.01 between the baseline value and that measured after calcium infusion. The normal range (8.1 to 24.8 nM BCE) is represented by the shaded area.
DISCUSSION
In individuals with SCI who had vitamin D levels in the lower range of normal, an acute calcium infusion decreased serum PTH and NTx concentrations. Six of 8 study participants had PTH levels above the upper limit of normal, and the remaining 2 participants showed values in the upper range of normal. Most study participants did not have absolutely elevated levels of serum NTx; however, it may be assumed that values for serum NTx would have been at the lower limit of normal in persons with reduced bone turnover of chronic immobilization, had it not been for a state of mild secondary hyperparathyroidism. Thus, serum NTx values within the high normal range in our study participants may actually represent an “inappropriately” elevated bone turnover for persons with SCI. The calcium bolus infusion was begun in our participants in the late morning and completed 6 hours later, which would have greatly lessened the effects of diurnal variation of NTx values on our study findings. Furthermore, all of our participants were men, and the absolute diurnal variation of NTx is considerably less in men than in women (17). Suppression of serum NTx, a marker for bone collagen catabolism, after modest elevation in the serum calcium concentration suggested a reduction in bone turnover and the associated potential for immobilization-related bone loss.
This intervention of a calcium infusion was a proof of concept: if a state of vitamin D deficiency and/or mild secondary hyperparathyroidism exists, then markedly raising the serum calcium concentration will immediately reduce bone resorption, and, hence, bone turnover. There was no attempt to show a sustained effect of this intervention; nor was there an attempt to translate this intervention to clinically relevant forms of calcium/vitamin D therapy. Rather, by demonstrating that a marked elevation of serum calcium has a dramatic effect on bone metabolism, it may be inferred from the present study that any condition that diminishes calcium absorption will serve to accelerate bone turnover and loss.
Albright et al postulated that loss of stress and strain on the skeleton would result in bone loss (18). Stewart et al observed 14 immobilized patients, 13 of whom had experienced a traumatic SCI, during their first or second week after entrance to a rehabilitation facility; all patients had suppression of the parathyroid–vitamin D axis as a result of exaggerated bone resorption, with associated hypercalciuria (19). Biering-Sorensen et al studied 6 patients with acute traumatic SCI longitudinally and found that the proximal tibia and femoral neck lost 40% to 50% and 60% to 70% of bone mineral density, respectively, over 31 to 53 months (median of 41 months) (1). Thus, persons with SCI suffer a permanent reduction in activity-related forces on the skeleton that have dramatic acute effects on bone, placing these persons at an increased risk of fracture (20–23).
Controversy exists concerning whether bone loss occurs during the chronic phase of SCI (4,24–26). Frotzler et al observed in a cross-sectional study and Eser et al in a longitudinal study a stabilization of bone mass, as shown by peripheral quantitative computed tomography of the distal epiphyses and midshafts of the femur and in the tibia (24,25). Zehnder et al reported that there was a continuous decline in Z-scores of bone mineral density of the distal tibial diaphysis, even beyond 10 years of injury (26). Bauman et al studied 8 pairs of monozygotic twins who were discordant for SCI and reported that increasing duration of injury was linearly associated with the degree of bone loss in the lower extremities (4). The observation that bone mass continues to decline at an accelerated rate, independent of age, in persons with SCI raises the question as to whether factors other than disuse are influencing this process, and whether they are modifiable.
Levels of the storage form of vitamin D (25[OH]D) have been shown to be reduced in individuals with SCI (12,13,27). In 100 study participants with SCI compared with 50 age-matched controls, about one third of the participants with SCI were absolutely vitamin D deficient ([OH]D level <16 ng/mL) (12). Twelve study participants with SCI had elevated serum PTH levels, and 20 of 50 participants with SCI had elevated 1,25 dihydroxyvitamin D levels, a marker for secondary hyperparathyroidism. Indeed, a negative correlation was found between 25(OH)D levels and serum PTH concentrations (12). The reasons for the vitamin D deficiency state were postulated to be numerous and multifactorial and included restricted calcium intake, limited sunlight exposure, medications that accelerated metabolism, and renal disease (28–30).
If vitamin D levels are low, gastrointestinal absorption of calcium would be anticipated to be reduced, which may be further depressed when calcium intake is restricted, as is the case with many persons with SCI because of medical advice to avoid dietary calcium and/or personal preference. A chronic reduction in gut absorption of calcium results in a state of mild secondary hyperparathyroidism (15,17). It has been proposed that a relative state of vitamin D deficiency exists if the plasma 25(OH)D value is less than 32 ng/mL because calcium absorption is not optimal (31). Chronically elevated levels of PTH stimulate bone turnover, which will result in a net loss of bone substance (32,33).
The optimal daily dose of elemental calcium for an older adult is at least 1,200 mg per day. The majority of elderly persons, as well as those with SCI, probably do not receive sufficient calcium in their diets (34). Furthermore, absorption of calcium and skin production of vitamin D both decline with advancing age, and the population of persons with SCI has an almost normal life expectancy (35,36). Calcium supplementation has been shown to decrease bone loss in postmenopausal women, and it lowered the rate of vertebral fracture in women with a history of vertebral fractures (5,6).
Vitamin D facilitates active calcium absorption, which, as previously noted, is of greater clinical relevance when a person's calcium intake is restricted. Deficiency of vitamin D, or at least a reduction in levels, is more common in persons who are institutionalized because of deprivation of sunlight (28). Possibly because of the sufficiency of calcium intake in some study populations compared with others, supplementation with vitamin D has been shown to reduce the rate of hip fractures and, in another study, arm fractures in elderly men and women (9,10). Thus, the importance of vitamin D balance will vary with dietary calcium intake. In a study of Dawson-Hughes et al, elderly men and women were supplemented with 500 mg calcium and 700 IU of vitamin D3 (cholecalciferol) per day for 3 years. Bone loss was moderately reduced in the femoral neck, spine, and total body, and there was a reduced incidence of nonvertebral fracture (9). Bauman et al supplemented individuals with chronic SCI with 800 IU vitamin D3 per day for 12 months and found, on average, a doubling of vitamin D levels; however, despite the improvement in vitamin D levels, 23% of the study participants continued to have an absolute and 58% had a relative vitamin D deficiency (12). The serum PTH level decreased by about one third after supplementation; NTx levels were not measured (12).
Ledger et al studied the effect of age-related increase of serum PTH levels on bone resorption in women (16). Urinary 24-hour NTx excretion was higher in older vs younger women (25.7 ± 2.1 vs 19.3 ± 1.7 nmol/mmol creatinine). A direct relationship was evident between serum PTH levels and urinary NTx excretion. The suppression of urinary NTx excretion by calcium infusion was greater in the older than in the younger women (7.5 ± 1.9 vs 4.1 ± 1.5 nmol/mmol creatinine), suggesting that age-related serum PTH elevation is a major contributor to bone loss (16).
In the report herein, individuals presumptively had reduced vitamin D and/or calcium intake. However, dietary intakes of vitamin D and calcium were not obtained; thus, it was not possible to differentiate whether reduction of one (or, more likely, both) nutrients were responsible for our metabolic findings. Although NTx excretion was suppressed by an acute supraphysiologic infusion of calcium, it was not evaluated as to what degree of suppression a more physiologic infusion of calcium would have produced, nor was an increase of oral calcium supplementation investigated. Furthermore, the effect of a more prolonged increase of serum calcium concentration on bone turnover and bone mineral density was not addressed.
CONCLUSION
Our study has demonstrated that persons with SCI who are relatively vitamin D deficient and/or have elevated levels of serum PTH have a bone turnover rate that may be rapidly and dramatically suppressed by raising the serum calcium concentration, as evidenced by the observed reduction in serum NTx concentrations. This finding, albeit not unexpected, clearly highlights the need to evaluate all people with SCI and, perhaps, individuals with other immobilizing conditions for the presence of treatable causes of heightened bone turnover. Vitamin D may be supplemented and/or calcium intake may be appropriately increased to raise the serum calcium concentration and reduce serum PTH levels. Although not studied, it would have been likely that an oral therapeutic intervention would have had a somewhat analogous effect on serum calcium metabolic parameters and bone markers to that of intravenous administration of calcium. Even if the suppression of bone resorption by oral supplementation is only partially as successful as that noted in this report, any suppression of bone turnover would be expected to have a salutary effect over decades on total skeletal mass, and in particular, in skeletal regions below the level of paralysis. Thus, dietary supplementation may represent a practical and cost-effective therapeutic approach that may reduce the risk of fractures over a lifetime in persons who are paralyzed at a young age.
Footnotes
This work was supported by the Department of Veterans Affairs Rehabilitation Research and Development (RR&D) Service, the Center of Excellence for the Medical Consequences of Spinal Cord Injury (B4162C), United Spinal Association, and the James J. Peters Veterans Affairs Medical Center, Bronx, NY.
REFERENCES
- Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin. Invest. 1990;20(3):330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Wecht JM, Kirshblum S. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305–313. doi: 10.1682/jrrd.2004.05.0062. [DOI] [PubMed] [Google Scholar]
- Pearson EG, Nance PW, Leslie WD, Ludwig S. Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil. 1997;78(3):269–272. doi: 10.1016/s0003-9993(97)90032-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Schwartz E, Wang J, Pierson RN Continuous loss of bone in chronic immobilization: a monozygotic twin study. Osteopor Int. 1999;10(1):123–127. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- Devine A, Dick IM, Heal SJ, Criddle RA, Prince RL. A 4-year follow-up study of the effects of calcium supplementation on bone density in elderly postmenopausal women. Osteoporos Int. 1997;7(1):23–28. doi: 10.1007/BF01623455. [DOI] [PubMed] [Google Scholar]
- Recker RR, Hinders S, Davies KM. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11(12):1961–1966. doi: 10.1002/jbmr.5650111218. [DOI] [PubMed] [Google Scholar]
- Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: a randomized controlled trial. Am J Med. 1995;98(4):331–335. doi: 10.1016/S0002-9343(99)80310-6. [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- Heikinheimo RJ, Inkovaara JA, Harju EJ. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int. 1992;51(2):105–110. doi: 10.1007/BF00298497. [DOI] [PubMed] [Google Scholar]
- Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons: a randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400–406. doi: 10.7326/0003-4819-124-4-199602150-00003. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(12):1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil. 1994;75(7):766–769. [PubMed] [Google Scholar]
- Wiske PS, Epstein S, Bell NH, Queener SI, Edmondson J, Johnston CC Increases in immunoreactive parathyroid hormone with age. N Engl J Med. 1979;300(25):1419–1421. doi: 10.1056/NEJM197906213002506. [DOI] [PubMed] [Google Scholar]
- Marcus R, Madvig P, Young G. Age-related changes in PTH and PTH action in normal humans. J Clin Endocrinol Metab. 1984;58(2):223–230. doi: 10.1210/jcem-58-2-223. [DOI] [PubMed] [Google Scholar]
- Ledger GA, Burritt MF, Kao PC, O'Fallon WM, Riggs BL, Khosla S. Role of parathyroid hormone in mediating nocturnal and age-related increases in bone resorption. J Clin Endocrinol Metab. 1995;80(11):3304–3310. doi: 10.1210/jcem.80.11.7593443. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Dresner-Pollack R, Parker RA, London D, Ferguson L. Diurnal variation of bone mineral turnover in elderly men and women. Calcif Tissue Int. 1997;60(5):419–423. doi: 10.1007/s002239900256. [DOI] [PubMed] [Google Scholar]
- Albright F, Burnett CH, Cope O. Acute atrophy of bone (osteoporosis) stimulating hyperparathyroidism. J Clin Endocrinol. 1941;1:711–716. [Google Scholar]
- Stewart AF, Adler M, Byers CM, Segre GV, Broadus AE. Calcium homeostasis in immobilization. An example of resorptive hypercalciuria. N Engl J Med. 1982;306(19):1136–1140. doi: 10.1056/NEJM198205133061903. [DOI] [PubMed] [Google Scholar]
- Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg. 103:732–739. doi: 10.1016/0002-9610(62)90256-8. 1962 Jun. [DOI] [PubMed] [Google Scholar]
- Freehafer AA, Hazel CM, Becker CL. Lower extremity fractures in patients with spinal cord injury. Paraplegia. 1981;19(6):367–372. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- Nottage WM. A review of long-bone fractures in patients with spinal cord injuries. Clin Orthop Relat Res. 1981;Mar–Apr, 155:65–70. [PubMed] [Google Scholar]
- Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62(9):418–423. [PubMed] [Google Scholar]
- Frotzler A, Berger M, Knecht H, Eser D. Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT) Bone. 2008;43(3):549–555. doi: 10.1016/j.bone.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34(5):869–890. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Zehnder Y, Luthi M, Michel D. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Morrison NG, Spungen AM. Vitamin D replacement therapy in persons with SCI. J Spinal Cord Med. 2005;28(3):203–207. doi: 10.1080/10790268.2005.11753813. [DOI] [PubMed] [Google Scholar]
- Lifshitz F, Maclaren NK. Vitamin D-dependent rickets in institutionalized mentally retarded children receiving long-term anticonvulsant therapy. I. A survey of 288 patients. J Pediatr. 1973;83:612–620. doi: 10.1016/s0022-3476(73)80223-9. [DOI] [PubMed] [Google Scholar]
- Hahn TJ, Hendin BA, Scharp CR, Haddad JG Effect of chronic anticonvulsant therapy on serum 25-hydroxycalciferol levels in adults. N Engl J Med. 1972;287(18):900–904. doi: 10.1056/NEJM197211022871803. [DOI] [PubMed] [Google Scholar]
- Gray RW, Boyle I, DeLuca HF. Vitamin D metabolism: The role of the kidney. Science. 1971;172(989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF. Vitamin D round table. In: Burckhardt P, Dawson-Hughes B, Heaney RP, editors. Nutritional Aspects of Osteoporosis. Vol. 2004. San Diego, CA: Elsevier; pp. 263–270. 2nd ed. [Google Scholar]
- Eastell R, Yergey AL, Vieira NE, Cedell SL, Kumar R, Riggs BL. Interrelationship among vitamin D metabolism, true calcium absorption, parathyroid function, and age in women: evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin D action. J Bone Miner Res. 1991;6(2):125–132. doi: 10.1002/jbmr.5650060205. [DOI] [PubMed] [Google Scholar]
- Lips P, Courpron P, Menuier PJ. Mean wall thickness of trabecular bone packets in human iliac crest: changes with age. Calcif Tissue Res. 1978;26(1):13–17. doi: 10.1007/BF02013227. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board Food and Nutrition Board, Institute of Medicine; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Calcium. 2000:71–145. In: Dietary Reference Intakes for Calcium, Phosphate, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press. [Google Scholar]
- MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: Effect of age and dietary calcium. J Clin Invest. 1979;64(3):729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]