Abstract
Background/Objective:
Spinal cord injury influences many hormones that are known to be involved in the modulation of neurotrophic, neurogenic, and neuroprotective events. Recent studies showed that leptin could be neuroprotective, enhancing neuronal survival in vitro and in vivo. The objective of this study was to evaluate the pattern of the serum leptin levels in rats during acute traumatic SCI.
Methods:
Forty male Sprague-Dawley rats were divided randomly into 4 groups. In the control group, neither laminectomy nor SCI was performed; only laminectomy was performed without SCI in the sham group. In the cervical and thoracic spinal trauma groups, laminectomies were performed following the same trauma procedure. Blood samples were drawn 2, 6, 12, and 24 hours after the procedures and assayed immediately.
Results:
In the first 2 hours, levels of leptin were similar in control and sham-operated groups and higher in neurotrauma groups (P < 0.05). At the sixth hour, leptin levels increased in the sham-operated group, decreased in the neurotrauma groups (P < 0.05), and did not change in the control group (P > 0.05). At the 12th hour, the levels of leptin increased in all groups (P > 0.05). At the 24th hour, they decreased in the control, sham-operated, and cervical groups (P < 0.05); levels did not change in the thoracic group (P > 0.05). The decrease was higher in the control group than in the other groups (P < 0.05).
Conclusions:
Activation of endogenous leptin secretion started immediately after the SCI. The level of neurologic lesion (either cervical or thoracic regions) affected the levels of serum leptin differently, but with the exception of the first 12-hour period, this difference did not reach a statistically significant level.
Keywords: Spinal cord injuries, Neuroprotection
INTRODUCTION
Leptin, the protein product of the obesity gene, is a circulating hormone produced primarily by adipose tissue that is known to play a key role in regulating feeding and body fat stores (1–5). In addition to body weight homeostasis, leptin is involved in various physiologic events, including reproduction, hematopoiesis, angiogenesis, wound healing, insulin secretion, and neuroprotection (6).
Many recent studies showed that leptin could be neuroprotective, that is, it appears to enhance neuronal survival in vitro and in vivo. Dicou et al (2) showed that systemically administered leptin reduced cortical lesions induced by ibotenate-mediated excitotoxicity and protected mouse cortical neurons in culture against n-methyl-d-aspartate cytotoxicity. Russo et al (6) showed that leptin stimulated the proliferation of SH-SY5Y neuroblastostoma cells and reduced apoptosis caused by serum withdrawal. Also, neuroprotective effects of leptin against specific neurotoxins have been demonstrated using the same cell line (7). Zhang and colleagues (8) pointed out that rat cortical neurons express leptin receptor and that leptin decreased apoptotic neuronal death in vitro induced by a combined oxygen-glucose deprivation protocol. Additionally, they showed that leptin significantly reduced cerebral infarction volume in a focal cerebral ischemia model. Weng et al (9) demonstrated a possible neuroprotective effect of leptin on dopaminergic neurons.
There is much information about leptin concentrations in humans with chronic spinal cord injury (SCI) in relation to body mass and energy expenditures. These articles indicate that people with high-lesion SCI have higher plasma leptin levels than controls do (4,10,11). In addition, Jeon et al (12) demonstrated that these differences were associated with increased fat mass and sympathetic nerve system dysfunction. However, there is no information in the literature about serum leptin concentration in acute SCI.
The goal of this study was to evaluate the pattern of serum leptin concentrations in rats during the acute period of SCI caused by trauma.
METHODS
Forty male Sprague-Dawley rats weighing 250 g to 340 g were handled according to the Principles of Laboratory Animal Care. All experimental procedures were approved by the Institutional Animal Care and Use Committee. They were housed 3 per cage under diurnal lighting conditions (12 h darkness/12 h light), fed pellets (Purina Rodent Chow, St Louis, MO) and allowed free access to water before the experiment. They were kept under a controlled temperature (25 ± 2°C). All rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight, Sigma Chemical Co, St Louis, MO) and allowed to breathe room air. The depth of anesthesia was monitored by frequent testing of leg reflexes and muscle tonus. The rats were divided randomly into 4 groups. Group C was the control group (n = 9), and neither laminectomy nor SCI was performed. It constituted the basal levels for the other study groups. Group S was the sham group (n = 9) that underwent C6-T2 laminectomy without SCI. Group SCI-C was the cervical spinal trauma group (n = 13; 9 of them survived within a 24-hour period). The animals had a C5-C6 laminectomy, and then spinal trauma was performed at this level using the method described below. Group SCI-T was the thoracic spinal trauma group (n = 9). The animals had a T6-T7 laminectomy, and then the same trauma procedure was performed.
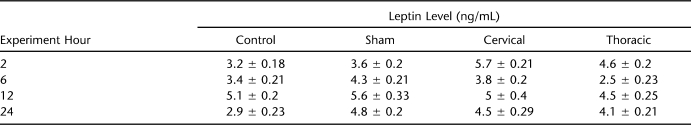
The surgical operations were conducted over 3 days: day 1, sham group; day 2, SCI-C group; day 3, SCI-T group. The operations started at 8 am, once the appropriate level of anesthesia was reached, and all animals were completed at approximately 9 am for 3 groups (approximately 20 min for each animal). Thus, we were able to collect blood samples at the same time. The first blood sample (2 h after the operation) was collected at approximately 11 am, the second blood sample (6 h after the operation) was collected at approximately 3 pm, the third blood sample (12 h after the operation) was collected at approximately 9 pm, and the last blood sample (24 h after the operation) was collected the next day at approximately 9 am (Table 1).
Table 1.
Mean Serum Leptin Levels in Four Study Groups
The animals underwent skin incision and paravertebral muscle dissection and had either cervical or thoracic laminectomies under loop magnification. The prominent spinous process of T2 was used as a surgical guide. The clip compression model described by Rivlin and Tator (13) was used. Spinal cord injury was accomplished by extradural compression of the exposed spinal cord for 30 seconds using an aneurysmal clip with a closing force of 50 g on the cord. The same clip was used in all the animals in which SCI was induced.
Blood samples were taken for serum leptin measurements from the orbital sinus of each animal under light ether anesthesia. The withdrawn blood was substituted by an equal volume of 0.9% NaCl immediately injected intraperitoneally after blood collection. Blood samples were centrifuged at 4°C for 30 minutes at 4,000 rpm, and serum aliquots were frozen at −20°C. Hormones were measured by commercial enzyme-linked immunosorbent assay kits according to the manufacturer's instructions. Serum concentrations of leptin were measured in duplicate with a lower detection limit of 0.5 ng/mL (Immuno-Biological Laboratories-Turkey Ltd Sti Yenimahalle, Ankara, Turkey). Each microtiter plate was filled with 100 µL of blanking reagent; standard solutions containing 0, 56.25, 112.5, 225, 450, 900, 1,800, and 3,600 ng/mL of leptin; and samples. An automatic well washer was used for washing (Wellwash 4 Mk2, Thermo Electron Corp, Vantaa, Finland). Optical densities were determined at 450 nm in an automatic microplate reader (Multiscan Ascent, Thermo Electron Corporation, Vantaa, Finland). Both the intra- and interassay coefficients of variation were less than 10% in the 3 assays.
Statistical Analysis: Data were analyzed using Sigma Plot (SYSTAT Software Inc, Ver. 11.0, Chicago, IL). Hormone levels were analyzed by Friedman repeated measures ANOVA on ranks followed by a Tukey multiple range test. Values were considered statistically significant at P < 0.05. Data were presented as mean ± SEM.
RESULTS
All the animals in the SCI-C group had flaccid tetraplegia and loss of sphincter control immediately after the trauma. Three animals developed apnea or dyspnea after the procedure, but none were connected to a respirator. Because 4 animals died in the first 6-hour period, they were excluded from the experiment. No significant breathing alteration was observed in the rest of the animals in this group. All the animals in the SCI-T group presented severe flaccid hind limb plegia and loss of sphincter control after the trauma. No apparent variation in respiratory rhythm was observed in this group.
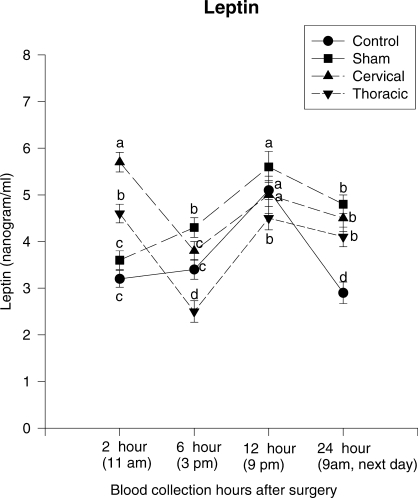
Figure 1 represents the serum leptin levels of the control, S, SCI-C, and SCI-T groups. At the second hour of the experiment, ANOVA did not detect a significant difference between the leptin levels in the control (∼3.2 ng/mL) and S (∼3.6 ng/mL) (F = 0.810, P = 0.502, df = 5) groups. However, leptin levels were statistically higher in the SCI-C (∼5.7 ng/mL) (F = 4.407, P = 0.004, df = 3) and SCI-T (∼4.6 ng/mL) (F = 2.679, P = 0.026, df = 4) groups. At the sixth hour of the experiment, ANOVA detected significant changes in all but the control group; leptin levels increased in the S group (F = 2.430, P = 0.035, df = 6), decreased in the SCI-C (F = 3.203, P = 0.013, df = 3) and SCI-T (F = 3.981, P = 0.011, df = 4) groups, and did not change significantly in the control group (F = 1.44, P = 0.074, df = 5). Leptin levels were similar in the control and SCI-C groups (F = 0.576, P = 0.875, df = 5), higher in the S group (F = 2.125, P = 0.059, df = 4), and lower in SCI-T group (F = 6.183, P = 0.002, df = 2). At the 12th hour, ANOVA detected significant changes in the groups; leptin levels increased in all groups and were similar in all groups (F = 1.762, P = 0.213, df = 5), with the exception of the SCI-T group (F = 2.453, P = 0.047, df = 5). At the 24th hour, ANOVA detected significant changes in all but the SCI-T group; leptin levels decreased in the control (F = 10.48, P = 0.001, df = 2), S (F = 2.45, P = 0.043, df = 5), and SCI-C groups (F = 2.126, P = 0.048, df = 3) but did not change in the SCI-T group (F = 1.4235, P = 0.08, df = 6).
Figure 1.
Serum leptin levels of the control, sham-operated, cervical and thoracic laminectomized groups. Twenty-four–hour serum leptin levels (mean ± SEM) of 4 study groups. At the 24th hour, leptin levels decreased in all groups but the thoracic spinal trauma one, and the decrease was greater in the control group than in the sham and cervical spinal trauma groups (P < 0.05). Different letters indicate the statistically significant difference among the groups (P < 0.05).
DISCUSSION
Leptin is a 16-kDa protein primarily produced by adipocytes (14) and is reported to vary diurnally in humans (15). Leptin is secreted in a pulsatile pattern. Kalsbeek et al (16) showed that the biological clock suprachiasmatic nuclei in hypothalamus controls the diurnal rhythm of plasma leptin levels directly, independently of other clock-controlled rhythms (corticosterone, insulin, feeding) in rats (16).
Ablation of suprachiasmatic nuclei eliminates the diurnal rhythmicity of circulating plasma leptin levels. Leptin may regulate both food intake and energy expenditure via central nervous system pathways (17,18). Although a wide variety of central and peripheral tissues expressing leptin receptors have been found (19,20), a major target for leptin is the brain, especially the ventromedial hypothalamus (18,21). Both Satoh et al (22) and Tang-Christensen et al (18) demonstrated that administration of central leptin caused a significant increase in plasma epinephrine and norepinephrine concentration. This finding strongly supported leptin's activating the sympathetic nervous system. Surely this process requires an intact sympathetic nervous system for normal leptin action (20–24). Leptin acts on hypothalamic neuronal targets to regulate energy balance and neuroendocrine functions. Leptin mediates many of its physiological effects by activating neurons in the hypothalamus that innervate and stimulate sympathetic preganglionic neurons in the spinal cord.
The stress response to trauma is characterized by sympathetic activation and influenced secretion of pituitary hormones (25). The adrenergic response is related to the extent of trauma and is determined by afferent signals (nociceptive pathways and humoral mediators) originating from the site of injury. The stress response to trauma may have activated postganglionic sympathetic neurons through a spinal reflex, resulting in the release of norepinephrine, regardless of the injured level (26). Nociceptive pathways may also be partially responsible for the activation of the stress response (27).
People with SCI experience dysfunction of the sympathetic nervous system, which results in an altered hormonal and metabolic response. In individuals with high-lesion SCI, these neurons are atrophied and stimulation below the lesion may be impaired because of disruption of the sympathetic component of the efferent limb in the cervical spinal cord (28). This interruption leads to pathologic changes in sympathetic innervations through the anatomic reorganization of pathways in the spinal cord (28). Consequently, impairment of the sympathetic nervous system may alter leptin secretion, and decentralization of the sympathetic nervous system may interrupt the pathway of leptin-mediated energy expenditure regulation (12).
Schramm (29) claims that spinal sympathetic interneurons play only a minor role in sympathetic regulation when the spinal cord is intact but that they play an important role after SCI, both in generating ongoing activity in sympathetic nerves and in mediating segmental and intersegmental sympathetic reflexes. The spinal sympathetic interneurons most directly influence the activity of sympathetic preganglionic neurons after SCI. Finally, spinal sympathetic interneurons are more likely to be excited and less likely to be inhibited by both noxious and innocuous somatic stimuli after chronic spinal transection. The onset of this hyperexcitability corresponds to morphologic changes in both sympathetic preganglionic neurons and primary afferents (29). Also, many studies suggest the presence of a tonic inhibitory adrenergic influence on leptin secretion (2,5,30,31). Removal of this tonic inhibitory adrenergic influence would probably contribute to increased plasma leptin levels.
There is much information in the medical literature about leptin concentrations in chronic SCI, and these articles show that people with high-lesion SCI have higher plasma leptin levels than controls do (4,10,11). However, there are no data available on the variation in serum leptin level during the acute phase of SCI.
In the present study, at the second hour of the experiment, leptin levels were similar in the control (∼3,2 ng/mL) and S (∼3,6 ng/mL) groups but higher in the neurotrauma groups (SCI-C ∼6 ng/mL and SCI-T ∼4,5 ng/mL) (P < 0.05). This situation might be explained by the stress response to trauma, which was characterized by the activation of postganglionic sympathetic neurons through a spinal reflex (25–27), or the activity of spinal sympathetic interneurons that most directly influenced the activity of sympathetic preganglionic neurons after SCI (29). At the sixth hour, leptin levels increased in the S group but decreased in both neurotrauma groups (P < 0.05) and did not change in the control group (P > 0.05). The decrease was more evident in thoracic group (P < 0.05). Also, this decrease might be explained by a temporary tonic inhibitory adrenergic influence dominating over the sympathetic system (2,5,30,31). There was a rising trend in all groups after the sixth hour. At the 12th hour, the levels of leptin in all groups increased and only the SCI-T group differed statistically from the others (P < 0.05).
At the 24th hour, leptin levels decreased in the control, S, and SCI-C groups (P < 0.05) but did not change in the SCI-T group (P > 0.05). The decrease was higher in the control group than in the others (P < 0.05) (Figure 1). We thought that the control group resumed the normal diurnal rhythm, but the other trauma groups continued to remain high. Yan et al (32) showed that serum leptin levels of patients who had undergone resection of hepatic tumor or cholecystectomy reached the highest level on the first day after operation and began to decrease on the second day but that the level was still higher than the levels before the operation. Their results might be related to the effects of surgical trauma without SCI, as in our S group. In our study, serum leptin levels increased progressively in the acute period in the S group, but this increase returned to normal levels in a definite period. In contrast, Maimoun et al (4), Wang et al (11), and Huang et al (33) showed that serum leptin levels were constantly high in chronic tetraplegia and paraplegia but not related to the level of neurologic lesion. Our results revealed that the level of neurologic lesion (either cervical or thoracic) affected the levels of serum leptin differently during the first 6 hours (P < 0.05). After the 6th hour, however, this difference began to diminish, and at the end of the 12th hour, it disappeared completely. Briefly, in the early stage of the trauma, SCI caused a quick increase in the serum levels, after which there was a short period of sharp decrease. After showing a partial increase, it remained constantly high (Figure 1).
Most traumatic SCIs do not involve actual physical transection of the cord. Rather, the spinal cord is damaged as a result of contusive, compressive, or stretch injury. Typically, residual white matter containing portions of the ascending sensory and descending motor tracts allows for the possibility of neurologic recovery. However, during the first minutes and hours after injury, a secondary multifactorial degenerative process is initiated by the primary mechanical injury that is proportional to the magnitude of the initial insult. Upon completion of the NASCIS III trial, it was found that all 3 treatment arms produced comparable degrees of recovery when treatment was begun within the shorter 3-hour window. When the 24-hour dosing of steroid began more than 3 hours after SCI, recovery was poorer than in the cohort treated within 3 hours of the SCI. These pharmacologic treatments, which interrupt the secondary cascade, if applied early as mentioned in NASCIS III (34), can improve spinal cord tissue survival and thus preserve the necessary anatomic substrate for functional recovery to take place.
CONCLUSIONS
The present study revealed that (a) activation of endogenous leptin secretion of the organism started immediately after the SCI, but it showed a greatly decreasing trend between the second and sixth hours; (b) the level of neurologic lesion (either cervical or thoracic regions) affected the levels of serum leptin differently, but with the exception of the first 12 hours, this difference did not reach a statistically significant level; (c) surgical trauma without SCI caused a progressive rising trend until the 12th hour. This may support the importance of beginning the neuroprotective treatment in the first 3-hour period after SCI, as reported in NASCIS III. These findings invite further investigation of their potential roles and the timing of medical treatment in the neuroprotective process of neurologic injury.
REFERENCES
- Commins SP, Marsh DJ, Thomas SA. Norepinephrine is required for leptin effects on gene expression in Brown and white adipose tissue. Endocrinology. 1999;140(10):4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- Dicou E, Attoub S, Gressens P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport. 2001;12(18):3947–3951. doi: 10.1097/00001756-200112210-00019. [DOI] [PubMed] [Google Scholar]
- Jeon JY, Steadward RD, Wheeler GD, Bell G, McCarger L, Harber V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab. 2003;88(1):402–407. doi: 10.1210/jc.2002-020939. [DOI] [PubMed] [Google Scholar]
- Maimoun L, Puech AM, Manetta J. Circulating leptin concentrations can be used as a surrogate marker of fat mass in acute spinal cord injury patients. Metabolism. 2004;53(8):989–994. doi: 10.1016/j.metabol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Fink BD, Morgan DA, Fox JM, Donohoue PA, Haynes WG. Sympathetic inhibition, leptin, and uncoupling protein subtype expression in normal fasting rats. Am J Physiol. pp. E668–E677. 1999;277(4 pt 1) [DOI] [PubMed]
- Russo VC, Metaxas S, Koboyashi K, Haris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145(9):4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- Lu J, Park CS, Lee SK, Shin DW, Kang JH. Leptin inhibits 1-methyl-4-phenylpyridinium-induced cell death in SH-SY5Y cells. Neurosci Lett. 2006;407(3):240–243. doi: 10.1016/j.neulet.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38(8):2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y. Leptin protects against 6-hydroxydopamineinduced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282(47):34479–34491. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG. Mobbs CV. Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm Metab Res. 1996;28(12):732–736. doi: 10.1055/s-2007-979889. [DOI] [PubMed] [Google Scholar]
- Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehab. 2000;81(12):1582–1586. doi: 10.1053/apmr.2000.9173. [DOI] [PubMed] [Google Scholar]
- Jeon JY, Harber VJ, Steadward RD. Leptin response to short-term fasting in sympathectomized men: role of the SNS. Am J Physiol Endocrinol Metab. 2003;284(3):E634–E640. doi: 10.1152/ajpendo.00302.2002. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10(1):39–43. [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positioning cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Heiman ML. Nocturnal rise of leptin in lean, obese, and non-insulindependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Romijn JA. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142(6):2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increase uncoupling protein expression and energy expenditure. Am J Physiol. pp. E226–E230. 1997;273(1 pt 1) [DOI] [PubMed]
- Tang-Christiansen M, Havel PJ, Jacobs RR, Larsen PJ, Cameron JL. Central administration of leptin inhibit food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab. 1999;84(2):711–717. doi: 10.1210/jcem.84.2.5458. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C. Anatomic localization of alternatively spliced leptin receptor (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94(13):7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montes JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48(2):287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G. Sympathetic activation of leptin via the ventromedial hypothalamus-leptin induced increase in catecholamine secretion. Diabetes. 1999;4(9)8:1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46(12):2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Murakami T, Otani S, Muwajima M, Shima K. Leptin affects pancreatic endocrine function through the sympathetic nervous system. Endocrinology. 1998;139(9):3863–3870. doi: 10.1210/endo.139.9.6201. [DOI] [PubMed] [Google Scholar]
- Desborough JP, Hall GM. Endocrine response to surgery. In: Kaufman L, editor. Anesthesia Review. Vol. 1993. Edinburgh, Scotland: Churchill Livingstone; pp. 131–148. [Google Scholar]
- Yoo KY, Hwang JH, Jeong ST. Anesthetic requirements and stress hormone responses in spinal cord-injured patients undergoing surgery below the level of injury. Anesth Analg. 2006;102(4):1223–1228. doi: 10.1213/01.ane.0000198429.09694.d3. [DOI] [PubMed] [Google Scholar]
- Scarborough DE. Cytokine modulation of pituitary hormone secretion. Ann N Y Acad Sci. 1990;594:169–187. doi: 10.1111/j.1749-6632.1990.tb40477.x. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Bunge RP, Pucket WR, Bygrave MA. The changes in human spinal sympathetic preganglionic neurons after spinal cord injury. Spinal Cord. 1999;37(1):6–13. doi: 10.1038/sj.sc.3100718. [DOI] [PubMed] [Google Scholar]
- Schramm LP. Spinal sympathetic interneurons: their identification and roles after spinal cord injury. Prog Brain Res. 2006;152:27–37. doi: 10.1016/S0079-6123(05)52002-8. [DOI] [PubMed] [Google Scholar]
- Correia ML, Morgan DA, Mitchell JL, Sivits W, Mark AL, Haynes WG. Role of corticotrophin-releasing factor in effects of leptin on sympathetic nerve activity and arterial pressure. Hypertension. 2001;38(3):384–388. doi: 10.1161/01.hyp.38.3.384. [DOI] [PubMed] [Google Scholar]
- Pinkney JH, Coppack SW, Mohamed-Ali V. Effect of isoprenaline on plasma leptin and lipolysis in humans. Clin Endocrinol (Oxf) 1998;48:407–411. doi: 10.1046/j.1365-2265.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- Yan GT, Hao XH, Xue H, Lin J, Zhang K, Wang LH. Changes in serum leptin levels in patients with surgically induced stress responses. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18(3):172–175. [PubMed] [Google Scholar]
- Wang YH, Huang TS, Lian HW, Su TC, Chen SY, Wang TD. Fasting serum levels of adiponectin, ghrelin and leptin in man with spinal cord injury. Arch Phys Med Rehabil. 2005;86(10):1964–1968. doi: 10.1016/j.apmr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR. Methylprednisolone or trilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89(5):699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]