Abstract
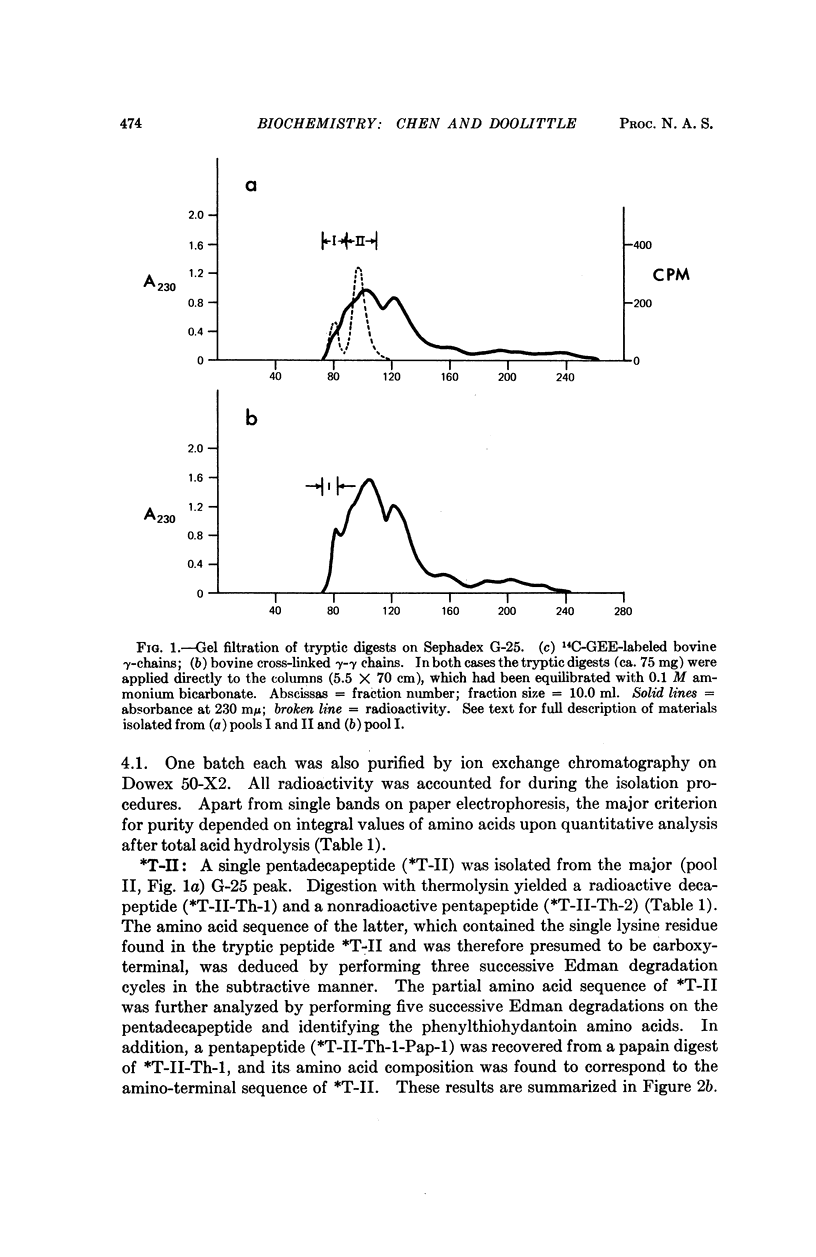
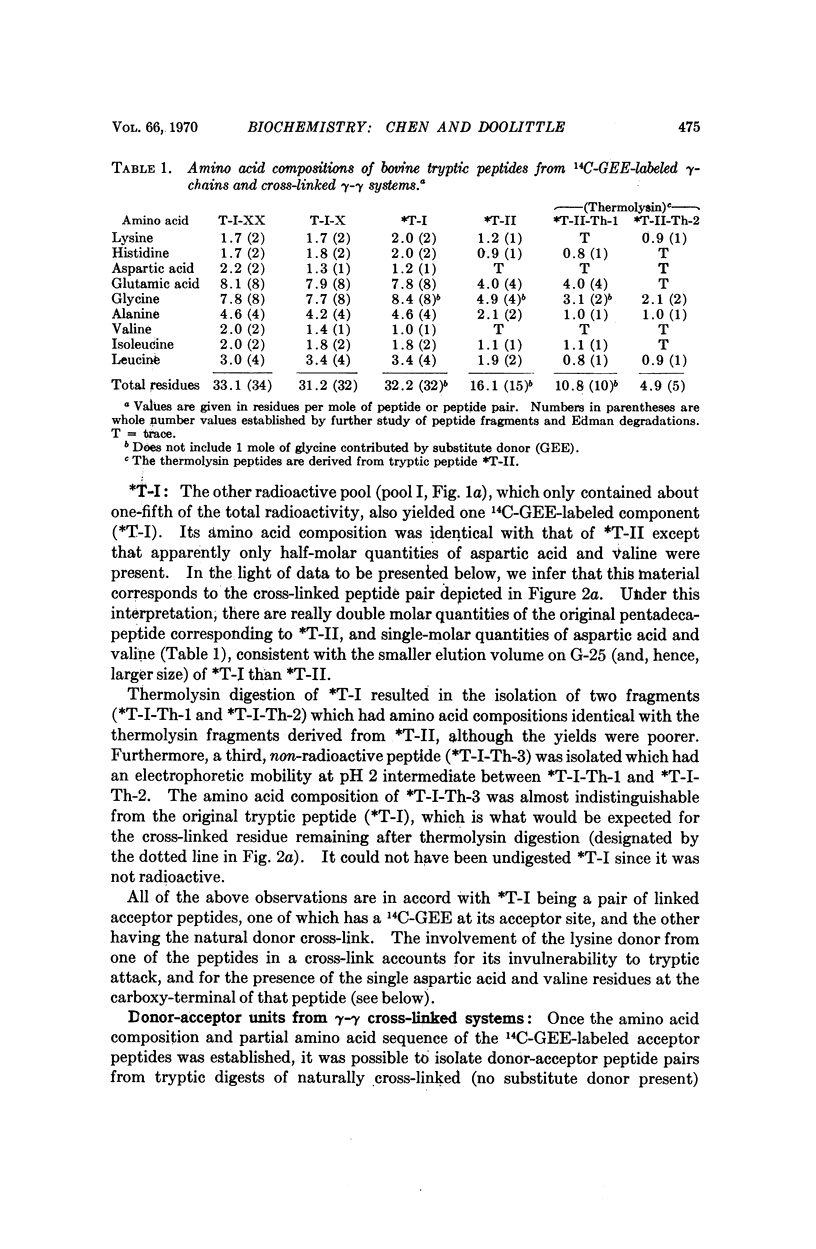
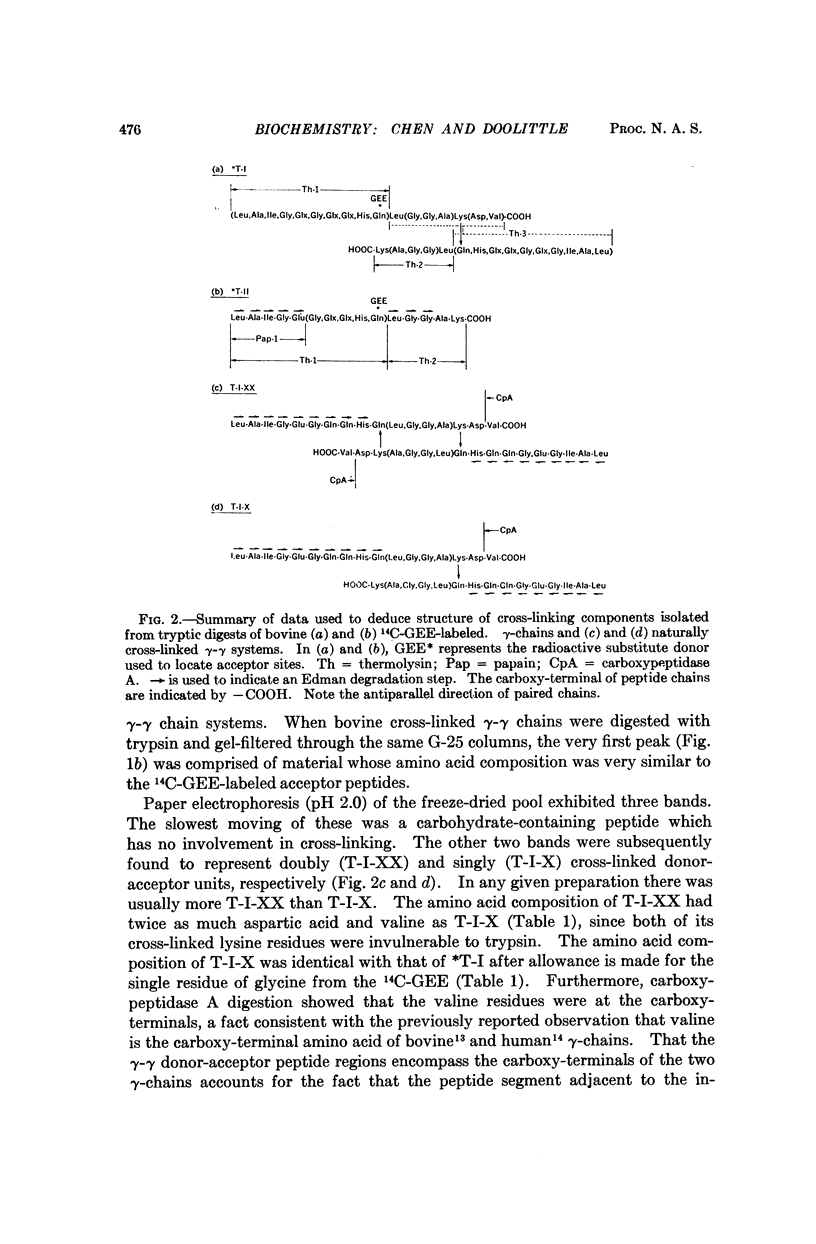
The cross-linking systems of bovine and human fibrins were studied by the introduction of a radioactive substitute donor as an inhibitor of fibrin cross-linking, separation of the constituent polypeptide chains after sulfitolysis, and tryptic digestion of the labeled γ-chains. The information gathered from this approach enabled us to isolate and characterize the complete donor-acceptor unit in tryptic digests of fibrin γ-γ cross-linked systems. In both bovine and human fibrin, this kind of cross-linking is accomplished by reciprocal bridging between overlapping carboxy-terminal segments of neighboring γ-chains. The amino acid sequence of the carboxy-terminal heptadecapeptide of the bovine γ-chain was determined and an alignment of the corresponding region of the human γ-chain established.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blombäck B., Blombäck M., Henschen A., Hessel B., Iwanaga S., Woods K. R. N-terminal disulphide knot of human fibrinogen. Nature. 1968 Apr 13;218(5137):130–134. doi: 10.1038/218130a0. [DOI] [PubMed] [Google Scholar]
- Chen R., Doolittle R. F. Identification of the polypeptide chains involved in the cross-linking of fibrin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):420–427. doi: 10.1073/pnas.63.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN P. Phenylthiohydantoins in protein analysis. Ann N Y Acad Sci. 1960 Aug 31;88:602–610. doi: 10.1111/j.1749-6632.1960.tb20056.x. [DOI] [PubMed] [Google Scholar]
- Gerbeck C. M., Yoshikawa T., Montgomery R. Bovine fibrinogen--heterogeneity of the gamma-chains. Arch Biochem Biophys. 1969 Oct;134(1):67–75. doi: 10.1016/0003-9861(69)90252-5. [DOI] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppel G. Elektronenmikroskopische Untersuchungen zur Gestalt und zum makromolekularen Bau des Fibrinogen molekïls und der Fibrinfasern. Z Zellforsch Mikrosk Anat. 1967;77(4):443–517. [PubMed] [Google Scholar]
- Lorand L., Chenoweth D., Domanik R. A. Chain pairs in the crosslinking of fibrin. Biochem Biophys Res Commun. 1969 Oct 8;37(2):219–224. doi: 10.1016/0006-291x(69)90722-0. [DOI] [PubMed] [Google Scholar]
- Lorand L. Physiological roles of fibrinogen and fibrin. Fed Proc. 1965 Jul-Aug;24(4):784–793. [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun. 1968 Feb 26;30(4):356–362. doi: 10.1016/0006-291x(68)90750-x. [DOI] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. Transglutaminase activity of the fibrin crosslinking enzyme. Biochem Biophys Res Commun. 1966 Sep 22;24(6):858–866. doi: 10.1016/0006-291x(66)90327-5. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Singer A., Sasaki R., Jukes T. H. Observations on the specificity of a thermostable bacterial protease "thermolysin". Biochem Biophys Res Commun. 1965 Nov 8;21(3):242–247. doi: 10.1016/0006-291x(65)90278-0. [DOI] [PubMed] [Google Scholar]
- Mills D. A., Liener I. E. Partial chemical characterization of the isolated gamma-chain of human fibrinogen. Arch Biochem Biophys. 1969 Mar;130(1):629–635. doi: 10.1016/0003-9861(69)90080-0. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. Chemical and enzymic detection of protein cross-links. Measurement of epsilon-(gamma-glutamyl)lysine in fibrin polymerized by factor XIII. Biochemistry. 1969 Mar;8(3):871–876. doi: 10.1021/bi00831a016. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- Takagi T., Iwanaga S. Polypeptide chain involved in the cross-linking of stabilized bovine fibrin. Biochem Biophys Res Commun. 1970 Jan 6;38(1):129–136. doi: 10.1016/0006-291x(70)91094-6. [DOI] [PubMed] [Google Scholar]



