Abstract
The intrinsic cytotoxicity of cell-free haemoglobin (Hb) has hampered the development of reliable Hb-based blood substitutes for over seven decades. Notably, recent evidence shows that the Hb deploys this cytotoxic attack against invading microbes, albeit, through an unknown mechanism. Here, we unraveled a rapid molecular reprogramming of the Hb structure-function triggered by virulent haemolytic pathogens that feed on the haem-iron. On direct contact with the microbe, the Hb unveils its latent antimicrobial potency, where multiple antimicrobial fragments are released, each harbouring coordinated ‘dual-action centres': microbe binding and pseudoperoxidase (POX) cycle activity. The activated Hb fragments anchor onto the microbe while the juxtaposed POX instantly unleashes a localized oxidative shock, killing the pathogen-in-proximity. This concurrent action conceivably restricts the diffusion of free radicals. Furthermore, the host astutely protects itself from self-cytotoxicity by simultaneously releasing endogenous antioxidants. We found that this decryption mechanism of antimicrobial potency is conserved in the ancient invertebrate respiratory protein, indicating its fundamental significance. Our definition of dual-antimicrobial centres in the Hb provides vital clues for designing a safer Hb-based oxygen carrier blood substitute.
Keywords: dual-antimicrobial activity, haemoglobin free radicals, limited proteolysis, microbe-binding and oxidative shock, reprogramming structure-function of haemoglobin
Introduction
The intrinsic toxicity of cell-free haemoglobin (Hb) because of its redox reactivity is well known. Auto-oxidation of HbFeII to HbFeIII readily produces superoxide radical (O2•−), which dismutates into H2O2. Further exposure to H2O2 (either from its intrinsically dismutated O2•− or from the plasma) initiates a pseudoperoxidase (POX) catalytic cycle between the HbFeIII (metHb) and HbFeIV (ferryl-Hb), to simultaneously produce transient ferryl-Hb protein radical and protonated superoxide radical (HO2•, also called hydroperoxyl radical) (Cashon and Alayash, 1995; Alayash et al, 2001). Furthermore, the O2•− can also assist the formation of highly reactive hydroxyl radical (•OH) through Fenton reaction (Puppo and Halliwell, 1988). This inherent property of the Hb challenges the successful development of a safe and reliable Hb-based oxygen carrier (HBOC), which would otherwise have been a much sought after blood substitute for blood transfusion (Moore et al, 2009). In fact, recent findings by Jiang et al (2007) showed that the POX cycle of Hb is specifically activated by microbial virulence factors (e.g., lipopolysaccharide (LPS) and proteases). Thus, even the slightest encounter or contamination of the cell-free Hb with such microbial virulence factors will cause the Hb to release free radicals and render the HBOC unsuitable as a blood substitute.
In vivo, as a front line defence molecule, the propensity for Hb to become activated to produce ferryl-Hb and superoxide radicals (henceforth referred to as free radicals) also raises concerns, for example, (i) these free radicals are not only toxic to the invading microbes, they are also detrimental to the host itself, similar to the safety concerns on the HBOC blood substitute; (ii) antioxidants present in the blood (Alayash, 1999), such as catalase (CAT), superoxide dismutase (SOD) and reduced glutathione (GSH), could reduce the Hb free radical-mediated bactericidal activity. Therefore, how the Hb counters the invading microbe while simultaneously prevents host self-cytoxicity because of the generated free radicals is intriguing. This dichotomous property of the Hb prompted us to hypothesize that the host assumes a tight regulation on this major blood protein to ensure a proficient self-defence.
The antimicrobial latency of the Hb is potentiated on preferential assault by virulent haemolytic pathogens like Staphylococcus aureus, as the microbe usurps haem-iron for its nutrient source at the initial phase of infection (Skaar et al, 2004; Lima et al, 2007; Mocny et al, 2007). This is exemplified by recent findings that the activated Hb elicits a broad spectrum of antimicrobial activity (Parish et al, 2001; Mak et al, 2007) and it generates microbicidal free radicals (Jiang et al, 2007). The direct interplay between Hb and microbe through known bacterial virulence factors, LPS (Kaca et al, 1994) and microbial proteases (Nishina et al, 1992; Jiang et al, 2007), enhances the oxidation rate of Hb. We therefore reasoned that structural perturbation or proteolysis of the Hb tetramer possibly promotes the oxidation of the haem-iron, which facilitates the POX cycle to generate more cytotoxic-free radicals (Alayash et al, 2001). It has been shown that proteases, detergents or antimicrobial peptides all trigger conformational changes to a functional homologue of Hb, the haemocyanin (HMC) of the invertebrates. This activates the HMC-phenoloxidase (PO), which catalyses the oxidation of phenols to O-quinones (Decker et al, 2001; Nagai et al, 2001; Jiang et al, 2007) leading to the formation of microbicidal reactive oxygen species (ROS) (Bolton et al, 2000). Therefore, on the basis of the functional homology between Hb and HMC, we envisage that the structure-based regulatory mechanism has a major role in the respiratory protein-mediated innate immune defence.
Here, we show that Hb is specifically and progressively cleaved by the extracellular proteases of the invading microbe. We discovered that the released Hb fragments contain dual functions: (i) binding of pathogen-associated molecular patterns (PAMPs, LPS or lipoteichoic acid (LTA)) and (ii) POX cycle activity to produce free radicals. We found that co-localization of these dual-activity sites in the same Hb fragments confers an ‘anchor-and-attack' strategy where the binding of the activated fragments onto PAMPs displayed on the microbe strategically positions the juxtaposed POX cycle activity to inflict a vicinal and instant production of free radicals, which kill the microbe. This mechanism seems to simultaneously restrict the diffusion of cytotoxic free radicals thus protecting the host from harm. The host is further protected by the endogenous antioxidants simultaneously released from the haemolysed RBC. Such an elegant infection-mediated activation of antimicrobial potency must be fundamentally crucial as it is well conserved in the ancient respiratory protein, HMC. The rapid molecular reprogramming of the structure-function of respiratory proteins to expose potent dual-antimicrobial regions for microbial PAMP-binding and catalysis of free radical production, provide insightful information on how these well-known oxygen carriers can be transformed to counteract microbial intruders. Furthermore, our elucidation of the hot spots of antimicrobial activities and the understanding of their mechanisms of action provide clues for intervention of the POX cycle of Hb. This may also facilitate the development of HBOC blood substitute.
Results
Activation of Hb POX cycle—microbial proteases and PAMPs coordinate the structural alteration of Hb
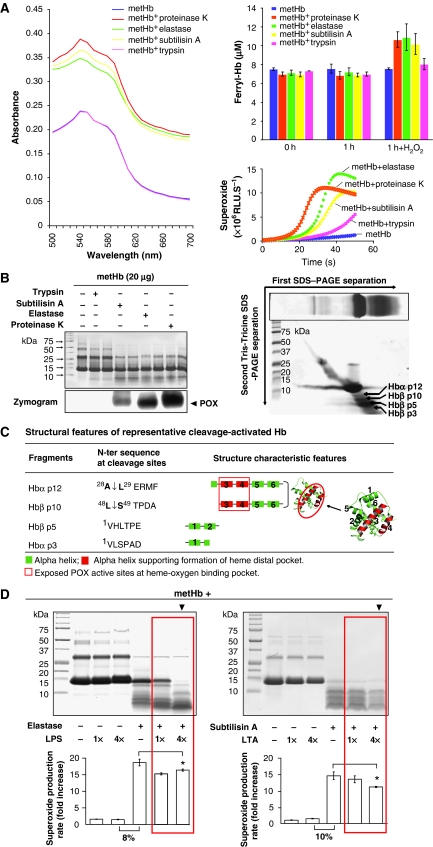
To test our hypothesis that microbial proteases and PAMPs structurally alter the Hb molecule to activate the POX cycle, we treated purified human metHb (HbFeIII) with three representative microbial proteases: subtilisin A, elastase and proteinase K (from Gram-positive and -negative bacteria and fungi, respectively). The spectral changes of Hb and its derivatives were monitored. Results showed that in the absence of H2O2, the characteristic peaks of the metHb (HbFeIII) remained similar before and after partial proteolysis, with or without protease treatment (Supplementary Figure S1). However, the addition of 0.5 μmol H2O2 caused an instant surge in the ferryl-Hb (HbFeIV) intermediate (Winterbourn, 1990), whose levels were gradually decreased (Supplementary Figure S1). This indicates a rapid oxidation of Hb to activate the POX cycle in the presence of H2O2. The absorption spectral levels and the concentrations of the transient ferryl-Hb formed by the microbial protease proteolysed metHb were significantly higher than that of the metHb alone or metHb treated with the host trypsin (Figure 1A, absorption spectra and histobars; Supplementary Figure S1). This was further accompanied by the enhanced production of superoxide in the presence of H2O2 (Figure 1A, curves). These results indicate that the microbial protease activates the Hb-POX cycle activity, which caused a surge in the production of ferryl-Hb and superoxide radicals when exposed to H2O2.
Figure 1.
The microbial proteases and PAMPs regulate the Hb-POX cycle activation. (A) Left panel: the absorption spectra of ferryl-Hb intermediate of the partially proteolysed metHb by the microbial proteases (on addition of H2O2). The metHb alone or metHb treated with trypsin (a control mammalian host protease) served as negative controls. Right upper panel: the concentration of ferryl-Hb produced on the addition of H2O2 was further determined by reaction with 2 mM sodium sulfide (Na2S) to form sulphhaemoglobin, which was detectable at 620 nm. Right lower panel: the simultaneous production of superoxide was determined by CLA-CL assay. (B) Left panel: the partial proteolytic profile of metHb was resolved on Tris–Tricine SDS–PAGE (10%). metHb treated with microbial proteases were electrophoresed in duplicate lanes. The POX cycle activities were visualized by chemiluminescence-aided zymography. Right panel: the cleaved metHb fragments were further resolved by second dimensional Tris–Tricine SDS–PAGE. The ‘first SDS–PAGE' (framed) shows the separation of cleaved metHb on an SDS–PAGE gel following which the lane of protein bands was excised and overlaid on the second SDS–PAGE gel for second dimensional separation of each protein band. The protein of interest was identified by MS. (C) The confirmed cleavage sites in metHb, and the structural features in the Hb fragments. The protein structures of Hb α, β subunits and the activated fragments were modelled (Supplementary Data). (D) Dose-dependent proteolytic activation of Hb-POX cycle by PAMPs. The profile of cleaved metHb is shown on Tris–Tricine SDS–PAGE and the Hb-POX cycle activity was quantified based on the production of superoxide by CLA-CL assay. ‘4 × ' is 1:4 molar ratio of metHb:PAMP. The rate of superoxide production was calculated from the linear portion of the CLA-CL curve. The fold increase was obtained by normalizing the data against the negative controls (metHb without treatment). Red boxes highlight progressive cleavages, disappearance of larger intermediate fragments and marginal reduction in the superoxide production in the presence of LPS or LTA.
Figure 1B shows metHb proteolysed by the microbial proteases, displaying similar cleavage profiles (left panel). Zymography detected the released POX cycle-active fragments. In contrast, the host trypsin did not proteolyse the metHb and no POX-active fragment was observed on the zymogram. Further resolution of the released fragments by second dimensional SDS–PAGE (Figure 1B, right panel), followed by mass spectrometry (MS), consistently identified POX cycle-active fragments derived from the Hb α1 and β subunits. N-terminal protein sequencing confirmed one cleavage site in each of the subunits: at A28-L29 in α1, and L48-S49 in β, resulting in four fragments—Hbα p12, Hbα p3, Hbβ p10 and Hbβ p5 (Figure 1C). Hbα p12 and Hbβ p10 contain the haem-oxygen binding pocket formed by two pairs of residues from the 3rd helix (H58 and V62 in α; H63 and V67 in β) and the 4th helix (L83 and H87 in α; L88 and H92 in β) on each of the subunits (Paoli et al, 1996).
Incubation of metHb with two combinations of PAMPs and proteases: LPS and elastase and LTA and subtilisin A (to represent the surface virulence factors of Gram-negative and -positive bacteria, respectively), showed potential collaboration between PAMPs and proteases on the proteolytic activation of the Hb-POX cycle activity (Figure 1D). The chemiluminescence analog–chemiluminescence (CLA-CL) assay, which detects the production of superoxide, was used to measure the instant activation of the POX cycle and the formation of free radicals. PAMPs alone neither proteolysed nor showed dosage effect on metHb, but induced 8–10% of POX cycle activity compared to the protease-treated metHb. As higher amounts of PAMPs evidently enhanced the cleavage of metHb by the microbial proteases (Figure 1D, ▾), we postulate that PAMPs act like detergents to relax the Hb structure possibly through binding to the Hb molecule, thus facilitating the proteolytic cleavage. Furthermore, such greater proteolysis of metHb in the presence of higher level of PAMPs (Figure 1D, ▾) was found to correlate with marginal reductions in the POX cycle activities (Figure 1D, ★), indicating that either further cleavages of active POX-bearing fragments have occurred, or there was blockage of substrate entry because of the accumulated PAMPs bound.
The proteolysed Hb fragments harbour dual-action centres: PAMP binding and POX cycle activity
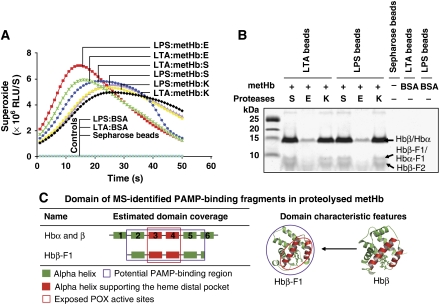
The binding of Hb to PAMPs prompted us to isolate the PAMP-binding fragments released on proteolysis of metHb. Hb fragments pulled down using PAMP-Sepharose, generated significant levels of superoxide (Figure 2A), implying the co-localization of the two activities (PAMP binding and POX free radical production) in the same fragments. A recurrent profile constituting three major Hb fragments of ⩽15 kDa was observed in both the LTA- and LPS-Sepharose pull-down fragments (Figure 2B), suggesting that all three microbial proteases targeted overlapping cleavage site(s) at the initial limited proteolysis. MS identified the bound proteins to be subunits of α and β and their respective fragments, Hbα-F1, Hbβ-F1 and -F2 (Supplementary Figure S2A). The Hbβ-F1 contains the haem-oxygen binding pocket at the 3rd and 4th helices (Figure 2C). Like the cleavage-activated Hbβ p10 (Figure 1C), the Hbβ-F1 contributed to the POX cycle activity. Furthermore, the isolated α and β subunits and β-F1 fragment indicate the presence of a cluster of PAMP-binding sites to the C-terminus of the Hb chains.
Figure 2.
The isolation and characterization of PAMP-binding fragments released on proteolytic activation of Hb-POX cycle. Pull-down of proteolysed fragments by PAMP-Sepharose. (A) The production of free radicals (represented by superoxide) in the fragments bound to the PAMP-Sepharose (denoted as PAMP:metHb:protease; ‘S', ‘E' and ‘K' are subtilisin A, elastase and proteinase K, respectively) was detected by CLA-CL assay. (B) The eluted metHb fragments were resolved on Tris–Tricine SDS–PAGE (10%), and the proteins were identified by MS. (C) The MS-inferred region of the isolated PAMP-binding fragments. The modelled domain structures of the respective fragments are indicated. Co-migration of fragments covering both N-/C-terminal sequences was detected as Hbβ-F2. Only one peptide mass fingerprint was identified for Hbα-F1.
Here, we found that the POX cycle activities in the isolated fragments have either been cleavage activated (e.g., in Hbβ-F1), or underwent further proteolysis (e.g., in Hbα, β). Taken together, these results clearly show the co-localization of both the PAMP-binding and POX cycle activity sites to the same fragments, thereby supporting the significance of such dual-action centres released on proteolytic attack of Hb by the microbial virulence factors.
Simulated infection releases pathogen-activated antimicrobial centres from Hb
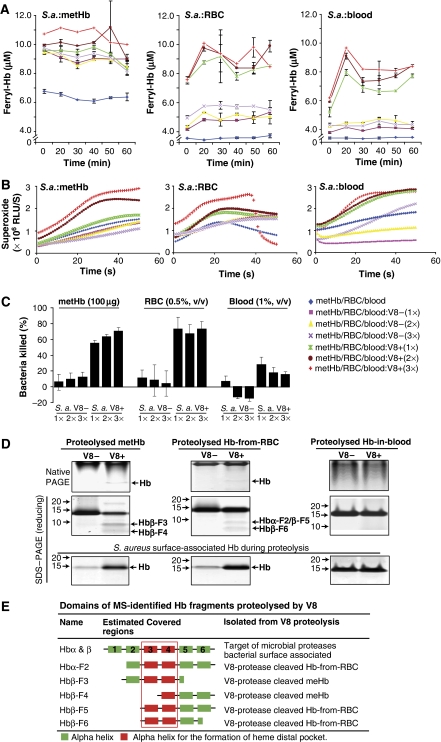
To confirm that the dual-action antimicrobial centres harboured in the Hb molecule are exposed through microbial proteolysis, the isolated metHb or the endogenous Hb contained in RBC (Hb-from-RBC) or in blood (Hb-in-blood) was examined in a reconstituted in vitro ‘infection-microenvironment', where a typical haemolytic S. aureus V8+/− and pathophysiological amount of H2O2 (Yamamoto et al, 1987) were introduced. The spectral changes of Hb and its derivatives were monitored (Winterbourn, 1990; Giulivi and Davies, 1994).
In this simulated infection, the V8+ S. aureus significantly induced rapid oxidation of metHb, Hb-from-RBC (HbFeII) and Hb-in-blood (HbFeII) as evidenced by the change in their absorption spectra (Supplementary Figure S3A), and concomitantly released higher levels of ferryl-Hb and superoxide (Figures 3A and B). The enhanced oxidation of Hb-from-RBC or Hb-in-blood by V8+ S. aureus in the presence of H2O2 was further supported by the increasing amount of metHb formed in these reactions (Supplementary Figure S3B), indicating the potential of Hb-POX cycle to be proteolytically activated on haemolytic infection. These activations simultaneously invoked 60–80% antimicrobial activity with metHb or Hb-from-RBC, and 20–40% antimicrobial activity with Hb-in-blood (Figure 3C). This difference in the antimicrobial activity could be due to the relatively lower level of production of free radicals because there was lesser amount of Hb present in the whole blood (only ∼0.33% RBC is present in 1% blood) (Figure 3A). Furthermore, other plasma factors such as haptoglobin (Gutteridge, 1987) may be involved in regulating the POX cycle activity, for which additional study will be required to delineate the mechanism.
Figure 3.
Proteolytic activation of metHb, Hb-from-RBC and Hb-in-blood by haemolytic S. aureus. 1 × , 2 × and 3 × annotate increasing doses of the V8 protease, where 1 × contains 2.5 U of V8 protease produced by 1.875 × 107 CFU of S. aureus. The protease activity was determined by azocasein assay and the viable bacterial count (CFU) was determined by spread plate method. For the V8− bacteria, the same volume of culture equivalent to the 1 × , 2 × and 3 × of the V8+ was applied. (A) The concentration of ferryl-Hb, which was formed during the course of incubation, was determined by its reaction with 2 mM Na2S. ‘S.a.' is S. aureus. (B) The end point production of superoxide was determined by CLA-CL assays. (C) Viable bacterial count was determined by spread plate method whereas the % of bacteria killed was calculated based on the negative controls. (D) The proteolysed Hb fragments were separated on both 12% native PAGE and Tris–Tricine SDS–PAGE (10%). Hb bound to the surface of S. aureus was eluted and resolved on SDS–PAGE. The proteins of interest (arrowed) were identified by MS. (E) Summary of the MS-inferred and modelled structures of the proteolysed Hb fragments activated by V8.
The V8-mediated proteolysis of Hb-in-blood was less apparent than that of the metHb or Hb-from-RBC (Figure 3D). MS analysis (Supplementary Figure S2B) traced the proteolysed fragments to the Hbα and β subunits (Figure 3D). Fragments of Hbβ-F3-6 and Hbα-F2, which are from the C-terminal region containing the 3rd and/or 4th helices of the haem-oxygen binding pocket (Figure 3E), indicate that V8 targets the N-terminii of both the α and β subunits. The proteolytic removal of the short N-terminii could have exposed the haem-oxygen-binding pocket, which immediately enhanced the POX cycle activity with concomitant production of the free radicals. Notably, the released Hb subunits (Figure 3D) were procured from the surface of the S. aureus, which were applied in the in vitro simulated infection. These Hb subunits/fragments were surface associated with the S. aureus probably through the potent PAMP-binding region at the C-terminus of each subunit. Therefore, it is conceivable that the cleavage-activated Hb subunits/fragments anchor onto the PAMPs, which are displayed on the pathogen while the juxtaposed POX cycle activity unleashes free radicals to kill the invader effectively.
Antioxidants curb the overproduction of Hb free radicals
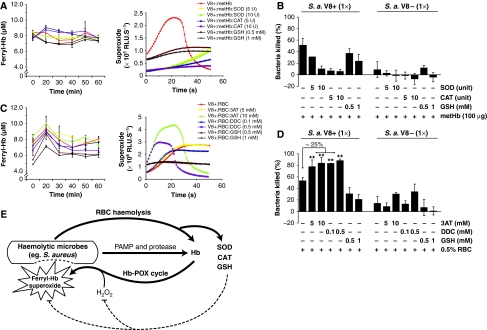
To show the involvement of antioxidants in the simulated infections, the levels of CAT, SOD, reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) were examined. We found that 57.82±5.61 and 16.90±1.32 Units/ml, respectively, of CAT and SOD were simultaneously released from the ruptured RBC, whereas the bacteria contributed 3.4±0.65 Units/ml of CAT and only trace level of SOD. The level of GSH and GSSG in the ruptured RBC were 22.42±1.82 and 1.09±0.79 mM, respectively. In total, these appreciable levels of antioxidants, particularly CAT from both the host and bacteria present in the reconstituted reactions, explain the attenuation of the free radical production in the V8+-activated Hb-from-RBC or Hb-in-blood compared with that of the metHb (Figures 3A and B). Thus, by mitigating the free radicals, the endogenous antioxidants contribute to oxidative resistance and may compromise the Hb-POX cycle activity. Furthermore, when CAT, SOD or GSH was supplemented into simulated infections, there was an apparent decline in the production of superoxide as detected by the CLA-CL assay (Figures 4A and C). However, the concentration of ferryl-Hb (Figures 4A and C) and the absorption spectra (Supplementary Figure 3C) did not show marked differences. This could be attributed to the lack of sensitivity of the spectral scanning and/or different inhibitory mechanism imposed by the antioxidants, for example, CAT inhibits the oxidation of metHb to ferryl-Hb by decomposing the H2O2 (Giulivi and Davies, 1994), hence resulting in a rapid conversion of ferryl-Hb to metHb (Supplementary Figure 3C, upper panel, red arrows); SOD would mainly scavenge the superoxide (McCord and Fridovich, 1969) from this reaction; and GSH inhibits the oxidation of Hb and scavenges the free radicals (Winterbourn, 1985). Overall, in the presence of these antioxidants, we observed a significant drop in the killing of V8+ S. aureus, but improved growth of V8− S. aureus (Figures 4B and D). These effects were dose dependent on CAT/SOD/GSH, confirming the protective role of the antioxidants, which act by counteracting the production and/or scavenging of the free radicals.
Figure 4.
Antioxidants (CAT, SOD and GSH) curb production of free radicals by Hb-POX cycle. (A, C) The free radical production, represented by the formation of ferryl-Hb and superoxide of V8+-activated metHb or Hb-from-RBC was reduced by addition of CAT, SOD and GSH. The CAT and SOD inhibitors, 3-amino-1,2,4-triazole (3AT) and diethyldithiocarbamate (DDC), respectively, rescued the production of free radicals from the POX-cycle. (B, D) The effect of antioxidants (CAT, SOD and GSH), and their inhibitors, on the bacteria killing induced by the activated metHb and Hb-from-RBC. **Statistical significance (P<0.05) as compared to the control (without inhibitors) was calculated by Student's t-test;1 × contains 2.5 U of V8 protease produced by 2.5 × 107 CFU of S. aureus, S.a. The protease activity was determined by azocasein assay and viable bacterial count (CFU) was determined by spread plate method. (E) On attack by virulent haemolytic microbes, the released Hb is proteolytically activated to Hb-POX cycle. H2O2 initiates the production of microbicidal free radicals, killing the microbes in proximity. Antioxidants, SOD, CAT and GSH, simultaneously released from RBC, remove excessive free radicals to further protect the host from self-cytotoxicity.
To further confirm the specific action of the antioxidants, we used inhibitors: 3-amino-1,2,4-triazole (3AT) against CAT (Van den Branden et al, 1984) and diethyldithiocarbamate (DDC) against SOD (Kelner and Alexander, 1986) in the reactions in which the inhibitory effect of the endogenous antioxidants on the production of free radicals declined dose dependently (Figure 4C), concomitant with the increasing amounts of oxidized metHb formed in the Hb-from-RBC (Supplementary Figure S3D). The results showed that in the presence of either CAT or SOD inhibitors, the V8 protease-activated Hb-from-RBC caused ∼25% increase in bacterial killing (Figure 4D), coincident with an instant substantial increase in ferryl-Hb formation and superoxide production (Figure 4C; Supplementary Figure S3C, lower panel, red arrows). Furthermore, besides inhibiting SOD, the DDC could also deplete GSH in the RBC (Kelner and Alexander, 1986), as manifested by the decrease in the ratio of GSH:GSSG dose dependently of DDC (Supplementary Figure S3E). This indicates an additional inhibitory action of DDC on the antioxidant, GSH. Taken together, all these results confirm that the simultaneously released antioxidants from the RBC had quenched the Hb free radical. We reasoned that nature has probably put in place such a countermeasure to curb the avalanche of free radicals and thereby protects the host from self-cytotoxicity (summarized in Figure 4E).
The biological significance of the activated antimicrobial centres in Hb
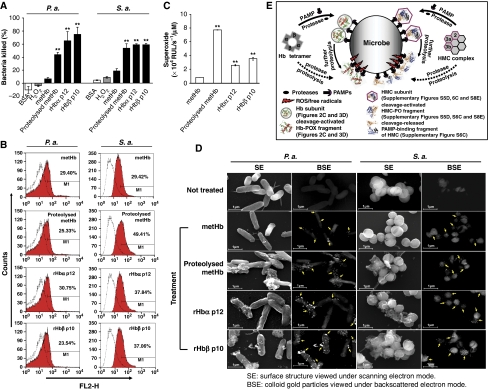
To test the biological significance of the decrypted dual-action centres from the Hb, the activated fragments were used to show the concurrent PAMP binding and free radical-induced microbicidal activity against live S. aureus and Pseudomonas aeruginosa. Supplementary Figure S4A summarizes the experimental strategy to compare the dual activities in the following activated Hb fragments: (i) partially proteolysed metHb; (ii) recombinant Hbα p12 (rHbα p12); and (iii) recombinant Hbβ p10 (rHbβ p10). Remnant viable bacterial counts showed that 1 μM of the activated Hb fragments killed up to 80% bacteria within 30 min in the presence of 0.5 μmol H2O2 (Figure 5A). Flow cytometry of the treated bacteria showed a significant fluorescence shift from 23.54 to 49.41% (Figure 5B), confirming that the activated fragments were bound to the bacterial surface. Altogether, the evidence on the significant POX cycle activity and the production of the free radicals/superoxide by the activated fragments, which were immobilized on the bacteria (Figure 5C), corroborates the dual-antimicrobial actions elicited in situ by the activated Hb. The intact metHb (negative control) was also bound to the bacterial surface through superficial PAMP-binding sites, but it only exhibited minimal POX cycle activity (see Figure 1D) and thus, negligible bactericidal activity, implying the capability of oxidative resistance in the bacteria (Lesniak et al, 2002; Chang et al, 2006).
Figure 5.
The biological effects of the activated antimicrobial centres in Hb, and a model for the ‘structure-function reprogramming' of respiratory proteins. (A) The bactericidal activities of Hb fragments were determined by bacterial viable count. The % killing was calculated based on the negative controls. (B) The binding of Hb fragments to the bacteria was analysed by flow cytometry. The fluorescence shift (red) was plotted against the negative control (white) bacteria incubated without the protein. FL2-H denotes the relative fluorescence intensity. (C) The POX cycle activity, represented by the production of superoxide, of 1 μM of the Hb fragments measured by CLA-CL assay. (D) SEM analysis of the bacteria treated with immunogold-labelled Hb fragments. The SE and BSE modes were used to view the bacterial surface morphology and the colloidal gold particles, respectively. The colloidal gold (arrows) shows the specific binding of Hb fragments to the bacteria. Magnification is × 25 600. **Statistical significance compared to that of the metHb, P<0.05, was calculated by Student's t-test. S.a.: S. aureus and P.a.: P. aeruginosa. (E) During infection, the extracellular microbial proteases cause limited proteolysis of the Hb/HMC complexes. The superficial subunits/regions of Hb/HMC can be directly proteolysed ( ), or alternatively, the released subunits bind to the bacterial surface (
), or alternatively, the released subunits bind to the bacterial surface ( ) through PAMPs and undergo further proteolysis, both of which expose ‘dual-action antimicrobial centres' (PAMP-binding and free radical production by POX cycle or PO) in the fragments. Co-localization of these two activities in the same fragments elicits concurrent ‘anchor-and-attack' strategy to kill the invading microbe and effectively stall the initial phase of infection.
) through PAMPs and undergo further proteolysis, both of which expose ‘dual-action antimicrobial centres' (PAMP-binding and free radical production by POX cycle or PO) in the fragments. Co-localization of these two activities in the same fragments elicits concurrent ‘anchor-and-attack' strategy to kill the invading microbe and effectively stall the initial phase of infection.
By using immunogold labelled SEM, we observed anchorage of the activated Hb fragments onto the bacteria, which caused morphological damage to the bacteria (Figure 5D). In contrast, the intact metHb was bound but did not injure the microbe. Similarly the two negative controls, 1 μM BSA or 0.5 μmol H2O2, did not injure the bacteria (Supplementary Figure S4B). The results clearly show the coordinated ‘anchor-and-attack' mechanism of the two antimicrobial centres in the Hb fragments.
The decryption mechanism of dual-antimicrobial centres is evolutionary conserved in respiratory proteins
To explore the fundamental significance of the infection-mediated proteolytic activation mechanism of oxygen-carrying proteins in the blood, we performed a comparative study on a functional homologue, HMC of an ancient invertebrate, the limulus. The HMC is known to be activated into PO by proteases, detergents or antimicrobial peptides (Decker et al, 2001; Nagai et al, 2001; Jiang et al, 2007). Hence, it was pertinent for us to examine whether microbial proteases and PAMPs also coordinate the structure-function activity transformation of HMC-PO during the host–pathogen interaction. Towards this, we examined purified HMC from the horseshoe crab, and its endogenous counterpart contained in the haemolymph (HMC-in-haemolymph).
Microbial proteases cleave HMC, releasing multiple fragments harbouring PAMP-binding and ROS-producing activities. Purified HMC was proteolysed by the microbial proteases, releasing PO-active fragments with a four- to five-fold enhancement of the ROS-producing activity (Supplementary Figure S5A). The second dimensional electrophoretic separation followed by MS showed five major fragments, which originated from subunits 2, 3a and 3b (Supplementary Figures S5B and C). N-terminal sequencing and analysis of domain organization showed that (i) HMC2 p60 and p56 harbour the di-copper oxygen-binding regions (CuA and CuB, P111–E374) for catecholoxidase (di-phenoloxidase) (Rosenzweig and Sazinsky, 2006), and (ii) HMC3a p32 and HMC3b p42 harbour the CuB oxygen-binding signature site (D354–D365 or D356–D367) for tyrosinase (monophenol monooxygenase) (Rosenzweig and Sazinsky, 2006) (Supplementary Figure S5D).
We have shown that PAMPs coordinate with proteases to proteolytically activate the HMC-PO (Supplementary Figure S5E). The effect of LPS is more pronounced than LTA (Supplementary Figure S5E, ★ and ▾), indicating a strong interaction between LPS and HMC. In contrast to metHb, the HMC-PO activity was induced up to 57 or 86%, respectively, by LPS or LTA alone compared with that generated by proteases alone (Supplementary Figure S5E, bar chart). Similar to Hb, the HMC fragments bound to PAMP-Sepharose showed significant PO activities (Supplementary Figure S6A). MS identified nine major fragments retrieved from the PAMP-Sepharose (Supplementary Figures S6B, C and S7A). The isolation of HMC3a-F1, HMC3b-F3 and -F4 fragments indicates that the PAMP-binding sites are located to the N- or C-domain. The HMC3a-F2, HMC3b-F1 and -F2, which harbour the CuA or CuB oxygen-binding sites in the M-domain, probably contribute to the detected PO activity of the PAMP-Sepharose bound fragments. Thus, similar to Hb, the virulence factors released HMC fragments carrying dual-action antimicrobial centres.
Simulated blood infection indicates evolutionary entrenchment of the mechanism of pathogen-mediated decryption of multiple antimicrobial sites. When the isolated HMC or HMC-in-haemolymph was incubated with virulent microbes, the PO activity was enhanced two- to five-fold causing 60–80% killing of S. aureus V8+ or P. aeruginosa elastase+ (Supplementary Figures S8A and B). Notably, the HMC-in-haemolymph killed ∼80% of the S. aureus even when the resulting PO activity was enhanced by only <two-fold (Supplementary Figure S8B, bar chart), indicating that other co-existing antimicrobial pathways (e.g., coagulation and complement pathways) also contribute to microbial clearance (Muta and Iwanaga, 1996; Smith et al, 1999). Simulated infection by live pathogens released a total of 24 fragments: HMC2 F1-F5; HMC3a F3-F18 and HMC3b F5-F7 (Supplementary Figures S7B and S8C and D). The MS data inferred a consensus coverage of the M-domain in combination with either partial N- or C-domain derived from the three subunits (Supplementary Figure S8E), emphasizing the importance of the M-domain to the cleavage-activated PO activity. The bacterial-binding fragments, such as HMC3a and 3b, HMC2-F5 and HMC3a-F17 (Supplementary Figure S8D), all contain intact M-domain, showing that both the potent PAMP-binding and the inducible PO activity are co-localized to the same subunit or fragment (Supplementary Figure S8E). This notion is further strengthened by the convergence of PO activity identified among the PAMP-binding fragments (Supplementary Figures S6C and S8E, **). Furthermore, it is possible that the other cleavage-activated PO-bearing fragments could bind PAMPs through the N- or C-domain, and consequently juxtapose the dual-antimicrobial actions.
Discussion
Our study has revealed a rapid reprogramming of the structure-activity relationship of the Hb molecule, which occurs at the immediate onset of infection. This process transforms the Hb from a tetrameric O2 carrier to activated subunits and fragments each harbouring dual-action antimicrobial centres. In the process of host–pathogen interaction, the activation of Hb-to-POX cycle was achieved through concerted actions of microbial proteases and PAMPs. The extracellular proteases of an invading microbe specifically attack the Hb, causing limited proteolysis and relaxing the structural rigidity between the α1-β2 and α2-β1 interfaces in the Hb tetramer. Ensuing from this conformational change, we observed a significant rise in free radical production, possibly because of a rapid oxidation of the haem-iron, which enhanced the POX cycle while consuming exogenous H2O2 (Alayash et al, 2001). The haem-containing fragments, Hbα p12 and Hbβ p10, that harbour the activated POX cycle activity probably contributed to the surge in free radical production.
The concerted action of PAMP and protease in activating Hb-POX cycle is evidenced by the in vitro reaction (Figure 1D). Conceivably, PAMPs relax the Hb tetramer, facilitating its proteolytic cleavage, which further increases the binding of more PAMPs to the released Hb fragments. This indicates that there are superficial as well as encrypted PAMP-binding sites within the tetrameric Hb molecule. The accumulation of PAMPs bound to the Hb fragments could in turn cause steric hindrance to the entry of substrates, resulting in a negative feedback regulation to prevent excessive POX cycle activity. The rationale for the concerted actions of PAMPs and proteases led us to successfully isolate the proteolysed Hb fragments, each of which contains both the free radical-producing and PAMP-binding activities (Figure 2). The co-localization and functional collaboration of the two active centres within the same Hb fragments were further confirmed in the in vitro reconstituted infection microenvironment (Figure 3). This suggests the significance of the decryption of multiple dual-action centres from the Hb during the host–pathogen interaction. Furthermore by SEM, immunogold labelling showed that the cleavage-activated Hb fragments docked on the bacterial surface, presumably causing on-site free radical-mediated bacterial killing.
It is plausible that such an instant and invader-localized oxidative shock through the anchorage of activated Hb onto the microbe ensures the microbicidal specificity and efficiency of the Hb free radical, although it appears that the antimicrobial activity could be swiftly downregulated by antioxidants (CAT, SOD or GSH) released from both the host's RBC and/or the microbe's, which probably scavenge the excessive free radicals. Thus, this ‘anchor-and-attack' mechanism is pre-empted from causing random cytotoxic damage to the host (Figure 4E). Conceivably, this prompt countermeasure likely occurs in the first instance of a systemic infection because first, persistent haemolysis or overwhelming ingestion of Hb/RBC by macrophages could compromise the host defence in vivo (Hand and King-Thompson, 1983); second, other parallel pathways such as complements (Smith et al, 1999), coagulation cascade (Muta and Iwanaga, 1996) or protease inhibitors (Jiang et al, 2009) would be anticipated to resume concurrently or soon after, to eliminate the infectious microbes, as continuous activation of Hb by microbial virulence factors could disrupt the local oxidant–antioxidant balance resulting in toxicity to the host tissues.
Interestingly, a comparative study on the functional homologue, HMC, from an ancient invertebrate, unveiled a well-conserved proteolytic activation mechanism of HMC-PO to counteract virulent microbes (Supplementary Figures S5, S6 and S8). The initial proteolytic cleavage on the surface of the HMC hexamer (Martin et al, 2007) removed the entire or part of the N-domain, to expose PO-active sites in the M-domain. This results in the immediate elevation of PO activity, which generates cytotoxic O-quinones from phenolic substrates, thus killing the bacteria effectively. We observed that PAMPs induced significant PO activity, which is similar to the action of detergents (Decker et al, 2001), thus strongly supporting our postulation that PAMP causes an initial conformational relaxation of the HMC to facilitate the microbial protease action. The co-localization of PAMP binding and PO-active sites in the HMC fragments provides strong evidence for the evolutionary origin of such dual-antimicrobial centres.
The conservation of the decryption mechanism to reveal multiple dual-action centres in the two evolutionarily distant respiratory proteins strongly indicates the importance of this incisive functional transformation mechanism underlying the host defence against microbial infection (Figure 5E). Insights gained from the antimicrobial action of respiratory proteins and identification of the antimicrobial sites, viz, PAMP binding and free radical-production regions close to the C-terminii of both proteins, may (i) be used for the development of surveillance strategies against clinically recalcitrant pathogens, in particular, S. aureus that possesses multi-antibiotic resistance and evades host complement killing (Foster, 2005), but is sensitive to the respiratory protein-mediated killing; (ii) provide vital clues for developing intervention strategies to improve the safety and efficacy of HBOC as a blood substitute. As the oxidative damaging potency of the Hb could be substantiated when confronted or contaminated by even trace amounts of bacterial PAMPs and/or proteases, it is particularly vital to ensure absolute absence of such ubiquitously occurring bacterial virulence factors from the HBOC.
Materials and methods
For each experiment, at least three independent tests were carried out. For the qualitative data, a typical representative result was presented; for the quantitative data, mean±s.d. was presented.
Biologicals and biochemicals
The purified human Hb was obtained from Sigma-Aldrich. Cell-free haemolymph (CFH) from horseshoe crab, Carcinoscorpius rotundicauda (collected from Kranji estuary, Singapore) was extracted (Ding et al, 1993) and HMC was purified from CFH (Jiang et al, 2007). Red blood cells were obtained from New Zealand white rabbits under national and institutional guidelines on ethics and biosafety (Institutional Review Board, Reference Code: NUS-IRB 06-149). S. aureus PC1839 [V8 active (+), kanamycinR] and AK3 [V8 inactive (−), kanamycinR and erythromycinR] (Karlsson et al, 2001), P. aeruginosa PAO1-Iglewski [elastase (+)] and PAO-B1A1 [elastase(−), tetracyclineR] (Toder et al, 1994) were used to simulate an in vitro infection microenvironment. The Escherichia coli Top10 and BL-21 (DE3) strains were used for DNA cloning and expression of recombinant proteins, respectively. The antibiotics used were kanamycin at 50 μg/ml, erythromycin at 5 μg/ml and tetracycline at 100 μg/ml. All the chemicals were obtained from Sigma-Aldrich unless otherwise stated.
MALDI-TOF-TOF MS and N-terminal protein sequencing
The protein bands of interest separated on PAGE gel were excised and subjected to the MALDI-TOF-TOF MS analysis and N-terminal sequencing (Supplementary data).
Partial proteolysis of metHb and HMC and determination of the POX cycle and PO activities
The ratio of protein:protease was in a pathophysiological range of μg:ng (Doring et al, 1984; Alayash, 1999). The conditions for partial proteolysis of metHb and HMC were optimized (Supplementary data; Supplementary Figures S9A and B). The spectral changes of Hb were monitored by spectrophotometric scanning between 500 and 700 nm (Winterbourn, 1990). The level of ferryl-Hb was determined based on the formation of sulphhaemoglobin by reacting with 2 mM sodium sulfide (Na2S). The concentration of sulphhaemoglobin was calculated using the extinction coefficient of 10.5 mM−1 at 620 nm (D'Agnillo and Alayash, 2001). The production of superoxide was measured by specific chemiluminescent-Cypridina luciferin analog–chemiluminescence (CLA-CL) assay (Nakano, 1990; Kawano et al, 2002). The PO activity was quantified biochemically (Supplementary data).
The resulting metHb-POX cycle and HMC-PO were zymographically analysed (Supplementary data). Cleaved metHb-POX cycle-active fragments and the phenol-stainable HMC-PO were separated on the second dimensional SDS–PAGE (Zhu et al, 2005). The protein of interest was identified by MS, and the precise cleavage sites determined by N-terminal sequencing.
The effect of PAMPs on the proteolytic activation of metHb-POX and HMC-PO was tested with combinations of elastase and LPS and subtilisin A and LTA. E. coli LPS 055:B5 and S. aureus LTA were used. The molar ratios of 4:1 and 1:1 and 6:1 and 1:1 between PAMP:metHb and PAMP:HMC, respectively, were used. The reaction mixtures were electrophoresed, and the POX cycle and PO activities were quantified.
Isolation of PAMP-binding fragments by PAMP-Sepharose affinity matrix
The LPS or LTA was separately conjugated to CNBr-activated Sepharose 4B according to the manufacturer's instructions (GE Healthcare). Partial proteolysis of metHb or HMC was ceased by 1 mM PMSF, and incubated with 50 μl slurry of LPS-/LTA-Sepharose at 4°C overnight. Incubation with BSA served as a negative control. The slurries were divided into two halves. For metHb reaction, one half was used for Hb-POX assay whereas the other was eluted with extraction buffer (10 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 0.1% SDS and 0.5% sodium deoxycholate). For HMC reaction slurry, one half was used for HMC-PO assay whereas the other was similarly eluted for analysis of bound proteins. After SDS–PAGE, the proteins of interest were identified by MS.
The in vitro simulated infection microenvironment
An amount of 100 μg metHb, 0.5% (v/v) RBC and 1% (v/v) blood or 60 μg isolated HMC, and 10% (v/v) CFH were incubated with bacteria (Supplementary data). Proteases at 2.5, 5.0 and 7.5 U, produced by the bacterial culture, were used to test the dosage effect. The protease activity was determined by azocasein assay (Supplementary data). Viable bacterial count (CFU) was determined by spread plate method. The Hb-POX cycle or HMC-PO activities were quantified biochemically. The spectral changes of Hb were monitored spectrophotometrically (500–700 nm). The released fragments containing Hb-POX cycle activity were analysed by native and reducing PAGE whereas the HMC-PO was examined by native PAGE and stained by phenol substrate followed by second dimensional SDS–PAGE. Proteins bound to the bacterial surface were eluted by extraction buffer, electrophoresed and MS identified. To show the effect of antioxidants against the free radicals produced by Hb-POX cycle, CAT and SOD activities, and the levels of reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) were measured (Supplementary data). To confirm the specific anti-oxidative role of CAT and SOD, the inhibitors, 3AT for CAT and DDC for SOD, were added in parallel reactions to derepress the CAT inhibition and SOD inhibition of the free radicals.
The antibacterial activities of the activated Hb fragments
In total 1 × 107 CFU of S. aureus PC1839 or P. aeruginosa PAO1 in PBS was added into a reaction mixture containing 0.5 μmol H2O2 and 1 μM of the Hb proteins (metHb, metHb partially proteolysed by subtilisin A, rHbα p12 or rHbβ p10). Details on the preparation of rHbα p12 and rHbβ p10 (Supplementary Figures S9C–E) are described in Supplementary data. To avoid over-disruption of bacterial cells, the reactions were incubated at room temperature for 30 min for viable bacterial count, or for 15 min for flow cytometry and immunogold-labelled scanning electron microscopy. The POX cycle-cum-free radical production, represented by the level of superoxide, was determined by CLA-CL assay.
For flow cytometry, the various Hb fragments-treated P. aeruginosa was fixed with 4% paraformaldehyde and blocked for 1 h with 5% BSA-PBS. The treated S. aureus was fixed and blocked with 10% fetal bovine serum–PBS. Two successive incubations of 1 h each were carried out with rabbit anti-Hb (1:500 diluted in PBS) and TRITC-conjugated goat anti-rabbit IgG (1:400 diluted in PBS) (Sigma). The tagged bacteria were resuspended in 200 μl of 0.5% paraformaldehyde–PBS before analysing on the BD FACSCalibur system. For SEM analysis by immunogold labelling, the treated bacteria were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde and blocked as mentioned above. Two incubations of 1 h each were carried out with rabbit anti-Hb and 20 nm colloidal gold-conjugated goat anti-rabbit IgG (Ted Pella, USA), both of which were 1:100 diluted in 0.1% BSA–PBS. The colloidal gold-labelled bacteria were subjected to standard procedures of post fixation, dehydration, infiltration and carbon coating for SEM sample preparation. The samples were viewed under the SEM (Philips XL-30 FEG SEM at 15 kV). The backscatter electron mode was used to observe colloidal gold particles labelled on the bacteria.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary data
Review Process File
Acknowledgments
This work was supported by MoE Tier 2 grant (JL Ding and B Ho). We thank Mdm J Howe for technical support in EM work, Dr BH Iglewski (University of Rochester School of Medicine and Dentistry) for P. aeruginosa PAO-Iglewski and PAO-B1A1; Dr S Arvidson (Karolinska University, Sweden) for S. aureus PC1839 and AK3.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alayash AI (1999) Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol 17: 545–549 [DOI] [PubMed] [Google Scholar]
- Alayash AI, Patel RP, Cashon RE (2001) Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal 3: 313–327 [DOI] [PubMed] [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ (2000) Role of quinones in toxicology. Chem Res Toxicol 13: 135–160 [DOI] [PubMed] [Google Scholar]
- Cashon RE, Alayash AI (1995) Reaction of human hemoglobin HbA0 and two cross-linked derivatives with hydrogen peroxide: differential behavior of the ferryl intermediate. Arch Biochem Biophys 316: 461–469 [DOI] [PubMed] [Google Scholar]
- Chang W, Small DA, Toghrol F, Bentley WE (2006) Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J Bacteriol 188: 1648–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agnillo F, Alayash AI (2001) Redox cycling of diaspirin cross-linked hemoglobin induces G2/M arrest and apoptosis in cultured endothelial cells. Blood 98: 3315–3323 [DOI] [PubMed] [Google Scholar]
- Decker H, Ryan M, Jaenicke E, Terwilliger N (2001) SDS-induced phenoloxidase activity of hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J Biol Chem 276: 17796–17799 [DOI] [PubMed] [Google Scholar]
- Ding JL, Navas MA III, Ho B (1993) Two forms of factor C from the amoebocytes of Carcinoscorpius rotundicauda: purification and characterisation. Biochim Biophys Acta 1202: 149–156 [DOI] [PubMed] [Google Scholar]
- Doring G, Dalhoff A, Vogel O, Brunner H, Droge U, Botzenhart K (1984) In vivo activity of proteases of Pseudomonas aeruginosa in a rat model. J Infect Dis 149: 532–537 [DOI] [PubMed] [Google Scholar]
- Foster TJ (2005) Immune evasion by staphylococci. Nat Rev Microbiol 3: 948–958 [DOI] [PubMed] [Google Scholar]
- Giulivi C, Davies KJ (1994) Hydrogen peroxide-mediated ferrylhemoglobin generation in vitro and in red blood cells. Methods Enzymol 231: 490–496 [DOI] [PubMed] [Google Scholar]
- Gutteridge JM (1987) The antioxidant activity of haptoglobin towards haemoglobin-stimulated lipid peroxidation. Biochim Biophys Acta 917: 219–223 [DOI] [PubMed] [Google Scholar]
- Hand WL, King-Thompson NL (1983) Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun 40: 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Tan NS, Ho B, Ding JL (2007) Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat Immunol 8: 1114–1122 [DOI] [PubMed] [Google Scholar]
- Jiang N, Thangamani S, Chor CF, Wang SY, Winarsih I, Du RJ, Sivaraman J, Ho B, Ding JL (2009) A novel serine protease inhibitor acts as an immunomodulatory switch while maintaining homeostasis. J Innate Immun 1: 465–479 [DOI] [PubMed] [Google Scholar]
- Kaca W, Roth RI, Levin J (1994) Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J Biol Chem 269: 25078–25084 [PubMed] [Google Scholar]
- Karlsson A, Saravia-Otten P, Tegmark K, Morfeldt E, Arvidson S (2001) Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect Immun 69: 4742–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Pinontoan R, Hosoya H, Muto S (2002) Monoamine-dependent production of reactive oxygen species catalyzed by pseudoperoxidase activity of human hemoglobin. Biosci Biotechnol Biochem 66: 1224–1232 [DOI] [PubMed] [Google Scholar]
- Kelner MJ, Alexander NM (1986) Inhibition of erythrocyte superoxide dismutase by diethyldithiocarbamate also results in oxyhemoglobin-catalyzed glutathione depletion and methemoglobin production. J Biol Chem 261: 1636–1641 [PubMed] [Google Scholar]
- Lesniak J, Barton WA, Nikolov DB (2002) Structural and functional characterization of the Pseudomonas hydroperoxide resistance protein Ohr. EMBO J 21: 6649–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A, Zunino P, D'Alessandro B, Piccini C (2007) An iron-regulated outer-membrane protein of Proteus mirabilis is a haem receptor that plays an important role in urinary tract infection and in in vivo growth. J Med Microbiol 56 (Pt 12): 1600–1607 [DOI] [PubMed] [Google Scholar]
- Mak P, Siwek M, Pohl J, Dubin A (2007) Menstrual hemocidin HbB115-146 is an acidophilic antibacterial peptide potentiating the activity of human defensins, cathelicidin and lysozyme. Am J Reprod Immunol 57: 81–91 [DOI] [PubMed] [Google Scholar]
- Martin AG, Depoix F, Stohr M, Meissner U, Hagner-Holler S, Hammouti K, Burmester T, Heyd J, Wriggers W, Markl J (2007) Limulus polyphemus hemocyanin: 10 A cryo-EM structure, sequence analysis, molecular modelling and rigid-body fitting reveal the interfaces between the eight hexamers. J Mol Biol 366: 1332–1350 [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055 [PubMed] [Google Scholar]
- Mocny JC, Olson JS, Connell TD (2007) Passively released heme from hemoglobin and myoglobin is a potential source of nutrient iron for Bordetella bronchiseptica. Infect Immun 75: 4857–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Johnson JL, Moore FA, Moore HB (2009) The USA Multicenter Prehosptial Hemoglobin-based Oxygen Carrier Resuscitation Trial: scientific rationale, study design, and results. Crit Care Clin 25: 325–356, Table of Contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T, Iwanaga S (1996) The role of hemolymph coagulation in innate immunity. Curr Opin Immunol 8: 41–47 [DOI] [PubMed] [Google Scholar]
- Nagai T, Osaki T, Kawabata S (2001) Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. J Biol Chem 276: 27166–27170 [DOI] [PubMed] [Google Scholar]
- Nakano M (1990) Determination of superoxide radical and singlet oxygen based on chemiluminescence of luciferin analogs. Methods Enzymol 186: 585–591 [DOI] [PubMed] [Google Scholar]
- Nishina Y, Miyoshi S, Nagase A, Shinoda S (1992) Significant role of an exocellular protease in utilization of heme by Vibrio vulnificus. Infect Immun 60: 2128–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G (1996) Crystal structure of T state haemoglobin with oxygen bound at all four haems. J Mol Biol 256: 775–792 [DOI] [PubMed] [Google Scholar]
- Parish CA, Jiang H, Tokiwa Y, Berova N, Nakanishi K, McCabe D, Zuckerman W, Xia MM, Gabay JE (2001) Broad-spectrum antimicrobial activity of hemoglobin. Bioorg Med Chem 9: 377–382 [DOI] [PubMed] [Google Scholar]
- Puppo A, Halliwell B (1988) Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J 249: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig AC, Sazinsky MH (2006) Structural insights into dioxygen-activating copper enzymes. Curr Opin Struct Biol 16: 729–735 [DOI] [PubMed] [Google Scholar]
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O (2004) Iron-source preference of Staphylococcus aureus infections. Science 305: 1626–1628 [DOI] [PubMed] [Google Scholar]
- Smith LC, Azumi K, Nonaka M (1999) Complement systems in invertebrates. The ancient alternative and lectin pathways. Immunopharmacology 42: 107–120 [DOI] [PubMed] [Google Scholar]
- Toder DS, Ferrell SJ, Nezezon JL, Rust L, Iglewski BH (1994) lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect Immun 62: 1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Branden C, Kerckaert I, Roels F (1984) Peroxisomal beta-oxidation from endogenous substrates. Demonstration through H2O2 production in the unanaesthetized mouse. Biochem J 218: 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC (1985) Free-radical production and oxidative reactions of hemoglobin. Environ Health Perspect 64: 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC (1990) Oxidative reactions of hemoglobin. Methods Enzymol 186: 265–272 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Brodsky MH, Baker JC, Ames BN (1987) Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem 160: 7–13 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Thangamani S, Ho B, Ding JL (2005) The ancient origin of the complement system. EMBO J 24: 382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary data
Review Process File