Abstract
The chaperone Hsc70 drives the clathrin assembly–disassembly cycle forward by stimulating dissociation of a clathrin lattice. A J-domain containing co-chaperone, auxilin, associates with a freshly budded clathrin-coated vesicle, or with an in vitro assembled clathrin coat, and recruits Hsc70 to its specific heavy-chain-binding site. We have determined by electron cryomicroscopy (cryoEM), at about 11 Å resolution, the structure of a clathrin coat (in the D6-barrel form) with specifically bound Hsc70 and auxilin. The Hsc70 binds a previously analysed site near the C-terminus of the heavy chain, with a stoichiometry of about one per three-fold vertex. Its binding is accompanied by a distortion of the clathrin lattice, detected by a change in the axial ratio of the D6 barrel. We propose that when Hsc70, recruited to a position close to its target by the auxilin J-domain, splits ATP, it clamps firmly onto its heavy-chain site and locks in place a transient fluctuation. Accumulation of the local strain thus imposed at multiple vertices can then lead to disassembly.
Keywords: chaperone, clathrin-coated vesicle, electron cryomicroscopy, membrane traffic
Introduction
Clathrin-coated vesicles transport cargo molecules, such as receptor-bound transferrin or LDL, from the plasma membrane to endosomes. Clathrin coats assemble as invaginating ‘pits' and dissociate after the enclosed vesicle has pinched off from the parent membrane (Roth and Porter, 1964; Anderson et al, 1977; Kirchhausen, 2000; Brett and Traub, 2006). The ATP-dependent chaperone, Hsc70, facilitates uncoating, providing the energy required to drive the clathrin assembly–disassembly cycle (Schmid et al, 1985; Greene and Eisenberg, 1990; Barouch et al, 1994). Like other members of the 70 kDa heat-shock protein family (Hsp70s), Hsc70 is an ATP-driven molecular clamp (Hartl and Hayer-Hartl, 2002). Its N-terminal, nucleotide-binding domain (NBD) couples rounds of nucleotide hydrolysis to stages of opening and closing of its C-terminal, substrate-binding domain. The latter has a groove to receive a hydrophobic peptide and a ‘lid' to close down over the bound peptide, after hydrolysis of ATP (Zhu et al, 1996). Hsp70s facilitate protein folding, by reducing aggregation and transiently stabilizing exposed, hydrophobic segments, and protein translocation, by preventing back diffusion. But how can a purely local mechanism of action drive a large-scale process like the disassembly of a clathrin coat?
Clathrin coats are lattices formed by the interdigitation of trimeric assembly units (triskelions), which have extended legs radiating out from a three-fold hub (Figure 1) (Ungewickell and Branton, 1981; Smith et al, 1998; Musacchio et al, 1999). The packing of individual triskelions is sufficiently flexible that both pentagonal and hexagonal (and occasionally heptagonal) rings can form (Cheng et al, 2007); 12 (or 12 plus the number of heptagonal facets) pentagons generate a closed structure. The symmetrical, D6-barrel lattice shown in Figure 1 can be prepared in reasonably high yield (with respect to other lattices) when clathrin triskelions self-assemble together with the endocytic adaptor, AP-2, under defined conditions in vitro (Fotin et al, 2004b). The structure of such a D6 barrel has been determined by electron cryomicroscopy (cryoEM) and single-particle analysis (Fotin et al, 2004b), to a resolution (about 8 Å) sufficient to place α-carbons of most residues, using as guides high-resolution X-ray crystallographic structures of two different fragments (Ter Haar et al, 1998; Ybe et al, 1999). Each triskelion leg comprises an elongated heavy chain (1675 residues), extending from the globular ‘terminal domain' at the N-terminus to the hub at the C-terminus, and a light chain. Except for the terminal domain and for about 75 residues at the C-terminus, the entire heavy chain consists of ∼40-residues, α-helical zig-zags, in eight approximate repeats of five zig-zags each. The compliance of the zig-zags allows a leg to adapt to variable curvature at different positions in the coat. The only well-ordered part of the light chain is a 71-residue α-helix, which interacts with a portion of the heavy chain relatively close to the hub.
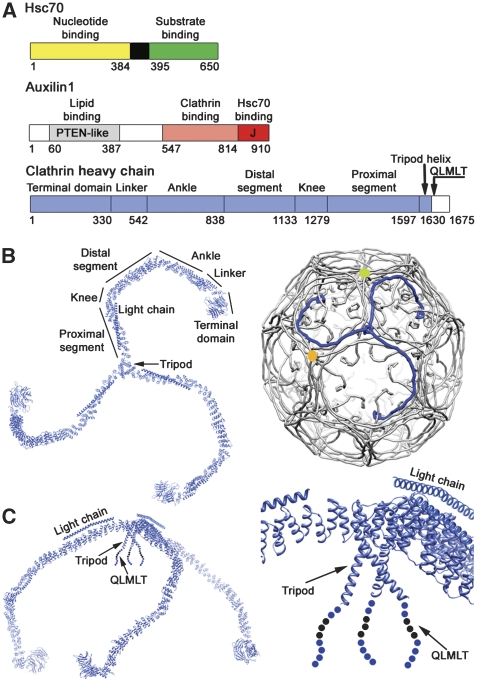
Figure 1.
Components of the clathrin uncoating process. (A) Domain organization of Hsc70 (top), auxilin (middle) and clathrin heavy chain (bottom). Residue numbers for domain or regional boundaries are shown below the bars. (B) A clathrin triskelion (left) and its packing within the lattice of a coat (right). The various regions of the heavy chain are labelled; the ordered, 71-residue α-helical segment of the light chain is also shown. Three symmetry-distinct vertices are colour-coded, yellow, blue (the hub of the blue triskelion) and green. (C) Side view of the triskelion (left), illustrating the pucker at the apex, and a close-up of the hub region, including the helical tripod and the QLMLT sequence near the C-terminus.
In the lattice, each triskelion leg (heavy chain) extends along three edges. As illustrated in Figure 1, the terminal domain, which projects inwards, connects into the first of the zig-zag repeats. The various zig-zag-repeat segments (linker, ankle, distal leg, knee, proximal leg) have acquired names mostly related to the meaning of ‘triskelion' as ‘three-legged'. The linker runs along part of an edge. The ankle crosses with two others beneath a vertex. The distal leg spans an edge at an intermediate radius, interacting closely with the proximal leg of another triskelion just ‘above' it. The knee bends gently at a vertex to allow the hub of the triskelion centred at that vertex to project inward. The proximal leg spans yet another edge and terminates at the three-fold hub structure, which has an inward projecting helical tripod, terminating in the only disordered segment in the entire heavy chain (residues 1630–1675). The C-terminus of the heavy chain thus faces terminal domains of three triskelions, each centred three vertices removed from the hub in question.
For so elaborately interdigitated a structure, the molecular contacts are relatively modest. The most extensive interface is the one between distal and proximal legs, mentioned in the preceding paragraph. At neutral pH, assembly requires the additional stability provided by interaction with clathrin adaptors or other accessory proteins and by the tendency of many of these proteins to aggregate, thus nucleating a relatively small structure like the D6 barrel (Vigers et al, 1986; Shih et al, 1995). At pH <6.2, assembly of triskelions into ‘cages' is spontaneous, but the distribution of sizes is broader.
Like all Hsp70 family members, Hsc70 requires a so-called J-domain containing protein to recruit it to a specific substrate (Hartl and Hayer-Hartl, 2002). The clathrin-linked J-domain protein is auxilin, a multi-domain protein that includes, in addition to C-terminal clathrin-binding and J-domain regions, a region with homology to the phosphoinositide phosphatase, PTEN (Ahle and Ungewickell, 1990; Ungewickell et al, 1995, 1997; Haynie and Ponting, 1996; Barouch et al, 1997). The timing of auxilin recruitment to a coated vesicle, immediately after budding, appears to determine its prompt uncoating (Lee et al, 2006; Massol et al, 2006). In vitro, a C-terminal fragment (residues 547–910), which includes the clathrin-binding and J-domain functions, is sufficient for Hsc70- and ATP-dependent uncoating (Holstein et al, 1996).
Also required for uncoating in vitro is the C-terminal segment of the heavy chain (Rapoport et al, 2008), which projects inward from the helical tripod within a funnel-like cavity defined by the three heavy-chain ankles that cross at that vertex (Figure 1B and C) (Fotin et al, 2004b). It contains a sequence (QLMLT, residues 1638–1642 in mammalian clathrin) that corresponds closely to the consensus sequence for optimal binding to the substrate groove in Hsc70 (Gragerov et al, 1994). Deletion or mutation of this short segment, or moving it closer to the triskelion hub, does not interfere with assembly, but it renders the assembled coats resistant to Hsc70, auxilin and ATP-dependent dissociation (Rapoport et al, 2008).
Binding of auxilin (547–910) to in vitro-assembled, D6-barrel coats saturates at one auxilin fragment per heavy chain (Fotin et al, 2004a). A cryoEM reconstruction has shown that each terminal domain binds an auxilin fragment, which also makes contacts with two other heavy chains in the lattice (Fotin et al, 2004a). The contact surface can explain the reported competition of auxilin with ‘clathrin-box' peptides that bind the terminal domain (Smith et al, 2004). This location for auxilin is appropriate for recruiting Hsc70 to the vicinity of the C-terminal peptide, its presumptive local substrate. An additional consequence of adding auxilin (547–910) is a change in the overall axial ratio of the barrel-like coat (Fotin et al, 2004a). Thus, even addition of auxilin locks in a global perturbation in the clathrin lattice.
We report in this paper the structure of a D6 clathrin barrel bound with Hsc70 recruited by auxilin (547–910), determined by cryoEM and single-particle analysis at 11 Å resolution. The Hsc70 associates with the C-terminal segment, as anticipated, with a stoichiometry of about one per three-fold vertex, giving rise to a globular density feature. ATP hydrolysis must take place to achieve strong Hsc70 binding, consistent with the ATPase cycle described above. Distortion of the clathrin lattice, even beyond the perturbation induced by auxilin (547–910), suggests that when Hsc70 splits ATP and clamps firmly onto the heavy-chain C-terminal segment, it locks in place a transient fluctuation to a locally strained configuration and that introduction of a critical number of such distortions favours disassembly. We propose that we have trapped an early uncoating intermediate, prevented by reduced pH from progressing toward dissociation.
Results
Preparation of auxilin-bearing clathrin coats with specifically bound Hsc70
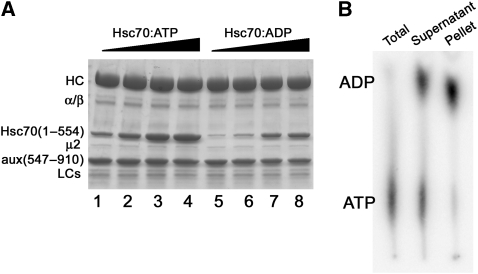
Hsc70 is relatively promiscuous in its binding propensity, and at high enough concentrations it associates extensively but non-specifically with clathrin coats. We, therefore, sought conditions under which we could obtain restricted, auxilin-dependent association of Hsc70. We screened for tight binding of Hsc70 (1–554):ATP with in vitro assembled clathrin/AP-2 coats bearing auxilin (547–910), prepared as described earlier (Fotin et al, 2004a). Hsc70 (1–554) is a C-terminally truncated form with diminished tendency to aggregate; it retains ATP- and auxilin-dependent uncoating activity (Jiang et al, 1997, 2005; Ungewickell et al, 1997). Auxilin (547–910) is a fragment sufficient to recruit Hsc70 and to stimulate uncoating; it encompasses the clathrin-binding and J-domains (Holstein et al, 1996) (Figure 1A). As a control for promiscuous, auxilin-independent binding, we used Hsc70 (1–554):ADP, which does not interact with auxilin (Holstein et al, 1996). We observed auxilin (547–910)-dependent binding only in the presence of ATP; non-hydrolysable ATP analogs (AMPPNP, AMPPCP, ATP-γS, ADP-AlF4, ADP-BeF3, ADP-vanadate) gave no increment over background binding. To prevent ATP-stimulated uncoating, we stabilized the coats by carrying out the incubation on ice at pH 6.0. We could saturate the coats by adding excess Hsc70 in a molar ratio to clathrin heavy chain of about 10:1. From Coomassie-blue-stained band intensities from SDS–PAGE, we estimated that at saturation, the Hsc70 bound in excess over the auxilin-independent background was ∼0.5 moles Hsc70 per mole clathrin heavy chain, or between one and two Hsc70 molecules per trimer (Figure 2A).
Figure 2.
Tight, auxilin-specific binding of Hsc70 depends on ATP hydrolysis. (A) SDS–PAGE of resuspended high-speed pellet from preparation of coats, bound with saturating amounts of auxilin (547–910) and incubated with increasing concentrations of Hsc70:ATP (lanes 1–4) or Hsc70:ADP (lanes 5–8). See Materials and methods for details. (B) Hsc70 associated with coats has hydrolysed ATP. TLC analysis showing 32P-labelled nucleotide in the mixture at the time of Hsc70:ATP addition and after separation by centrifugation into supernatant (free Hsc70 with both free and bound nucleotides) and pellet (Hsc70 and nucleotide bound to coats).
The failure of non-hydrolysable ATP analogs to stimulate auxilin-dependent association with coats suggests that ATP hydrolysis is necessary for tight binding. Analysis of the nucleotide composition of the Hsc70-containing coats showed essentially no residual ATP (Figure 2B), under conditions in which substantial quantities of unhydrolysed ATP remained in the solution. We conclude that the preparation we have described yields coats to which auxilin (547–910) has recruited Hsc70:ATP, with subsequent nucleotide hydrolysis. This conclusion is consistent with the known properties of Hsc70 and other Hsp70 homologs: the chaperones associate with J-domain-containing co-chaperones in their ATP-bound form, while subsequent tight attachment to the substrate requires ATP hydrolysis.
Electron cryoEM of D6 coats with bound auxilin and Hsc70
We obtained an image reconstruction from about 1500 ‘best' D6-coat images, selected from the original 14 000-particle stack. Image selection was based on phase residuals at successive stages of refinement (Fotin et al, 2006). The nominal resolution, using a Fourier-shell correlation (FSC) cutoff of 0.143, is 15.2 Å. As the coat has nine copies of the clathrin heavy chain within each D6 asymmetric unit, we could improve the resolution and enhance signal-to-noise by averaging corresponding segments of the triskelion legs, as described (Fotin et al, 2004b, 2006). The FSC-estimated resolution of the non-coat-symmetry (n.c.s.) averaged map is 11.3 Å (Supplementary Figure S1). This estimate is consistent with the appearance of the map, in regions of known molecular structure.
Clathrin coats are less rigid and less uniform than icosahedral virus particles or ribosomes, and elimination of particles with high phase residuals selects for minimally distorted coats (Fotin et al, 2004b, 2006). To verify that stringent selection of undistorted particles did not affect the molecular interpretation, we compared the model based on our final map with one of the intermediate maps obtained in the course of refinement—a reconstruction at 21 Å resolution derived from about 7000 particles (Supplementary Figure S2). All the features described and analysed here can be seen in this lower resolution map, which was not subjected to n.c.s. averaging (Supplementary Figure S2). To validate directly that discarded particles with high phase residuals are distorted in some way, we carried out a multi-reference alignment of the complete data set using six classes. The largest class contained about 44% of the particles; the remaining five classes showed clear evidence of distortion or damage (see Materials and methods for details).
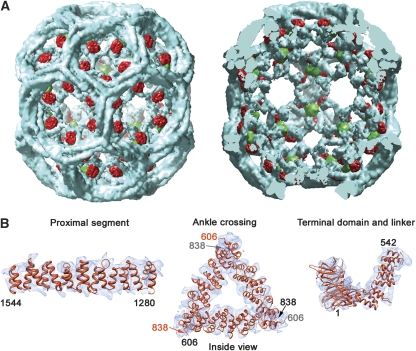
We fit models for individual segments of the clathrin heavy chain into the n.c.s. (nine-fold) averaged density by visual inspection, followed by computational rigid-body refinement. We used the proximal-leg, distal-leg pair (see next paragraph) as one rigid body, the terminal domain as a second, the ankles as a third, and the C-terminal tripod helix as a fourth. The knee bends variably at each of the nine D6-distinct locations, so a model for that region was fit to connect the appropriately placed, rigid-body refined segments just listed. The model matches well with density features throughout the structure (Figure 3). We also carried out exactly the same density averaging and model fitting procedure with the auxilin (547–910)-bound coat reconstruction (from Fotin et al, 2004a) to facilitate accurate comparison.
Figure 3.
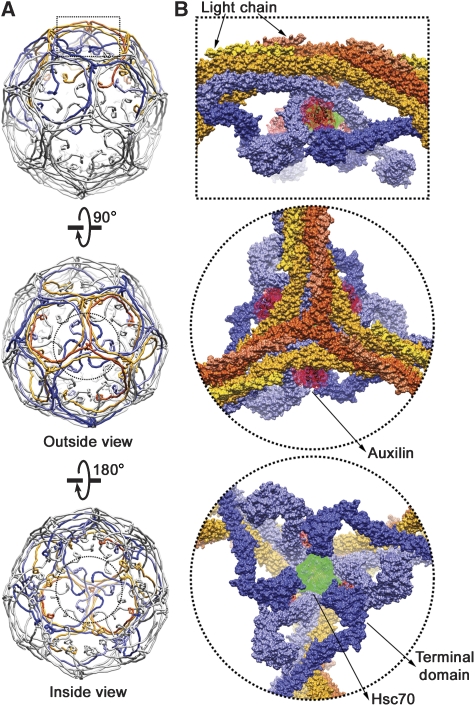
Image reconstruction of an Hsc70 (1–554):auxilin (547–910):clathrin coat. (A) Outside view (left) and cutaway view (right) of the complete coat. Clathrin is in blue, auxilin (547–910) is in red and Hsc70 (1–554) is in green. The boundaries of clathrin and the auxilin fragment are as in Fotin et al (2004b). The boundary of the Hsc70 was determined by comparing the new reconstruction with the previously published reconstruction of the auxilin complex. (B) Detailed views of the density map in specific regions, to illustrate the helical zig-zag and the fit of the heavy-chain model.
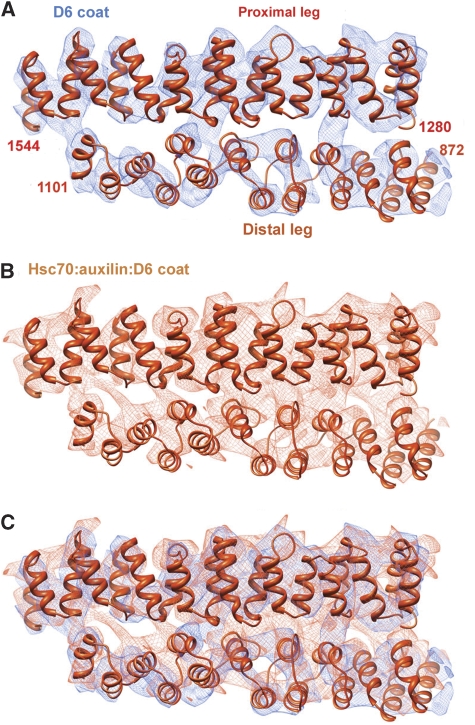
It is clear from comparison of the density maps for ‘native' (Fotin et al, 2004b), auxilin-bound (Fotin et al, 2004a) and Hsc70:auxilin-bound coats (this work) that the association of proximal and distal triskelion legs, which run parallel to each other along an edge and have an extended, radial contact, is essentially invariant, both among n.c.s.-related edges and among the three different states of the coat we have studied. Superposing the proximal segment densities from the three maps (after n.c.s. averaging) results in an excellent match of distal segment densities (Figure 4). This invariance suggests that our Hsc70:auxilin:clathrin coats maintain integrity in the presence of ATP through strengthening of the proximal–distal contact at pH 6. Low pH also favours clathrin assembly in vitro, even in the absence of adaptors or other assembly promoting components, and we propose that it is the invariant proximal–distal interface that determines the stability of these clathrin ‘cages'.
Figure 4.
Invariance of the proximal–distal contact. (A) The 8 Å resolution map of the D6 coat (Fotin et al, 2004a), with the model of corresponding heavy-chain segments. The view is in a direction tangential to the surface of a coat, with the exterior of the lattice above and the interior below. (B) Corresponding map and model for the Hsc70:auxilin:clathrin complex. (C) Superposition of the two, with the map from the uncomplexed coat in blue (as in A) and the map from the ternary complex in brown (as in B). The two maps were positioned to optimize agreement in the proximal-leg region, and the excellent superposition of the distal-leg maps shows that the interface does not shift when the ligands distort the coat.
Auxilin and Hsc70
Density features corresponding to auxilin (547–910) can be identified at three quasi-equivalent positions around each vertex, as described earlier (Fotin et al, 2004a). With reference to the triskelion centred at any particular vertex, each of the three adjacent auxilin fragments contacts the terminal domain from a triskelion centred three vertices away and the ankle region from one centred two vertices away (Figure 5). The C-termini of the reference triskelion project inward, within the triangle of auxilin fragments. The J-domain of auxilin is augmented, at its N-terminus, by two α-helices (Gruschus et al, 2004), and the composite structure (residues 797–910) docks into the maps in an orientation very similar to our earlier fit (Figure 5) (Fotin et al, 2004a). Density for the rest of the fragment (the clathrin-binding region) cannot yet be fit, as there is currently no atomic model for that part of auxilin.
Figure 5.
Relative positions of auxilin (547–910) and Hsc70 (1–554) in the complex. (A) Overview of the D6 coat, showing in dashed outline the region illustrated in close-up to the right. The lattices at the top and centre are viewed from outside; the lattice at the bottom is cut away at the front, and the indicated hub is viewed from the inside. (B) Close-up view, in surface rendering, of the hub indicated in (A). The triskelion centred at the vertex shown is in orange; triskelions centred at nearest-neighbour vertices are in yellow; triskelions centred at second nearest-neighbour vertices are in light blue and triskelions centred at third nearest-neighbour vertices are in dark blue. The auxilin fragment, outlined in red, lies between the dark blue terminal domains and the light blue ankle segments of clathrin. Hsc70, in green, binds in the funnel-like cavity bounded by these segments. The clathrin chains are in surface rendering from the molecular model; the auxilin and Hsc70 are in basket contours, based on the density.
We could assign the location of Hsc70 by computing local difference maps between the reconstructions with bound auxilin (547–910) alone and with Hsc70 added (Supplementary Figure S3). These maps were computed from non-n.c.s.-averaged reconstructions, low-pass filtered to 20 Å resolution. As shown in Figure 5, difference density at each vertex abuts the C-terminus of the helical tripod, within the triangular funnel formed by three crossing ankles and three terminal domains. A short segment in the disordered region of polypeptide chain, just C-terminal to the tripod helix, has a sequence that corresponds closely to the consensus for tight binding by Hsc70 (Gragerov et al, 1994; Fotin et al, 2004a). Mutation of this segment eliminates Hsc70:ATP-dependent uncoating and substantially reduces Hsc70 binding (Rapoport et al, 2008), and we can infer that Hsc70 clamps onto the C-terminal segment at some stage during the uncoating reaction. The location of Hsc70 density suggests that it is this clamped state that we have captured—an interpretation supported by our conclusion, from the data in Figure 2, that our structure contains Hsc70 at a stage immediately following nucleotide hydrolysis. The volume and roughly three-fold symmetric shape of the Hsc70 density both suggest that we have captured a single Hsc70 at each vertex, consistent with our estimate from band strengths in Figure 1A, and that the density feature is an average from molecules in three similarly occupied orientations. Indeed, the funnel leading to the Hsc70 site is too small to accommodate more than one uncoating enzyme. In the ADP state, the two domains of Hsc70 are not fixed with respect to one another. We propose that the J-domain interacting region of Hsc70 contacts one of the three, quasi-equivalent auxilins at a vertex and that the substrate-binding domain will then be oriented to find one of the three C-terminal tails of the tripod.
Conformational changes in the clathrin coat
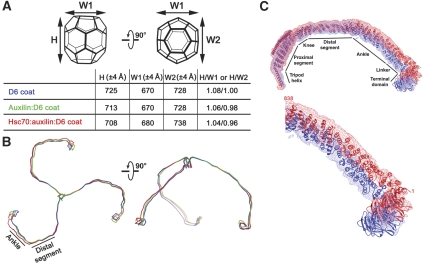
Association with uncoating factors alters the dimensions of the D6 barrel. The axial ratios shift by about 2% on binding auxilin (547–910) and by a total of about 4% on binding of both auxilin (547–910) and Hsc70 (1–554) (Figure 6A and B). Thus, the proportions of the entire barrel change when uncoating factors bind. We note that the axial ratio is insensitive to EM magnification and other scalar calibration factors. We also carried out a reconstruction of native D6 coats (in the absence of bound auxilin or Hsc70) at pH 6; the axial ratios are identical to those of native coats at pH 6.5 (Fotin et al, 2004b), and we can, therefore, rule out any purely pH-dependent (rather than uncoating-factor-dependent) mechanism.
Figure 6.
Conformational changes in clathrin that accompany binding of auxilin and Hsc70. (A) Axial ratios of D6 coats. The height (H) and two equatorial widths (W1 and W2), illustrated in the cartoon, are the distances between corresponding pairs of atoms at the outer margins of the molecular models. (B) Local changes in the conformation of the N-terminal parts of a triskelion in response to binding of auxilin (green) or auxilin plus Hsc70 (red). The reference triskelion is in blue. (C) Density maps and ribbon representations of a single triskelion leg from the unliganded coat (blue) and the auxilin:Hsc70-bound coat (red). Superposition determined at the hub of the triskelion, as in (B). Top: complete leg; bottom: detail of N-terminal region. The maps have been contoured generously, to show clearly the lower density of the terminal domain and linker; hence, the relatively ‘loose' fit of the proximal and distal legs.
To analyse the molecular basis for the axial-ratio change, we superposed the corresponding vertices of the three models, using as a common reference frame the three proximal–distal pairs that radiate around the vertex (Figure 6B and C). With bound uncoating factors, the crossed ankles shift radially outward to widen the opening around the foot of the helical tripod. The lever arm of the linkers, which connect into the ankle crossing from the terminal domains, amplifies the apparent shift, so that the terminal domains facing each vertex move even more noticeably away from each other. Auxilin alone appears to induce most of the change in the ankle crossing. Addition of Hsc70 widens the opening of the funnel around each tripod by enhancing the displacement of the terminal domains.
How do these local shifts produce large-scale changes in axial ratio and hence generate strain in the coat? The 36 vertices of the D6 barrel fall into three symmetry-distinct classes (see Figure 1). The axial ratio depends on the relative curvature at vertices of each class. The pucker at the apex of a triskelion is invariant: the local curvature at each vertex is determined not by a change in triskelion pucker but by a change in the angle between the two proximal–distal pairs that run antiparallel to each other along an edge (Fotin et al, 2004b). The distal-leg components of each of these pairs emanate from crossed ankles at the neighbouring vertices (Figures 1 and 5). The preferred geometry of the ankle crossing, therefore, propagates into curvature preferences, because the ankle crossing at one vertex is linked by a relatively rigid structural member (the distal–proximal pair) to the three neighbouring vertices. In an isotropic structure (like the 60-triskelion soccer ball), a change in curvature preference would simply raise or lower the free energy of the overall lattice. In the case of the D6 barrel, the minimum free energy structure in the presence of uncoating factors is evidently one in which the ratio of local curvatures among the symmetry-distinct vertices has changed, producing the observed change in axial ratio.
Discussion
We have determined a three-dimensional image reconstruction of a D6-barrel clathrin coat with bound auxilin (547–91) and Hsc70 (1–554):ADP. In addition to the densities for the added components, there are local shifts in parts of the clathrin heavy chain that propagate into changes in the axial ratios of the barrel. The resolution of about 11 Å has allowed us to adjust the molecular model of a clathrin lattice, derived from earlier work at 8 Å resolution (Fotin et al, 2004b), to fit these local shifts. We can, therefore, compare interactions and conformations at nine symmetry-distinct locations in each of three states of a coat (native; native:auxilin; and native: auxilin:Hsc70:ADP). A previous effort to visualize by cryoEM the location of Hsc70 did not use specifically bound auxilin to recruit Hsc70 and could not achieve sufficient resolution to pinpoint the position of Hsc70 (Heymann et al, 2005).
We draw four principal, qualitative conclusions. First, the interactions between parallel distal and proximal leg segments, which make up each of the two strut-like members of a lattice edge, are invariant. The precision afforded by the resolution is sufficient to determine that the geometry of contacts at the distal–proximal interface is conserved throughout each structure and among the three different states of the coat. Second, Hsc70 binds a C-terminal segment of the heavy chain within a funnel of surrounding protein (largely from the ankles and terminal domains of adjacent triskelions). It is likely that no more than one Hsc70 can occupy a binding position at each vertex. Third, ATP hydrolysis is required for the tight association of Hsc70 at the positions identified here. Fourth, the strain in the clathrin lattice, already evident from earlier work on coats with bound auxilin (547–910), is enhanced after binding of Hsc70.
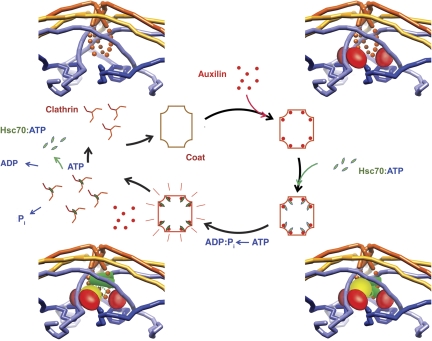
The complex we have analysed requires stabilization by lowered pH, which uncouples ATP hydrolysis from uncoating (Barouch et al, 1997). Our observations suggest a likely mechanism for this stabilization. Strengthening of the invariant distal–proximal contacts will allow the coat to resist the strain imposed successively by binding of auxilin and Hsc70, trapping it in a state that would fall apart were these contacts weaker. That is, we are looking at a structure that probably corresponds to a trapped intermediate in the uncoating pathway, unable to move toward dissociation (Figure 7).
Figure 7.
Model for the uncoating mechanism. The central diagram is a schematic representation of the underlying Hsc70/clathrin cycle, and the four corner diagrams show details of binding events at a vertex. Clockwise, from upper left: clathrin coat binds auxilin (red), which stabilizes a strained clathrin conformation (manifested by change in axial ratio of coat); auxilin recruits Hsc70:ATP (ATPase domain in yellow; substrate-binding domain in green); Hsc70 cleaves ATP and substrate-binding domain clamps tightly onto a specific segment of the disordered C-terminal tail of the heavy chain, trapping further strain in the clathrin lattice; when a large enough number of vertices have bound Hsc70, the accumulated strain causes the coat to dissociate, releasing auxilin, clathrin:Hsc70:ADP and Pi. Nucleotide exchange and dissociation of Hsc70 from clathrin complete the cycle.
All Hsp70-family proteins have an actin-like NBD and a peptide-binding domain (PBD), the latter comprising two subdomains—a β-sandwich and a helical bundle. Structures of the two domains (Flaherty et al, 1990; Zhu et al, 1996) and of the intact chaperone (or a homolog) in various states (Jiang et al, 2005; Liu and Hendrickson, 2007; Swain et al, 2007; Polier et al, 2008; Schuermann et al, 2008), and extensive mechanistic studies (reviewed in Hartl and Hayer-Hartl, 2002) lead to the following picture for one cycle of substrate interaction and nucleotide hydrolysis. When the NBD binds ATP, the NBD and PBD adopt a defined configuration relative to one another, fixing the linker segment between them (Vogel et al, 2006; Swain et al, 2007). ATP hydrolysis leads to loss of the tight inter-domain contact and flexion of the linker. ATP hydrolysis also allows the β-sandwich and helical-bundle subdomains of the PBD to close up against each other, clamping onto the bound peptide (which exchanges more freely from the ATP state than it does from the ADP-bound or apo-conformations). Although peptide binding stimulates the ATPase activity, a co-chaperone with a J-domain is essential for rapid transit through the full catalytic cycle that links ATP hydrolysis to peptide clamping and unclamping. The J-domain associates tightly with the Hsp70-family protein in its ATP-bound conformation, and the protein that bears the J-domain facilitates access of Hsc70 to substrate through other, target-specific, regions. Nucleotide exchange proteins also accelerate the cycle, by facilitating re-entry of ATP, opening of the clamp, and release of peptide substrate.
Auxilin, the J-domain protein that attaches Hsc70 to clathrin-coated vesicles, appears to have two distinct functions in the ATP-hydrolysis cycle that leads to uncoating. One is to introduce some distortion into the clathrin lattice—or at least to perturb that lattice from its ground state. The other is to recruit a generic chaperone (Hsc70) to a specific substrate (a clathrin coat) and to direct Hsc70 activity to coat disassembly. Supplementary Figure S4 shows the approximate location of the J-domain as we have placed it in part of the auxilin (547–910) density, similar to the fit proposed earlier (Fotin et al, 2004a). Will a J-domain in this position place a recruited Hsc70 in a suitable orientation to access its target? If we take the structure of a crosslinked complex between the auxilin J-domain and the Hsc70 NBD as representative of the relative position and orientation of the two components (Jiang et al, 2007), we can then align the NBD in that structure with the NBD of full-length Sse1:ATP (Liu and Hendrickson, 2007; Polier et al, 2008; Schuermann et al, 2008) to create an approximate hybrid model for the J-domain:Hsc70:ATP complex. Within the accuracy of these rough assumptions, this alignment places the Hsc70 PBD near the three-fold axis, in the vicinity of the clathrin C-termini (Supplementary Figure S4).
In cells, auxilin appears in endocytic clathrin-coated structures just after membrane scission (Lee et al, 2006; Massol et al, 2006). This timing in turn restricts Hsc70 recruitment to a stage at which the organizing function of the clathrin coat is complete. The affinity of auxilin for the coat is modest: we used 26 μM in the experiments described here. Retention of auxilin in freshly budded coated vesicles requires, in addition to the coat-binding interaction seen in our reconstructions, some activity of the N-terminal half of the polypeptide chain—presumably an interaction with the membrane bilayer. One proposed model for how this timing is determined posits that the PTEN-like module recognizes a specific lipid (e.g. a particular phosphoinositide) that is generated by a modifying enzyme (e.g. a phosphoinositide phosphatase) within the budding pit (Di Paolo and De Camilli, 2006; Massol et al, 2006). As long as the membrane of the pit is continuous with the parent membrane, the modified lipid will diffuse rapidly out of the bud site. Once the vesicle has pinched off, however, the lipid will accumulate in the coated vesicle membrane and hence help trap auxilin. In a typical endocytic-coated vesicle studied by live-cell imaging, there are about 200 clathrin heavy chains and an enclosed vesicle 600–700 Å in diameter (Ehrlich et al, 2004; Saffarian and Kirchhausen, 2008). If recruitment of each auxilin required a modified lipid molecule, the total number of such molecules would be <1% of the total in the vesicle bilayer, even to saturate the heavy chains with auxilin (probably an overestimate of the necessary occupancy). Thus, the specific lipid will not be a major component of coated vesicle membrane as isolated from cells or tissues.
The clathrin heavy chain, C-terminal ‘tail' contains the QLMLT sequence required for Hsc70-dependent uncoating (Rapoport et al, 2008). This segment projects from the helical tripod toward the crossed ankles of other heavy chains (Figures 1B and C and 6). In the initial description of coat organization from our laboratories, it was suggested that the C-terminal region might be an ‘ankle brace' to stabilize the assembly and that interaction with Hsc70 might then weaken the lattice (Fotin et al, 2004a). But the subsequent demonstration that recombinant clathrin lacking the entire C-terminal segment, including the QLMLT motif, assembles as well in vitro as does wild-type clathrin suggests that ‘ankle brace' may not be a correct description (Rapoport et al, 2008). Instead, our present structure suggests that Hsc70 binding fixes and amplifies a strained conformation initially induced by auxilin binding, and that the uncoating mechanism may depend not on withdrawing a stabilizing interaction (an ‘ankle brace') but rather on maintaining the strained conformation for an extended time, thereby increasing the likelihood of triskelion dissociation.
The model just proposed can be summarized as follows. First, distortion of the ‘ankle crossing' by auxilin binding allows access of Hsc70 to its consensus-binding site near the C-terminus of each clathrin heavy chain. Second, binding and ATP hydrolysis lock an Hsc70 uncoating enzyme onto (approximately) one such site per vertex, further distorting the ankle crossing. In our preparations, stabilization of other contacts (by lowered pH) prevents the distortions from propagating into uncoating. In this description, the effect of an Hsc70 bound beneath the tripod of a particular triskelion is not, primarily, on the interactions of that trimer, but rather on the stability of interactions that hold neighbouring trimers in the lattice. We can refer to this kind of mechanism as an ‘indirect' effect of Hsc70 binding (i.e. binding to one trimer primarily destabilizes neighbouring trimers, not the one with which it associates). An alternative set of models would postulate a ‘direct' effect on the triskelion to which the Hsc70 is bound. For example, Hsc70 binding might stabilize strain in the contacts of the proximal legs that come together at the hub to which the Hsc70 molecule in question is clamped. The perturbations seen in the cryoEM structure and the location of the bound Hsc70 do not seem to favour this model.
A further issue concerns the relationship of the distorted structure we have analysed to the pathway of productive dissociation. Is this an on-pathway intermediate or an off-pathway configuration trapped by the conditions under which the structure was determined? It is likely to be at least closely related to an on-pathway structure for three reasons. First, the structure has formed by precisely the sequence of biochemical events (auxilin binding by Hsc70:ATP, followed by ATP hydrolysis) believed to be critical for chaperone activity. Second, recruitment by auxilin has deposited Hsc70 onto the target site identified both by sequence consensus and by direct mutational analysis. Third, the distortions detected when auxilin binds and when it then recruits Hsc70 are consistent with each other and with a progressive destabilization of clathrin lattice interactions.
To test aspects of the mechanism we have outlined will require a number of new experiments, with observations on the dissociation kinetics of individual coats in vitro and in vivo. We do not yet know how many Hsc70 chaperones are needed to uncoat a single D6 barrel. As our determination of auxilin-dependent binding suggests, promiscuous association can confound direct measurement of bound chaperone. Moreover, the extensively interconnected character of the lattice implies that loss of one or two clathrins may not lead to cooperative dissociation of the rest. Reconstitution of coats from fluorescently tagged, recombinant clathrin should permit experiments on disassembly of individual particles, generating the sorts of data needed for quantitative assessment of the proposed mechanism.
The structure of the Hsc70:auxilin:coat complex and the qualitative features of the uncoating mechanism it suggests answer the question we posed at the outset: how can the local, ATP-driven clamping-unclamping cycle of Hsc70 drive a large-scale process like coat disassembly? The molecular organization of clathrin in the coat—in particular, the position of the specific Hsc70 attachment site at the base of a constricted funnel of criss-crossing ankles—has evolved to allow the tight attachment step in Hsc70 binding to capture a destabilizing fluctuation in clathrin conformation, so that build-up of strain at multiple vertices ultimately leads to dissociation. Moreover, auxilin may stabilize a first intermediate, in which the free energy of auxilin binding compensates for some of the distortion required ultimately to disassemble the lattice. Hsc70 homologs appear to function as ‘disassemblases' in other contexts—for example, in taking apart an origin recognition complex in DNA replication, the originally identified role for DnaK and DnaJ in bacteriophage λ replication (Alfano and McMacken, 1989). It has been suggested that they participate in the cytoplasmic uncoating of some viruses, although such proposals will remain purely speculative until the relevant J-domain containing protein has been identified. Viral capsids, like clathrin coats, are cooperatively assembling shells with interdigitating arms that could exploit accretion of local, ATP-driven clamps to produce a globally destabilizing cumulative strain.
Materials and methods
Specimen preparation
Bovine Hsc70 (1–554) with a C-terminal His-tag was expressed in Escherichia coli at 25°C, using a pET21a vector. The protein was purified using NiNTA, ion-exchange and gel filtration chromatography and stored in buffer S (20 mM MES pH 6.0, 2 mM MgCl2, 25 mM KCl, 10 mM (NH4)2SO4, 2 mM DTT) at −80°C. Bovine auxilin (547–910) was expressed as a GST fusion protein in E. coli at 25°C, using a pGEX4T-1 vector. Affinity purification was followed by thrombin cleavage to remove GST. Auxilin (547–910) was further purified using ion exchange and gel-filtration chromatography and stored in buffer S at −80°C.
Clathrin and AP2 were extracted from calf brain based on an established protocol (Matsui and Kirchhausen, 1990), and were further purified by hydroxyapatite chromatography on an Econo-Pac CHT-II column (BioRad). Coats were assembled from clathrin (0.5 ml, 2 mg/ml) and AP-2 (0.2 ml, 1.3 mg/ml) by dialysis overnight at 4°C against coat formation buffer (50 mM MES-Na, pH 6.5, 2 mM EDTA, 100 mM NaCl, 2 mM DTT) (Fotin et al, 2004b). Assembled coats were harvested by centrifugation and re-suspended at room temperature in 180 μl buffer S.
To determine the optimal ratio of Hsc70 to auxilin and clathrin for cryoEM analysis (Figure 2A), Hsc70(1–554) was incubated with auxilin-saturated coats in buffer S at different molar ratios with either 2 mM ATP or 2 mM ADP. Molar ratios of Hsc70 to clathrin heavy chain were 1:1 (lanes 1, 5), 3:1 (lanes 2, 6), 10:1 (lanes 3, 7) and 20:1 (lanes 4, 8). The coats were pelleted at 4°C for 25 min at 50 000 r.p.m. in a TLA70 rotor, and the resuspended pellets were analysed by SDS–PAGE.
To prepare Hsc70- and auxilin-bound clathrin coats for cryoEM, auxilin (547–910) at 3.5 mg/ml was incubated with coats at 2 mg/ml on ice for 30 min. Hsc70 (6 mg/ml) was incubated with a 100-fold excess of ATP at 25°C for 8 min, then chilled on ice before mixing with the auxilin-clathrin coats. The mixture was incubated on ice for 30 min with auxilin and Hsc70 at final concentrations of 26 μM each, clathrin coat at 2.6 μM (heavy chains), and ATP at 2.6 mM. The sample was diluted two-fold with buffer S just before flash-freezing to reach an optimal density of particles in a micrograph. It was applied to a holey carbon grid (Quantifoil Micro Tools GmbH, Germany) and flash-frozen in liquid ethane at −180°C using a FEI Vitrobot. Freezing conditions were optimized to embed the specimen in a very thin ice layer, to minimize background noise. A batch of 30 frozen grids was prepared and stored in liquid nitrogen.
Electron cryoEM and image processing
Grids of vitrified specimen were loaded on an Oxford cryo-transfer holder and imaged in a Philips Tecnai F20 electron microscope operated at an acceleration voltage of 200 kV. Images were recorded using low-dose procedures on Kodak SO-163 film at a nominal magnification of × 50 000 and underfocus values ranging from 2 to 5 μm. All micrographs were inspected visually, and only drift-free images were selected for digitization with a Zeiss SCAI scanner at 7 μm step size. Particles were selected from images using the display program Ximdisp associated with the MRC program suite (Crowther et al, 1996). The programs CTFFIND3 and CTFTILT (Mindell and Grigorieff, 2003) were used to determine average defocus value, astigmatism, tilt angle, and tilt axis for all digitized micrographs. Individual particle defocus values were adjusted from the average defocus at the micrograph centre by considering tilt and particle distance. FREALIGN V7.05 was used to determine particle rotation and translation as well as to compute a CTF-corrected three-dimensional reconstruction (Grigorieff, 2007). The high resolution limit was increased gradually from 40 to 8.4 Å over all parameter refinement cycles, and D6 symmetry was imposed during each reconstruction. Features at the centre of the particle were suppressed with a soft-edged mask (Fotin et al, 2004b, 2006), to eliminate density from randomly positioned AP2 complexes. FREALIGN was run on a 112-node Mac cluster managed by SBGrid (http://www.sbgrid.org).
The density we attribute to Hsc70 was calculated as a local difference map between the Hsc70/auxilin-D6 and auxilin-D6 reconstructions after low-pass filtering both maps to 20 Å and superposing them independently around each unique vertex. We used the program diffmap.exe (N Grigorieff), which scales the Fourier amplitudes of the transformed input map to match the average amplitudes of the reference map in each resolution shell. An alternative procedure implemented with SPIDER (Shaikh et al, 2008), which normalizes the two locally superimposed maps by matching average and standard deviation of the two maps before subtraction, resulted in the same difference density. The standard deviations (σ) of the noise in the reconstructions of Hsc70:Auxilin:D6 and Auxilin:D6 were estimated in each case from the difference between the reconstructions used for the FSC calculation (halfset reconstructions, see below). The σ of the noise in the Hsc70:Auxilin:D6–Auxilin:D6 difference map was estimated as the square root of the sum of squares of the noise standard deviations in each reconstruction. The isosurface representations of the Hsc70 density shown in Figures 3 and 5 and Supplementary Figure S3 were contoured at a level of 4 σ.
To evaluate the structural homogeneity of the Hsc70:Auxilin:D6 data set, we performed multi-reference alignment using six different references. To generate starting references, the data set was divided into six equal fractions, and reconstructions were calculated from each fraction. Each particle image was aligned against each of the six references, and its membership to a class was assigned according to the best phase residual. New reconstructions were calculated after class assignment, and the procedure was repeated for another 20 cycles, at which point the particle classes remained essentially unchanged. The largest class contained about 6500 particles (44% of the data set), resulting in a reconstruction at 21 Å resolution. The second largest class (17%) contained a coat with a height, H (as defined in Figure 6A), significantly smaller than observed for the largest class of Hsc70:auxilin:D6 coats, as well as for auxilin:D6 or unliganded D6 coats, suggesting distortions introduced during sample preparation. The remaining classes resulted in reconstructions that did not show distinct cage details, presumably due to the presence of misaligned, more significantly distorted or damaged particles. Decreasing or increasing the number of references (e.g. assuming four or eight references) did not significantly alter the sizes of the largest and second largest classes.
For model fitting, both clathrin and auxilin densities in the final map were improved using n.c.s. density averaging (Fotin et al, 2004a). We determined the relation between structurally equivalent segments of the docked atomic model in O (command LSQ_EXPLICIT) (Jones, 1992). The resulting operators were refined using MAVE (Uppsala Software Factory) (command IMPROVE) (Jones, 1992). The averaged density was projected onto a mask in reference position (command AVERAGE), then expanded onto masks in n.c.s.-related positions (command EXPAND). MAMA was used to remove density overlap before expansion. The FSC after n.c.s. averaging was computed from two soft-masked n.c.s. averaged halfset reconstructions each containing half the original number of particle images. Surface-rendered views of density were created using UCSF Chimera (Pettersen et al, 2004). Initial docking of clathrin model segments into the EM density map was carried out manually using the program O (Jones et al, 1991) and improved using rigid body refinement implemented in MAVE.
Maps and α-carbon coordinates for the auxilin:clathrin and Hsc70:auxilin:clathrin complexes were deposited in the EMDB (accession numbers EM5120 and EM5118).
Nucleotide composition analysis
Coats (1.2 mg/ml), assembled as described above in buffer S, were incubated with auxilin (547–910) (3.5 mg/ml in buffer S) for 30 min at 4°C. Hsc70 (1–554) (6 mg/ml in buffer S) was incubated with a 10-fold excess of ATP containing ATP (α-32P) (30 mCi) for 2 min at 4°C. Hsc70 (1–554)/ATP solution was mixed with auxilin-coat solution and the reaction mixture was incubated at 4°C for 15–20 min. The final volume of the reaction mixture was 100 μl, with 9.0 μM auxilin and 9.0 μM Hsc70 (1–554) both at 2.5-fold molar excess over clathrin heavy chain, and 90 μM ATP. The reaction mixture was carefully layered onto a 100 μl cushion of buffer S with 15% glycerol in a TLA100 centrifuge tube. After centrifugation at 100 000 r.p.m. for 12 min at 4°C, the supernatant was withdrawn and any remaining supernatant removed by injecting 800 μl of buffer S with 15% glycerol from the bottom of tube, allowing supernatant to flow over the top edge. Glycerol containing buffer was removed, the centrifuge tube rinsed gently with buffer S (without perturbing the pellet), and the pellet resuspended in 30 μl buffer S. Nucleotides in the supernatant and pellet were extracted by heating to 100°C for 1 min with 0.2% SDS and 10 mM EDTA.
Total 32P-labelled nucleotides in each sample were determined by scintillation counting using ScintiSafe Econo 1 scintillant and a Tricarb 1900CA liquid scintillation analyzer. The ATP to ADP/Pi ratio was determined by storage phosphor autoradiography (Molecular Dynamics Storm 860 scanner) after separation of nucleotides by thin layer chromatography on polyethylene imine cellulose plates developed with aqueous 1 M formic acid containing 0.7 M LiCl.
The fraction of nucleotides in the pellet sample due to contamination with supernatant was determined by addition of fluorescein to the reaction mixture at a final concentration of 3 μM before centrifugation, and the fluorescence of pellet and supernatant fractions measured in a glass-bottom, 96-well plate on a Bio-Tek Synergy 2 fluorescence plate reader. For these measurements, 5 μl of each sample was mixed with 200 μl 100 mM Tris–HCl buffer (pH 7.4). Buffer was used to determine background fluorescence. Nucleotide contamination from the supernatant was estimated to be 4 (±2)% based on the fraction of fluorescence intensity in the pellet sample.
Supplementary Material
Supplementary Figures S1–S4
Review Process File
Acknowledgments
We thank Werner Boll for help with clathrin preparations; Yifan Cheng and Zhongli Li for instruction and assistance with EM data collection; Tom Walz for access to the Tecnai F20 microscope. The work was supported by NIH Ruth Kirschstein National Research Service Award (to YX), by a Human Frontier Science Program Fellowship (to TB) and by NIH grants GM-36548 (to TK) and GM-62580 (to NG and SCH). NG and SCH are Investigators in the Howard Hughes Medical Institute.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahle S, Ungewickell E (1990) Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol 111: 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano C, McMacken R (1989) Heat shock protein-mediated disassembly of nucleoprotein structures is required for the initiation of bacteriophage lambda DNA replication. J Biol Chem 264: 10709–10718 [PubMed] [Google Scholar]
- Anderson RG, Brown MS, Goldstein JL (1977) Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell 10: 351–364 [DOI] [PubMed] [Google Scholar]
- Barouch W, Prasad K, Greene L, Eisenberg E (1997) Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry 36: 4303–4308 [DOI] [PubMed] [Google Scholar]
- Barouch W, Prasad K, Greene LE, Eisenberg E (1994) ATPase activity associated with the uncoating of clathrin baskets by Hsp70. J Biol Chem 269: 28563–28568 [PubMed] [Google Scholar]
- Brett TJ, Traub LM (2006) Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol 18: 395–406 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Boll W, Kirchhausen T, Harrison SC, Walz T (2007) Cryo-electron tomography of clathrin-coated vesicles: structural implications for coat assembly. J Mol Biol 365: 892–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA, Henderson R, Smith JM (1996) MRC image processing programs. J Struct Biol 116: 9–16 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T (2004) Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118: 591–605 [DOI] [PubMed] [Google Scholar]
- Flaherty KM, DeLuca-Flaherty C, McKay DB (1990) Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 346: 623–628 [DOI] [PubMed] [Google Scholar]
- Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T (2004a) Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432: 649–653 [DOI] [PubMed] [Google Scholar]
- Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T (2004b) Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432: 573–579 [DOI] [PubMed] [Google Scholar]
- Fotin A, Kirchhausen T, Grigorieff N, Harrison SC, Walz T, Cheng Y (2006) Structure determination of clathrin coats to subnanometer resolution by single-particle cryo-electron microscopy. J Struct Biol 156: 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragerov A, Zeng L, Zhao X, Burkholder W, Gottesman ME (1994) Specificity of DnaK-peptide binding. J Mol Biol 235: 848–854 [DOI] [PubMed] [Google Scholar]
- Greene LE, Eisenberg E (1990) Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem 265: 6682–6687 [PubMed] [Google Scholar]
- Grigorieff N (2007) FREALIGN: high-resolution refinement of single particle structures. J Struct Biol 157: 117–125 [DOI] [PubMed] [Google Scholar]
- Gruschus JM, Han CJ, Greener T, Ferretti JA, Greene LE, Eisenberg E (2004) Structure of the functional fragment of auxilin required for catalytic uncoating of clathrin-coated vesicles. Biochemistry 43: 3111–3119 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Haynie DT, Ponting CP (1996) The N-terminal domains of tensin and auxilin are phosphatase homologues. Protein Sci 5: 2643–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Iwasaki K, Yim YI, Cheng N, Belnap DM, Greene LE, Eisenberg E, Steven AC (2005) Visualization of the binding of Hsc70 ATPase to clathrin baskets: implications for an uncoating mechanism. J Biol Chem 280: 7156–7161 [DOI] [PubMed] [Google Scholar]
- Holstein SE, Ungewickell H, Ungewickell E (1996) Mechanism of clathrin basket dissociation: separate functions of protein domains of the DnaJ homologue auxilin. J Cell Biol 135: 925–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Maes EG, Taylor AB, Wang L, Hinck AP, Lafer EM, Sousa R (2007) Structural basis of J cochaperone binding and regulation of Hsp70. Mol Cell 28: 422–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Prasad K, Lafer EM, Sousa R (2005) Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell 20: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang RF, Greener T, Barouch W, Greene L, Eisenberg E (1997) Interaction of auxilin with the molecular chaperone, Hsc70. J Biol Chem 272: 6141–6145 [DOI] [PubMed] [Google Scholar]
- Jones TA (1992) A, yaap, asap, @#*? A Set of Averaging Programs. In CCP4 Proceedings pp 91–105. Warrington, UK: SERC Daresbury Laboratory [Google Scholar]
- Jones TA, Zou J-Y, Cowan SW (1991) Improved methods for building protein models in electron desntiy maps and the location of errors in these models. Acta Cryst A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T (2000) Three ways to make a vesicle (Review). Nat Rev Mol Cell Biol 1: 187–198 [DOI] [PubMed] [Google Scholar]
- Lee DW, Wu X, Eisenberg E, Greene LE (2006) Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci 119: 3502–3512 [DOI] [PubMed] [Google Scholar]
- Liu Q, Hendrickson WA (2007) Insights into Hsp70 chaperone activity from a crystal structure of the yeast Hsp110 Sse1. Cell 131: 106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol RH, Boll W, Griffin AM, Kirchhausen T (2006) A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci USA 103: 10265–10270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Kirchhausen T (1990) Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry 29: 10791–10798 [DOI] [PubMed] [Google Scholar]
- Mindell JA, Grigorieff N (2003) Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 142: 334–347 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BMF (1999) Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol Cell 3: 761–770 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Polier S, Dragovic Z, Hartl FU, Bracher A (2008) Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133: 1068–1079 [DOI] [PubMed] [Google Scholar]
- Rapoport I, Boll W, Yu A, Böcking T, Kirchhausen T (2008) A motif in the clathrin heavy chain required for the hsc70/auxilin uncoating reaction. Mol Biol Cell 19: 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TF, Porter KR (1964) Yolk Protein Uptake in the Oocyte of the Mosquito Aedes Aegypti. L J Cell Biol 20: 313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarian S, Kirchhausen T (2008) Differential evanescence nanometry: live-cell fluorescence measurements with 10-nm axial resolution on the plasma membrane. Biophys J 94: 2333–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, Braell WA, Rothman JE (1985) ATP catalyzes the sequestration of clathrin during enzymatic uncoating. J Biol Chem 260: 10057–10062 [PubMed] [Google Scholar]
- Schuermann JP, Jiang J, Cuellar J, Llorca O, Wang L, Gimenez LE, Jin S, Taylor AB, Demeler B, Morano KA, Hart PJ, Valpuesta JM, Lafer EM, Sousa R (2008) Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol Cell 31: 232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, Leith A, Frank J (2008) SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat Protoc 3: 1941–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T (1995) A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J Biol Chem 270: 31083–31090 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Dafforn TR, Kent H, Sims CA, Khubchandani-Aswani K, Zhang L, Saibil HR, Pearse BM (2004) Location of auxilin within a clathrin cage. J Mol Biol 336: 461–471 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Grigorieff N, Pearse BM (1998) Clathrin coats at 21 Å resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J 17: 4943–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M, Gierasch LM (2007) Hsp70 chaperone ligands control domain association via an allosteric mechanism mediated by the interdomain linker. Mol Cell 26: 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Haar E, Musacchio A, Harrison SC, Kirchhausen T (1998) Atomic structure of Clathrin—a b propeller terminal domain joins an a Zigzag linker. Cell 95: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E, Branton D (1981) Assembly units of clathrin coats. Nature 289: 420–422 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE (1997) Functional interaction of the auxilin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem 272: 19594–19600 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378: 632–635 [DOI] [PubMed] [Google Scholar]
- Vigers GP, Crowther RA, Pearse BM (1986) Location of the 100 kD50 kD accessory proteins in clathrin coats. EMBO J 5: 2079–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M, Mayer MP, Bukau B (2006) Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J Biol Chem 281: 38705–38711 [DOI] [PubMed] [Google Scholar]
- Ybe JA, Brodsky FM, Hofmann K, Lin K, Liu SH, Chen L, Earnest TN, Fletterick RJ, Hwang PK (1999) Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature 399: 371–375 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA (1996) Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272: 1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S4
Review Process File