Abstract
We report the structure of an integrin with an αI domain, αXβ2, the complement receptor type 4. It was earlier expected that a fixed orientation between the αI domain and the β-propeller domain in which it is inserted would be required for allosteric signal transmission. However, the αI domain is highly flexible, enabling two βI domain conformational states to couple to three αI domain states, and greater accessibility for ligand recognition. Although αXβ2 is bent similarly to integrins that lack αI domains, the terminal domains of the α- and β-legs, calf-2 and β-tail, are oriented differently than in αI-less integrins. Linkers extending to the transmembrane domains are unstructured. Previous mutations in the β2-tail domain support the importance of extension, rather than a deadbolt, in integrin activation. The locations of further activating mutations and antibody epitopes show the critical role of extension, and conversion from the closed to the open headpiece conformation, in integrin activation. Differences among 10 molecules in crystal lattices provide unprecedented information on interdomain flexibility important for modelling integrin extension and activation.
Keywords: αI domain, integrin, structure
Introduction
Integrins are αβ heterodimeric adhesion receptors that transmit signals bidirectionally across the plasma membrane and link ligand binding to force transmission by the cytoskeleton (Springer and Wang, 2004; Luo et al, 2007). Four domains in the α-subunit and eight in the β-subunit are conserved in the ectodomain of all metazoan integrins. Additionally, an αI domain is inserted in the α-subunit of 9 of 18 vertebrate integrins. αI domains are a late integrin evolutionary specialization found only in chordata, and have ligand-binding function when present. Ligand-binding affinity of the αI domain is allosterically regulated by its homologue in the β-subunit, the βI domain. An invariant Glu residue, located a few residues after the C-terminal α7-helix of the αI domain, is postulated to act as an ‘intrinsic ligand', which when bound to the active βI domain relays activation to the αI domain.
Integrin αXβ2, the complement receptor type 4 (CR4), is important for phagocytosis of particles opsonized with the complement product iC3b. αXβ2 also marks dendritic cells, and functions as a danger receptor for fibrinogen and denatured or cleaved proteins (Vorup-Jensen et al, 2005). Deficiency of αXβ2 and sister β2 integrins causes life-threatening bacterial infections. Ligands are recognized by an inserted (I) domain in the αX-subunit.
Integrins can be classified into two main subfamilies, distinguished by the presence or absence of an αI domain. Structural information to date is limited to ectodomains of two integrins that lack αI domains (αI-less integrins), αVβ3 (Xiong et al, 2001, 2002) and αIIbβ3 (Xiao et al, 2004; Springer et al, 2008; Zhu et al, 2008), and to small fragments of αI integrins, that is the isolated αI domain (Springer and Wang, 2004; Luo et al, 2007) and β2-leg fragments (Beglova et al, 2002; Shi et al, 2007).
An enduring mystery is how allostery is transmitted between the αI and the βI domains. A specific relay mechanism involving an αI domain C-terminal α-helix that slides axially has been proposed (Huth et al, 2000; Alonso et al, 2002; Yang et al, 2004). Furthermore, it has been proposed that the αI domain should be rigidly connected to other domains, because otherwise a change in interdomain orientation could replace α-helix sliding (Nishida et al, 2006). However, this proposed rigid connection raises the question of how two known conformational states of βI domains (Xiao et al, 2004) could couple to three known states of αI domains (Shimaoka et al, 2003; Jin et al, 2004).
Here, we report the crystal structure of a complete αI domain integrin ectodomain. This αI integrin, αXβ2, is in a bent, resting state, which differs significantly from that of known αI-less integrin structures. The αI domain is in the inactive conformation, consistent with the overall latent conformation of the ectodomain. Contrary to earlier expectation, the αI domain shows surprising flexibility. This flexibility has important implications for αI domain integrin function. The structure further reveals the relationships between the αI domain and other domains within an intact integrin ectodomain, and other unique features relevant to integrin activation and function.
Results and discussion
The crystal structures and their qualities
Crystals of αXβ2 were obtained in three lattices denoted A, B, and C (Table 1). Molecular replacement using integrin αIIbβ3 and αVβ3 structures as search models failed to yield solutions. Initial phases were obtained using Ta SAD data from a crystal soaked with tantalum bromide cluster (Table 1). Multiple-wavelength Se anomalous diffraction (MAD) data sets in lattices A and C were collected to 3.8 and 4.0 Å, respectively. The initial Ta SAD phases were then combined with the Se MAD data set in lattice A to obtain experimental phases. These phases were extended by density modification to 3.5 Å native data for lattice A. The αXβ2 structures built in lattice A were then used in molecular replacement to solve the structures in lattices B and C. The experimental phases for lattice C from Se MAD data were then obtained with help from the molecular replacement solution for lattice C and extended from 4.0 Å to native data at 3.7 Å. We thus have experimental phases for lattices A and C at 3.5 and 3.7 Å, respectively, and a molecular replacement solution for lattice B at 3.95 Å.
Table 1.
X-ray diffraction dataa
| Data set | Se MAD (A)b | Se MAD (C) | ||||
|---|---|---|---|---|---|---|
| Peak | Inflection | Remote | Peak | Inflection | Remote | |
| Space group | P212121 | I212121 | ||||
| Unit cell | ||||||
| a (Å) | 131.9 | 161.2 | ||||
| b (Å) | 162.8 | 166.4 | ||||
| c (Å) | 536.9 | 536.8 | ||||
| α (deg) | 90 | 90 | ||||
| β (deg) | 90 | 90 | ||||
| γ (deg) | 90 | 90 | ||||
| Wavelength (Å) | 0.97926 | 0.97942 | 0.96419 | 0.97926 | 0.97942 | 0.96419 |
| Resolution (Å) | 50–3.8 | 50–3.8 | 50–3.8 | 50–4.0 | 50–4.0 | 50–4.0 |
| Completeness (%) | 99.5 (99.4) | 99.5 (99.4) | 99.6 (99.5) | 99.3 (99.5) | 99.5 (99.7) | 99.3 (99.4) |
| I/σ(I) | 9.2 (2.0) | 7.6 (1.6) | 7.8 (1.6) | 8.8 (1.6) | 8.6 (1.7) | 10.7 (2.2) |
| Rmerge (%)c | 21.4 (131.2) | 26.8 (159.4) | 26.6 (156.5) | 19.5 (159.0) | 18.9 (153.7) | 15.0 (116.3) |
| Data set | Native (A) | Native (B) | Native (C) | Ta SAD (A) | ||
| Space group | P212121 | P212121 | I212121 | P212121 | ||
| Unit cell | ||||||
| a (Å) | 132.1 | 149.7 | 161.0 | 130.4 | ||
| b (Å) | 163.6 | 165.8 | 165.5 | 162.4 | ||
| c (Å) | 536.9 | 537.8 | 536.2 | 536.5 | ||
| α (deg) | 90 | 90 | 90 | 90 | ||
| β (deg) | 90 | 90 | 90 | 90 | ||
| γ (deg) | 90 | 90 | 90 | 90 | ||
| Wavelength (Å) | 0.97926 | 0.97928 | 0.97928 | 1.25467 | ||
| Resolution (Å) | 50–3.5 (3.61–3.50) | 50–3.95 (4.16–3.95) | 50–3.7 (3.92–3.70) | 50–5.0 | ||
| Completeness (%) | 99.9 (100.0) | 99.4 (99.4) | 99.4 (99.4) | 99.3 (99.0) | ||
| I/σ(I) | 12.0 (1.9) | 12.6 (1.9) | 12.6 (1.9) | 11.1 (3.5) | ||
| Rmerge (%)c | 16.1 (137.1) | 14.1 (143.2) | 10.4 (135.5) | 13.8 (62.6) | ||
| Number of reflections (total/unique) | 1 108 987/147 271 | 886 095/117 330 | 573 288/76 217 | |||
| Unique reflections (work/free) | 145 735/1536 | 116 309/1021 | 75 374/843 | |||
| Rworkd/Rfree (%)e | 29.7 (43.3)/ 33.5 (43.8) | 35.0 (39.9)/ 37.3 (42.4) | 31.8 (41.8)/ 33.3 (41.3) | |||
| RMSD | ||||||
| Bond (Å) | 0.003 | 0.003 | 0.004 | |||
| Angle (deg) | 0.648 | 0.643 | 0.722 | |||
| Ramachandran plotf | 78.7/0.98 | 78.7/1.1 | 78.1/1.65 | |||
| Molecules/asymmetric unit | 4 | 4 | 2 | |||
| Chain with αI domain | A | G | ||||
| Non-H atoms, protein/carbohydrate | 49 614/570 | 49 515/673 | 23 978/404 | |||
| PDB code | 3K6S | 3K71 | 3K72 | |||
| aNumbers in parentheses correspond to the outermost resolution shell. | ||||||
| bLetters in parentheses denote different crystal lattices. | ||||||
| cRmerge=ΣhΣi∣Ii(h)−<I(h)>∣/ΣhΣiIi(h), where Ii(h) and <I(h)> are the ith and mean measurement of the intensity of reflection h. | ||||||
| dRwork=Σh∣∣Fobs(h)∣−∣Fcalc(h)∣∣/Σh∣Fobs(h)∣, where Fobs(h) and Fcalc(h) are the observed and calculated structure factors, respectively. No I/σ cutoff was applied. | ||||||
| eRfree is the R value obtained for a test set of reflections consisting of a randomly selected ∼1% subset of the data set excluded from refinement. | ||||||
| fResidues in favourable and outlier regions of the Ramachandran plot as reported by MOLPROBITY (Davis et al, 2007). | ||||||
The three lattices have unit cells with similar b and c dimensions. However, the a dimensions are about 132, 150, and 161 Å in lattices A, B, and C, respectively (Table 1). All have 16 αXβ2 molecules per unit cell and either four or two molecules per asymmetric unit. The expansion along the a axis was accompanied by a change from P212121 spacegroup in lattices A and B, which have four molecules per asymmetric unit, to I212121 spacegroup in lattice C, which has two αXβ2 molecules per asymmetric unit (Table 1). This expansion changed the packing environments around each molecule in the three lattices, so a total of 10 unique αXβ2 molecules are defined.
Although our data are only at moderate to low resolution, the quality of the electron density maps has been greatly improved by multi-crystal averaging (Materials and methods). Typical density is shown for the αI domain (Figure 1A and B) and β-tail domain (Figure 1C). At this resolution, small sidechains such as those of Ala, Ser, Val, and Thr may appear as bulges from the mainchain, but are not well resolved, whereas larger residues such as Leu, Met, Arg, Ile, Phe, Tyr, and Trp often show densities with identifiable, characteristic shapes (Figure 1D). Disulfide bonds were almost always seen as continuous density connected to the mainchain (Figure 1C and E). Individual atoms such as metals do not have separable density from coordinating sidechains; however, extra density and difference peaks may indicate their presence. The majority of the αXβ2 mainchain has continuous density in the maps after multi-crystal averaging. The only exception is the αI domain, which is disordered in some αXβ2 molecules (see below). Tracing the mainchain was also aided by knowledge of domain folds from previous integrin structures. The only significant gap within an αXβ2 molecule with an ordered αI domain in the multi-crystal averaged maps was at the C-terminal connection between the αI and β-propeller domains. At this location, the sequence to structure register was readily verified by density for Phe-328 and the anomalous signal for Met-332 (Figure 1E). Furthermore, density for the disulfide bond at the N-terminal connection between the αI and β-propeller domains was readily apparent (Figure 1E). The mainchain trace and the sequence to structure register were validated with Se anomalous signals for the 25 Met residue positions. Density of average quality, over a stretch of residues with both small and large sidechains, is shown in Figure 1D.
Figure 1.
Representative electron density. (A, B) The αI domain. The main chain of αI domain is shown in white. Se anomalous map is shown at 3 σ level (red). Electron density map of αI domain after multi-crystal averaging is at 1 σ level (blue). Electron density around residues 318 and 319 is shown at 0.6 σ in green. The methionine residues are shown in stick, with yellow carbon atoms and orange sulphur atoms. (B) Different view to show the electron density around the α7-helix. (C, D) Multi-crystal averaging electron density around β-tail domain (C) and around residues 765–771 and 783–786 in the calf-1 domain (D) Colours and map σ levels are as in (A). (E) The αI/β-propeller/βI domain interface. Multi-crystal averaging electron density map shown at 1 σ level around α-subunit β-propeller residue Asn-373 and its N-linked glycan (blue), the N-linker residues 127–130 and disulfide-bonded β-propeller residues C126 and C97 (green), and residues 326–327 of the C-linker and β-propeller residues 328–331 (yellow). The sidechains of residues C97, C126, F328, M332, N373 and its N-linked glycan are shown in stick. The electron density in this region shows distinct paths of the three segments, and clear connected density with the β-propeller domain. Se anomalous map is shown at 3 σ level around the sidechain of Met-332. αI domain, β-propeller domain, and βI domain are in wheat, grey, and cyan, respectively. N- and C-linkers are in pink. The oxygen atoms, nitrogen atoms, and sulphur atoms are in red, blue, and orange, respectively. The carbon atoms of the glycan are in white. (F) Stereo image of β-tail domain CD loop. The oxygen, sulphur, nitrogen, and carbon atoms are in red, orange, blue, and wheat, respectively. Multi-crystal averaging map at 1 σ is blue and Se anomalous map at 3 σ level is red.
The αXI domain
Of 10 αXβ2 molecules visualized in the three lattices, only two molecules show electron densities for αI domains, in which lattice interactions stabilize their positions. The position of the αI domain was clearly evident from electron density maps and from Se anomalous signals for the four Se-Met residues (Figure 1A and B). The αXI domain extends away from the remaining domains of CR4, which adopt a bent and compact conformation (Figure 2A). The αI domain is inserted between blades 2 and 3 of the αX β-propeller domain (Figure 3A and B; Supplementary Figure S1). The two linkers connecting the αI and β-propeller domains are surprisingly flexible, and the αI domain itself has no contact with the β-propeller and only a small contact with the specificity determining loop (SDL) of the βI domain (Figure 3B; Table 2). Although not noted earlier (Nishida et al, 2006), EM also provided evidence for flexibility, with variation among class averages in orientation between the αI domain and the αXβ2 headpiece (Figure 2C–E; Supplementary Figure S2). Flexibility of the N-terminal (N) linker to the αI domain is limited by its short length of three residues and an immediately preceding disulfide bond to a loop in β-propeller blade 2 (Figures 1E, 2A, 3A and B). However, the long, 10-residue Ser- and Thr-rich C-terminal (C) linker is highly flexible; even in the two ordered αI domains, much of the C-linker shows weak density. An N-linked oligosaccharide, among several protected from EndoH digestion, extends from the β-propeller to the αI domain and partially shields the linkers (Figures 1E, 2A, 3A and B). Continuous densities to the β-propeller domain clearly distinguish the N-linker, C-linker, and N-glycan (Figure 1E). The linker regions of all αI domains are flanked by N-glycosylation sites in the β-propeller, I domain, or linker itself; glycans here may circumscribe flexibility and shield the flexible C-linker from proteolysis.
Figure 2.
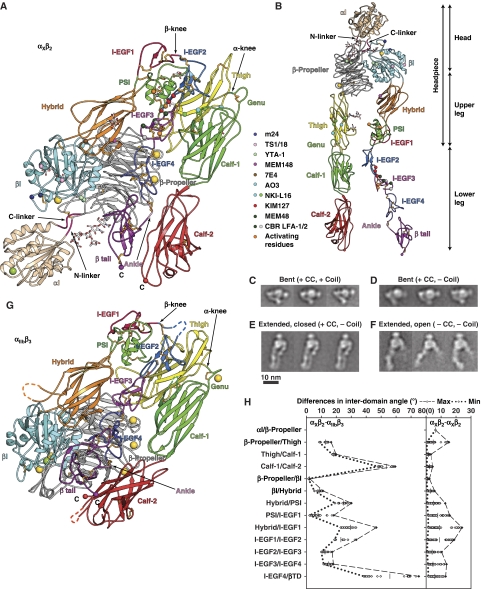
Structure of CR4, integrin αXβ2. (A) Cartoon of αXβ2 molecule 1 in lattice A and (B) an extended model made by adjusting domain interfaces at and near the knees. Disulfides are gold sticks, and glycans are sticks with white carbons. Ca and Mg ions are gold and green spheres, respectively. Smaller spheres show Cα atoms of conformation-associated epitopes and C-terminal residues. (C–F) Representative αXβ2 EM class averages. The presence (+) or absence (−) of a disulfide after the αX and β2 C-termini (CC) and subsequent linker and coiled-coil (coil) is indicated. (G) Cartoon of αIIbβ3 (Zhu et al, 2008) in same orientation and style as αXβ2 in (A). (H) Differences in interdomain angles between 10 molecules of αXβ2 and 2 molecules of αIIbβ3 (left panel), or among 10 molecules of αXβ2 (right panel). The mean values of interdomain angles are shown as bars and the maximal and minimal angles are shown in dashed or dotted lines, respectively.
Figure 3.
The αI, βI, and β-propeller domain interface, and I domain metal-binding sites. (A, B) The interface. Representations are as in Figure 2A and B; additionally, the indicated sidechains and backbone carbonyls are shown as sticks. (C) Superposition of headpieces from αXβ2 (magenta), unliganded-closed αIIbβ3 (Zhu et al, 2008) (yellow) and liganded-open (Springer et al, 2008) αIIbβ3 (cyan, with ligand in black). (D) Enlarged view of the superposition in (C) showing only αXβ2 (with its ADMIDAS metal as cyan sphere) and the ligand and SYMBS, MIDAS, and ADMIDAS metals from liganded-open αIIbb3 (yellow). (E–G) Metal coordination sites, with coordinations dashed. (E) αI domain MIDAS. (F, G) βI domain metal-binding sites in αXβ2 (F) and unliganded-closed αIIbβ3 (G).
Table 2.
Solvent accessible surfaces buried in bent αxβ2a
| Headpiece–tailpiece | α Headpiece–β headpiece; α tailpiece–β tailpiece | Domain junctionsb | |||||
|---|---|---|---|---|---|---|---|
| Domain | Solvent accessible surface area buried (Å2) | ||||||
| αXβ2 | αIIbβ3 | αXβ2 | αIIbβ3 | αXβ2 | αIIbβ3 | ||
| αI | 0 | — | 188 | — | αI—N- and C-linkers | 475 | — |
| N- and C-linkers | 0 | — | 34 | — | N- and C-linkers–αβ-propeller | 451 | — |
| αβ-propeller | 639 | 235 | 1606 | 1854 | αβ-propeller–α-thigh | 1054 | 833 |
| α-thigh | 383 | 314 | 0 | 0 | α-thigh–αcalf-1 | 880 | 914 |
| αcalf-1 | 19 | 113 | 609 | 367 | αcalf-1–αcalf-2 | 726 | 770 |
| αcalf-2 | 241 | 0 | 406 | 636 | β PSI–β hybrid | 781 | 759 |
| β PSI | 366 | 151 | 0 | 0 | β PSI–βI-EGF1 | 1048 | 848 |
| βI | 28 | 1 | 1752 | 1766 | β hybrid–βI | 1412 | 1610 |
| β hybrid | 91 | 671 | 16 | 172 | β hybrid–βI-EGF1 | 0 | 20 |
| βI-EGF1 | 0 | 0 | 0 | 0 | βI-EGF1–βI-EGF2 | 564 | 463 |
| βI-EGF2 | 662 | 395 | 303 | 188 | βI-EGF2–βI-EGF3 | 574 | 550 |
| βI-EGF3 | 313 | 460 | 320 | 153 | βI-EGF3–βI-EGF4 | 541 | 402 |
| βI-EGF4b | 300 | 358 | 171 | 338 | βI-EGF4–β-tail | 744 | 435 |
| β-tail | 51 | 56 | 239 | 312 | |||
| Total | 3093 | 2754 | 5644 | 5786 | 9250 | 6840 | |
| aCalculated with AREAIMOL of CCP4 using chains A and B of lattice A for αxβ2 and chains A and B of αIIbβ3 (Zhu et al, 2008). | |||||||
| bIncluding β-ankle. | |||||||
αI and βI domains have structurally homologous α/β folds, with a metal ion-dependent adhesion site (MIDAS) that binds a Mg2+ ion (Springer and Wang, 2004; Luo et al, 2007). Density suggests the presence of a metal ion at the αX MIDAS. Three different conformations termed closed, intermediate, and open have been defined for αI domains (Springer and Wang, 2004; Luo et al, 2007). The positions of the Mg2+, the Asp-X-Ser-X-Ser MIDAS motif, and the C-terminal α7-helix (Figure 1E) show that the αXI domain is in the closed, low-affinity conformation similar to that of the isolated αXI domain (Supplementary Figure S4) (Vorup-Jensen et al, 2003).
The β2I domain
In β3 integrins, the β3I domain has been shown to have two conformations, termed closed and open (Luo et al, 2007). The Mg2+-binding βI domain MIDAS is flanked on either side by Ca2+-binding adjacent to MIDAS (ADMIDAS) and synergistic metal-binding sites (SYMBS) (Xiong et al, 2001, 2002; Xiao et al, 2004; Zhu et al, 2008). In the bent αXβ2 structure, the β2I domain is in a low-affinity, closed conformation similar to that seen in β3 integrins, as shown by the positions of the β2I domain Asp-X-Ser-X-Ser motif and C-terminal α7-helix, and the swung-in hybrid domain (Figure 3C, F and G). Electron density suggests that metal ions occupy the βI ADMIDAS, and possibly the SYMBS, but not the MIDAS. Lack of metal ions at the SYMBS and ADMIDAS may in part be due to use of the crystallization precipitants citrate and phosphate, which compete for metal ions.
In a surprising contrast to previous integrin β3 and αI domain structures, an extra acidic residue is found at the β2 MIDAS, Asp243 (Figure 3F). Although density for a metal ion is absent, the position of the Asp243 sidechain suggests that it could directly coordinate Mg2+. This has important implications, because direct coordination of this Asp with Mg2+ would reduce its electrophilicity, and hence decrease affinity for its intrinsic ligand (see below).
The β2I domain also shows an extra acidic residue at the ADMIDAS, Glu-325 (Figure 3F). The backbone carbonyl oxygen of Glu-325 coordinates the ADMIDAS Ca2+ ion similarly to Met-335 in β3. Although the Glu-325 sidechain is only partially in density, it has the potential to coordinate the ADMIDAS in the closed conformation (Figure 3F) or during transition to the high-affinity, open conformation.
Overall bent conformation and differences from αI-less integrins
Apart from the αI domain, the 12 other domains in αXβ2 have excellent density in all 10 molecules, including the two domains at the β-knee, I-EGF domains 1 and 2. These 12 domains assume a bent conformation as found in the αI-less integrins αVβ3 (Xiong et al, 2001, 2002) and αIIbβ3 (Zhu et al, 2008) (Figure 2A and G). The leg domains tuck tightly against one another, and against the headpiece, owing to sharp bends at the α- and β-knees. As αI and αI-less integrins are among the most distantly related members of the integrin family, the bent conformation appears to be a general characteristic of the low-affinity integrin state (Nishida et al, 2006).
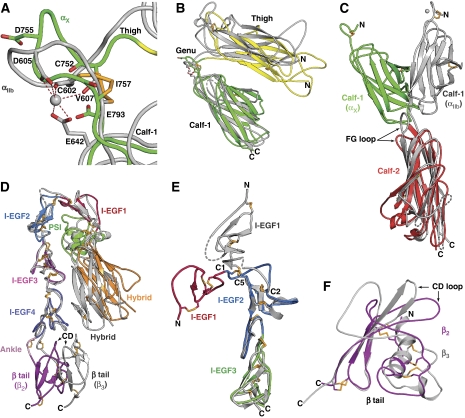
However, there are important differences in packing interactions and orientations. Compared to αV and αIIb, the αX genu has a different backbone conformation (Figure 4A). The metal-coordinating residues are conserved in the αX genu (Supplementary Figure S5a), but because of the different backbone conformation, their geometry is not appropriate for binding a Ca2+ ion. This surprising finding shows the conformational plasticity of this disulfide-bonded, 7-residue loop. Furthermore, the thigh and calf-1 domains approach more closely in αXβ2 than in αVβ3 or αIIbβ3 (Figure 4B). Moreover, the orientation between the calf-1 and calf-2 domains differs by 50° between aXβ2 and β3 integrins (Figures 2A, G, H and 4C; Supplementary Figure S4a–c), with the FG loop of calf-2 squashed by the calf-1 domain in αX (Figure 4C). This results in a wider cleft between the upper β-leg and lower α-leg in αXβ2 than in αIIbβ3; that is, a greater separation between the βI and calf-2 domains (Figure 2A and G). The lower β-leg is tucked in a cleft, between the upper β-leg on one side and the α-subunit on the other. Thus, this cleft is wider, particularly at its base near the membrane, in αXβ2 than in αIIbβ3. The base of αX calf-2 has several loops that differ markedly from those in αV and αIIb (Figure 4C), and includes a membrane-proximal α-helix in the XY loop that is cleaved by furin in most αI-less integrins but not αI integrins (Figure 4C; Supplementary Figure S1).
Figure 4.
Integrin leg domains. Domains in αXβ2 are coloured as in Figure 2A and B and in other integrins are silver with dashes for disordered loops and metals as spheres. (A) The genus. (B) The αX and αIIb thigh and calf-1 domains superimposed on calf-1. (C) The αX and αIIb calf-1 and calf-2 domains superimposed on calf-2. (D) The β2 and β3 (Zhu et al, 2008) legs superimposed on I-EGF domains 2–4. (E) Bent intact β2-leg and β2-leg fragment (Shi et al, 2007) superimposed on I-EGF2. (F) Superimposed β2 and β3 (Xiong et al, 2001)-tail domains.
The orientation between the I-EGF4 and β-tail domain differs by 40–70° between αXβ2 and αIIbβ3 (Figures 2H and 4D); indeed, the orientations at the last interdomain interfaces in both the α- and β-subunits show the greatest differences between αXβ2 and αIIbβ3 (Figure 2H), a specialization that may accommodate the αI domain. The base of the calf-2 domain is broad and may help together with the β-tail domain to orient bent integrins on the cell surface. Assuming that the calf-2 domains of αXβ2 and β3 integrins would be approximately normal to the membrane, the overall effect of the different orientations at the calf-1/calf-2 and I-EGF4/β-tail interfaces is to provide clearance between the αI domain and the plasma membrane in αI integrins (Figure 2A and G; Supplementary Figure S4). However, flexibility of the linkers between the ectodomain and the transmembrane domains may enable substantial rigid body movement of the ectodomain relative to the membrane, as has been found for αIIbβ3 (Zhu et al, 2009).
The ectodomain-transmembrane domain linkers
A disulfide bond in the construct used for crystallization was designed to mimic the clasp between associating transmembrane domains. Cysteines are present at the ends of the linkers following the αX calf-2 and β2 β-tail domains, immediately preceding the position of transmembrane domains in the native protein. Complete sets of negative stain EM class averages for αXβ2 molecules with four different types of C-terminal clasps are compared in Supplementary Figure S2. Constructs containing a C-terminal coiled-coil are the most constrained, with all particles in the bent conformation, whether or not a C-terminal disulfide is present (Figure 2C; Supplementary Figure S2c) or absent (Supplementary Figure S2d). When a C-terminal disulfide is present, and the coiled-coil is removed, as in the construct that was crystallized, particles that are bent (Figure 2D), and are extended with a closed headpiece (Figure 2E), are both present (Supplementary Figure S2e). Furthermore, this preparation, as similarly shown for a preparation with a coiled-coil (Nishida et al, 2006), adopts the extended, open headpiece conformation in presence of CBR-LFA-1/2 and KIM127 Fab (data not shown). Therefore, the C-terminal disulfide used in these studies does not constrain a particular integrin conformation. When neither a C-terminal disulfide nor a coiled-coil is present, particles that are bent, extended with the closed headpiece or extended with the open headpiece, are all present (Figure 2F; Supplementary Figure S2f).
Among 10 αXβ2 molecules in our crystal structures, the orientation between the calf-2 and β-tail domains differs (Supplementary Figure S3a), and thus is not constrained by the C-terminal disulfide bond. Furthermore, the ectodomain-transmembrane domain linkers are flexible. The last residue of the calf-2 domain is Tyr-1081, and the only linker residue seen in density is Lys-1082. Four further linker residues precede the TM domain in the native αX-subunit, Val-His-Asn-Pro (Supplementary Figure S1a). The corresponding Val-His-Gly-Cys sequence in the clasped construct is disordered. The last residue of the β-tail domain is Cys-673. Among the 10 αXβ2 molecules, the last residue seen in density is either Cys-673 or linker residue Val-674. Four further linker residues precede the TM domain in the native β2-subunit, Ala-Gly-Pro-Asn. The corresponding Ala-Gly-Pro-Asp-Gly-Cys sequence in the clasped construct is disordered. Among the 10 αXβ2 molecules, the distance between the last calf-2 and β-tail domain residues is 14–16 Å. A vast number of backbone conformations would be available for the five linker residues in each subunit to span this distance and join together either in a disulfide bond in the crystallized construct or at the beginning of two associated transmembrane α-helices in the native protein.
The disorder observed in the ectodomain-transmembrane domain linkers in our structure is entirely consistent with the lack of ordered structure of the same region in recently published NMR and Disulfide/Rosetta structures of the membrane-embedded region of integrin αIIbβ3 (Lau et al, 2009; Zhu et al, 2009). Among the four α-subunits that associate with the β2-subunit, neither the sequence nor the length of the linker is conserved (Supplementary Figure S6b). All this evidence strongly suggests that the linkers between the ectodomain and TM domain are flexible, and hence do not constrain the orientation between the ectodomain and the membrane. This conclusion is consistent with previous evidence from disulfide crosslinking within the linker region of αIIbβ3, and building models consistent with the restraints, which showed that flexion of the ectodomain was limited only by the steric barrier of the lipid bilayer (Zhu et al, 2009). As pointed out earlier, this also has important implications for transmembrane signalling, because large movements such as transmembrane domain separation, but not small movements such as transmembrane domain pivoting or pistoning, could be transmitted through the flexible linkers (Zhu et al, 2009).
Ectodomain flexibility
The 10 examples of αXβ2 visualized in three different crystal lattices (Table 1) provide unprecedented information on integrin flexibility (Figures 2H and 5). Overall, more variation in interdomain angle occurs between the small domains in β2 than between the robust domains in αX (Figure 2H). Within β2 the greatest interdomain variation is seen at hybrid/I-EGF1 and I-EGF1/I-EGF2 interfaces at the β-knee. Surprisingly, in αX the greatest flexibility is found at the β-propeller/thigh interface, rather than at the thigh/calf-1 interface at the α-knee (Figure 2H). This finding is consistent with the long, open loops in the thigh domain that flank the β-propeller domain in αX (Figures 2B and 3C).
The overall effect of flexibility at multiple domain interfaces results in variation in up to 16 Å in the position of domains that are membrane-proximal in the bent conformation (Figure 5A and B). This breathing substantially opens the cleft bearing the lower β-leg, between the upper β-leg and lower α-leg. The lower β-leg moves largely in sympathy with the lower α-leg, away from the upper β-leg (Figure 5A). Cleft opening enables egress of the lower β-leg before integrin extension.
Figure 5.
Breathing of bent αXβ2. (A) Two representative diverse structures after superposition of all 10 molecules on all domains except αI (shown are chains G and H of lattice A with the αI domain from chain A and chains C and D of lattice A with the αI domain from chain G of lattice B). (B) Schematic showing maximal differences in position after superposition.
The β2-knee and αXβ2 extension
In integrin extension, by far the largest changes in interdomain orientation occur at the α- and β- knees, which transition from highly bent to extended conformations (Figure 2C–F). In the β2-leg, there is an acute bend between I-EGF domains 1 and 2, whereas there is a distinctive, extended conformation at the junctions between I-EGF domains 2 and 3 and between domains 3 and 4 (Figures 2A and 4D).
Integrin EGF domains are a distinctive subfamily of EGF domains, with an additional disulfide bridge between cysteines 1 (C1) and 5 (C5) in each domain (Takagi et al, 2001; Beglova et al, 2002; Zhu et al, 2008). Only a single residue intervenes between C8 of one domain and C1 of the next domain, at a Cys-X-Cys junction. It has been suggested earlier that the extra C1–C5 disulfide in I-EGF domains is a specialization that enables moderate, but not extreme, motion at the interdomain junctions, and that the amount of motion that can occur is modulated by the length of the C1–C2 loop. The interdomain junction is referred to as gimbal-like, because there are two types of motion, one in the Cys-X-Cys backbone, and a second in the C1–C2 loop, involving both the C1–C2 backbone, and rotameric changes of the C1 sidechain (Zhu et al, 2008).
β2-leg fragment crystal structures (Shi et al, 2007) that extend either through I-EGF2 or I-EGF3 provide two examples of the I-EGF domain 1/domain 2 interface and one example of the I-EGF domain 2/domain 3 interface for comparison to the interfaces in the bent conformation. The bend between I-EGF domains 1 and 2 in the bent conformation is far more acute than seen in either of the two examples of β2-leg fragments, which by comparison are more extended in conformation (Shi et al, 2007; Zhu et al, 2008) (Figure 4E). More movement at the gimbal-like β-knee occurs by flexion of the tip of I-EGF2 within its first disulfide-bonded C1–C2 loop than at the Cys-X-Cys junction with I-EGF1 (Figure 4E). Thus, the backbone conformation of the 13 residues between C1 and C2 of I-EGF2 varies greatly between the bent and extended conformations, and a portion of this loop is disordered in the more extended, and higher resolution, β2-leg fragment (Figure 4E). As emphasized earlier, the C1–C2 loop is much longer in I-EGF2 than in other I-EGF domains, enabling substantial flexibility important for integrin extension (Zhu et al, 2008). The flexibility of this loop is further emphasized by the presence of different I-EGF2 C1–C2 loop conformations in different examples of bent αXβ2 molecules (Figure 5A), and by missing density in this loop in a bent integrin αIIbβ3 crystal structure (Zhu et al, 2008).
Among I-EGF domains, I-EGF3 has the shortest C1–C2 loop, with four residues between C1 and C2. Remarkably, the orientation between I-EGF domains 2 and 3 is essentially identical in crystals of bent αXβ2 and the β2-leg fragment (Figure 4E). This suggests that this interface is relatively rigid, and that the extended conformation at this interface would also be seen in extended αXβ2. Furthermore, the results support the suggestion that the length of the loop between C1 and C2 of I-EGF domains correlates with interdomain flexibility (Zhu et al, 2008).
Structural evidence that destabilizing the bent conformation favours integrin activation
αXβ2 containing a human αX-subunit and chicken β2-subunit is constitutively active in binding iC3b. The minimal substitutions for activation of CR4 activity were found to be β2 Q525S and V526L, and it was suggested earlier that these mutations were in an interface with αX that stabilized CR4 in the resting state (Zang and Springer, 2001). Indeed, as predicted, the structure shows that these residues locate to a loop of I-EGF3 in the lower β2-leg that is deeply buried in the bent conformation in a crevice between the αX thigh and calf-1 domains (Figure 2A), but is exposed in the extended conformation (Figure 2B). Thus, these substitutions appear to destabilize the bent conformation, and induce CR4 activation by favouring the extended conformation.
KIM-127 is an antibody that reports integrin activation; CBR-LFA-1/2 is an antibody that induces activation. Their epitopes have been mapped earlier to individual mouse/human amino acid substitutions (Lu et al, 2001a). The KIM-127 epitope is buried on I-EGF2, in an interface with the PSI domain (Figure 2A and B). The CBR-LFA-1/2 epitope is buried on I-EGF3 in an interface with the hybrid domain (Figure 2A and B). Both epitopes would be exposed on integrin extension.
The AO3 and NKI-L16 Abs to αL induce or report activation of integrin αLβ2, and have also been mapped to individual human/mouse substitutions (Xie et al, 2004). Mapping these residues onto αX shows that these Abs bind to the face of the thigh domain that is buried by calf-1 in the bent conformation, but would be exposed after extension (Figure 2A and B).
These results show a close association of the inactive β2 integrin state with the bent conformation, and close association of the active β2 integrin state with the extended conformation. The results are in excellent agreement with EM studies on complexes between Fab and integrin αXβ2 that have shown that KIM-127 Fab that reports β2 integrin activation on cell surfaces binds only to the extended conformation, and that CBR-LFA-1/2 Fab that activates β2 integrins on cell surfaces induces extension (Nishida et al, 2006). Together with the earlier EM studies, and functional experiments with these antibodies on intact cells, this work definitively establishes that integrin extension is sufficient to induce activation and occurs during physiologic integrin activation.
Antibodies that discriminate between the closed and open headpiece conformations
The effects of further activating antibodies cannot be explained solely by inducing integrin extension, because they either bind to epitopes that are completely exposed in the bent conformation, or bind to regions that move in allostery. Furthermore, some inhibitory antibodies do not act by blocking ligand binding, because they do not bind to the αXI domain. The locations of all of these epitopes map to regions of the βI and hybrid domains that shift in allostery, and they support the importance of conversion from the closed to open headpiece conformation in β2 integrin activation.
Some of these antibodies bind to epitopes that are fully exposed in the bent conformation. The activation-dependent m24 epitope maps to residues that are exposed in both the bent and extended conformations in the βI SDL and α1-helix (Lu et al, 2001b; Kamata et al, 2002) (Figure 2A and B); α1-helix movement alters the relative disposition of these residues in the open headpiece (Xiao et al, 2004). Conversely, the allosterically inhibitory TS1/18 Ab binds to βI residues on the α1- and α7-helices that are exposed in both the bent and extended conformations (Figure 2A and B) (Lu et al, 2001b); these residues would be further apart on helix movement in the open headpiece conformation (Xiao et al, 2004). The inhibitory 7E4 Ab binds to an outer hybrid/βI domain interface that is well exposed in the bent and extended, closed headpiece conformations (Figure 2A and B); this Ab is predicted to stabilize the closed headpiece conformation (Tng et al, 2004).
Other antibodies, all of which are activating, bind to epitopes that are masked in the bent conformation, but may (YTA-1) or must (MEM148) also favour the open headpiece conformation. The activation-associated YTA-1 epitope maps to residues in the β5-α6 loop of the βI domain that are shielded by the β-tail domain in the bent, closed headpiece conformation (Figure 2A) and might also change conformation in the open headpiece (Zang et al, 2000). The activation-reporting MEM148 Ab binds to an inner hybrid/βI domain interface (Tang et al, 2005) that is masked in the bent/closed and extended/closed conformations by the β-propeller domain (Figure 2A and B); headpiece opening is required for epitope exposure. Together, the localization of the epitopes for antibodies that allosterically activate or inhibit β2 integrin fuction shows that not only extension but also headpiece opening is required for β2 integrin activation.
Disulfide bond exchange in integrins is not supported
On the basis of the presence of a CGXC sequence in each of four cysteine-rich repeats in integrins, a motif that is also found at the active site of thioredoxin, a thiol-isomerase activity has been hypothesized and found in integrin αIIbβ3 (O'Neill et al, 2000). In thiol and disulfide isomerases, the two cysteines of the CGXC motif are alternatively disulfide bonded to one another, or reduced. However, I-EGF domains have a fold quite distinct from that in these isomerases, and the two cysteines in the CGXC motif are not bonded to one another, but correspond to C5 and C6, which are present in the C1–C5 and C3–C6 disulfides. Multiple reduced cysteines have been reported to be present in the cysteine-rich repeats of αIIbβ3 isolated from outdated platelets (Yan and Smith, 2001). However, other studies have found no significant number of reactive cysteines on transfectants (Luo et al, 2004). A classic study, on fresh platelets, found that though a single reduced cysteine in the cytoplasmic domain of platelet GPIbβ (25 000 molecules/cell) was readily detected, none were present in the integrin αIIb or β3-subunits (100 000 molecules/cell) (Kalomiris and Coller, 1985). In agreement, the crystal structure of the integrin αIIbβ3 ectodomain shows that all cysteines are paired (Zhu et al, 2008). In the multi-crystal averaged density for the αXβ2 structures reported here, density for disulfide bonds is in general well defined and easily traced (Figure 1C and E). All cysteines, except Cys-444 that is buried in the β-propeller domain, are present in disulfide bonds. Likewise, all cysteines present in three different high-resolution structures of β2 integrin leg fragments are disulfide bonded (Shi et al, 2005, 2007). Thus, as described above, extension at the β-knee, with large leg rearrangements at the I-EGF1/I-EGF2 interface, can occur without reduction (Figure 2C–F). It should be noted that substantial rearrangement of the C1–C5 disulfide bond, with change in the C1 cysteine rotamer, can occur without reduction, because of the backbone flexibility afforded by the long C1–C2 loop in I-EGF2.
Structural refutation of the deadbolt model
On the basis of the αVβ3 structure, a CD loop ‘deadbolt' in the β-tail domain has been proposed to contact the βI domain and restrain it in the closed conformation (Xiong et al, 2003). However, the interface of 40 Å2 (Xiong et al, 2001) or 10 Å2 (Xiong et al, 2009) is so small that it would be surprising if it were significant (Lo Conte et al, 1999). Deletion and mutation of the CD loop in integrins αVβ3 and αIIbβ3 have been shown to have no effect on their activation (Zhu et al, 2007). Furthermore, a lack of any interface between the β-tail CD loop and the βI domain was found in an αIIbβ3 crystal structure (Zhu et al, 2008). Thus, the deadbolt hypothesis has been disproved in integrins with the β3-subunit, on which this hypothesis was based.
The only mutational test of the deadbolt hypothesis interpreted in its support is a study with the integrin β2-subunit, in which β-tail CD loop substitution and deletion were found to activate integrin αMβ2 (Gupta et al, 2007). However, αXβ2 and αVβ3 differ markedly in the conformation of the β-tail domain, including the CD loop (Figure 4F). Furthermore, the orientation of the β-tail domain within the β-leg differs markedly between αXβ2 and αVβ3, with divergence beginning at the β-ankle loop that separates I-EGF4 from the β-tail (Figures 2H and 4D). The β-tail domain is well defined in multi-crystal averaged density (Figure 1C and F). Furthermore, the conformation of its CD loop is well defined, with unambiguous density for multiple large sidechains, and a strong anomalous signal for Met-660 at the tip of the CD loop (Figure 1C and F). The CD loop and the entire β-tail domain are far from the βI domain in the β2-subunit (Supplementary Figure S4f); Met-660 is 35 Å away from the nearest residue in the βI domain α7-helix, Lys-340.
Why then would CD loop mutations be activating in β2 integrins? The β2-tail CD loop is a major contributor to the contact with the preceding domain in the β-tail, I-EGF4 (Figure 4D; Supplementary Figure S4f). This contact substantially increases the size of the I-EGF4/β-tail interface from 435 Å2 in αIIbβ3 to 744 Å2 in αXβ2 (Table 2). This interface is deeply buried in the cleft occupied by the lower β-leg, between the hybrid domain on one side and the calf-2 domain in the other. Thus, mutation of the β2-tail CD loop is predicted to greatly destabilize the orientation between the I-EGF4 and β-tail domains in the bent conformation. Thus, it is also likely to destabilize the buried conformation of the lower β-leg, and result in integrin extension. Thus, the deadbolt hypothesis has been disproved for the integrin for which it was originally proposed, αVβ3 (Zhu et al, 2007, 2008), and the single CD loop mutational study interpreted in favour of the deadbolt hypothesis, carried out in a β2 integrin (Gupta et al, 2007), has now been shown not to support this hypothesis, and to instead support activation by integrin extension.
Signal transmission between the βI and αI domains in integrin activation
How is activation transmitted to the αI domain? αI-less integrins have two distinct conformational states of the βI domain, in the closed and open headpieces. The headpiece is closed in the bent-closed and extended-closed conformations, and open in the extended-open conformation (Luo et al, 2007). Similarly, EM shows that when αXβ2 extends, its headpiece can remain closed (Figure 2E), as seen in the bent crystal structure or can open, with the hybrid domain swung out (Figure 2F) (Nishida et al, 2006). In ligand binding in αI-less integrins, the open state of the βI domain MIDAS Mg2+ ion coordinates the Asp sidechain in ligands such as Arg-Gly-Asp (Luo et al, 2007; Springer et al, 2008). In contrast to such binding to an extrinsic ligand, the βI MIDAS of αI integrins may bind an intrinsic ligand, that is to an invariant Glu residue that corresponds to the first residue in the αI C-linker, αX Glu-318 (Figure 3A and B). Mutation of this Glu abolishes integrin activation (Huth et al, 2000; Alonso et al, 2002). In contrast, when mutation of this Glu to Cys is combined with mutation of β2I MIDAS-loop residue Ala-210 to Cys, an intersubunit disulfide bond forms, and ligand binding is constitutively activated (Yang et al, 2004).
In our structure, αX Glu-318 does not bind the β2I MIDAS, consistent with the finding that both the αX and β2I domains are in the closed, low-affinity conformations. However, the orientation of the αXI domain with respect to the remainder of the ectodomain, and of αX Glu-318 with respect to the β2I MIDAS lends credence to the intrinsic-ligand hypothesis (Figure 3A and B). The αXI domain is oriented such that the C-linker is at the interface between the β-propeller and βI domains, where αI-less integrins bind ligands. Indeed, αX C-linker residues 325–329 occupy a position at this interface similar to that of the fibrinogen peptide at the αIIbβ3 recognition interface (Springer et al, 2008) (Figure 3D). In the two αXβ2 molecules with density for the αXI domain, the last residue with significant interaction with the αXI domain is α7-helix residue Ile-317, which is buried in a hydrophobic region at the base of the αI domain. Multi-crystal averaging density for the αXI domain shows continuous 1 σ backbone density up to residue 317, 0.6 σ density for residues 318 and 319, less density thereafter, and resumption again of 1 σ backbone density at residue 328 (Figure 1B and E). On the basis of somewhat better 2Fo–Fc density, the sidechain of Glu-318 is modelled as pointing towards the β2I MIDAS, 16–19 Å away in the two αXβ2 molecules. The Cβ atoms of αX Glu-318 and β2 Ala-210, which when mutated to Cys form an activating intersubunit disulfide bond (Yang et al, 2004), are 14–18 Å apart. Thus, αX Glu-318 is poised to coordinate the β2 MIDAS on conformational rearrangement.
As residue Ile-317 is buried in a hydrophobic pocket in the αXI domain, and continuous backbone density is present through this residue, the position of αX residue Glu-318 is highly constrained by the position of the αXI domain and its α7-helix. Owing to the flexible connection of the αXI domain to the β-propeller domain, particularly at the C-linker, rigid body motion of the αXI domain relative to the β-propeller domain can bring αX Glu-318 closer to the β2 MIDAS. However, clashes between the domains would occur before the αX Glu-318 sidechain could coordinate the β2 MIDAS. It thus appears that axial displacement of the αXI domain α7-helix would be required for MIDAS engagement.
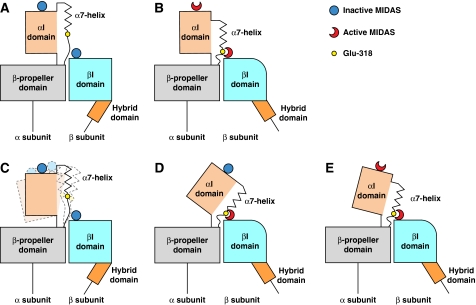
Flexibility of αI domains had not been noted in an earlier EM study, and it had been thought that a ‘relatively firm interface between the αI and β-propeller domains…would favour downward displacement of the α7-helix, rather than tilting of the αI domain' on engagement of the αI Glu residue with the βI MIDAS (Nishida et al, 2006) (Figure 6A and B). This postulated rigid connection presented a quandary—how could a two-position switch in the βI domain connect to a three-position switch in the αI domain? Structures of isolated integrin αI domains have shown three conformational states, termed closed, intermediate and open (Luo et al, 2007). Relative to the closed conformation of the αI domain seen here in bent αXβ2, intermediate and open αI domains have C-terminal α7-helices that are displaced one and two turns along the helical axis in the C-terminal direction, respectively, and have intermediate and high affinity for ligand (Shimaoka et al, 2003; Jin et al, 2004).
Figure 6.
Communication of allostery between αI and βI domains. (A, B) Previous model with firm interface between the α-subunit I and β-propeller domains (Nishida et al, 2006). There should be a 1:1 correspondence between αI and βI domain conformational states according to this model (A, closed; B, open). (C–E) Current model, with a flexible interface between the α-subunit I and β-propeller domains. This enables three states of the αI domain to couple to two states of the βI domain. (C) When the αI and βI domains are each in the closed state, the αI domain is flexible. (D, E) Both the intermediate state αI domain, with its MIDAS closed and α7-helix displaced one turn (D), and the open state αI domain, with its MIDAS open and α7-helix displaced two turns (E), can couple to the open βI domain. The greater tilting of the αI domain in (D) suggests greater strain than in (E). Lessening of this strain in (E) would help compensate for the higher energy of the open αI MIDAS.
The flexibility of the αI domain revealed in this study resolves this conundrum. One-turn and two-turn displacements of the α7-helix in the intermediate and open states, respectively, may each be sufficient for binding of Glu-318 to the βI MIDAS, when combined with some helix unwinding and with different orientations of the αI domain with respect to the β-propeller and βI domains (Figure 6C–E). Moreover, strain energy stored in domain tilting or helix unwinding required for Glu-318 to reach the β MIDAS in the intermediate αI state with one-turn α-helix displacement (Figure 6D) could be released on conversion to two-turn α-helix displacement in the open αI state, and compensate for the higher energy of the open αI MIDAS (Figure 6E). On the basis of crystal structure studies on intermediate state αI domains (Shimaoka et al, 2003), initial coupling between the βI and αI domains might stabilize the intermediate αI domain state with a closed MIDAS (Figure 6D), which on ligand binding would convert to the open αI state with an open MIDAS (Figure 6E). This flexibility enables fine-tuning of integrin adhesiveness. These ideas are important to test with further structures of αI integrins in active states.
Other functions for αI domain flexibility
Flexibility of the αI domain may also be important in integrin mechanochemistry. The actin cytoskeleton binds to the integrin β-subunit, and exerts a tensile force between the β-subunit cytoplasmic domain and ligand-binding site of αI-less integrins that stabilizes the open, high affinity state of the headpiece (Zhu et al, 2008). A tensile force on the C-terminal α7-helix, but not N-terminal β-strand, of isolated, ligand-bound αI domains stabilizes the open αI domain state (Astrof et al, 2006). Tensile force exerted between the ligand-binding site of the αI domain and the β-subunit cytoplasmic domain in an intact, activated integrin would be transmitted through the N-linker to the β-propeller domain and through the C-linker to the βI domain MIDAS, and favour pivoting of the αI domain to an orientation different than that captured in crystals, in which the tensile force would be better aligned with the α7-helix axis, facilitating mechanochemical stabilization of the open αI domain state (Zhu et al, 2008).
The flexibility of the αI domain revealed here, together with its capacity for allostery, may be the key features that account for the appearance of αI integrins in chordates and their expansion in mammals to become half of all integrin α-subunits. The combined β-propeller and βI domain ligand-binding unit in αI-less integrins is far bulkier and less flexible than the αI domain, and often binds flexible ligands such as Arg-Gly-Asp. CR4 recognizes iC3b covalently linked to pathogen surfaces. The flexibility of the αI domain in αI integrins, and its extension above the integrin head, better suits αI integrins for binding their less flexible and less accessible ligands, such as collagen fibrils (α1, α2, α10, α11), and ligands on cell surfaces (αL, αM, αX, αD, αE).
Materials and methods
Production of soluble αXβ2 and crystallization
αXβ2 ectodomain constructs contained TEV protease sites, ACID–BASE coiled-coils, and Strep-II and His6 tags as described earlier (Nishida et al, 2006). An additional C-terminal disulfide was introduced by altering the GTGG and DTSG linker sequences following αX and β2 (Nishida et al, 2006), to GCGG and DGCG, respectively (Supplementary Figure S2a,b). CHO Lec 3.2.8.1 cells were cotransfected with these vectors and screened for single clones with high expression (Nishida et al, 2006). The clone with highest expression level (8 mg/l) was expanded and cultured in roller bottles at 37°C, and culture supernatants were collected every week. Soluble integrins were purified as described earlier (Nishida et al, 2006).
To obtain αXβ2 protein for crystallization, purified recombinant protein was treated with His-tagged TEV protease (Invitrogen) at 250 U/mg and Endo H (Roche) at 0.1 U/mg at room temperature for 16 h, followed by re-pass-through the Ni-NTA column. The flow-through containing deglycosylated αXβ2 protein was dialysed against and stored in TBS/Ca buffer (20 mM Tris–HCl pH7.5, 150 mM NaCl, 5 mM CaCl2), and further purified by Superdex 200 (Amersham Biosciences, Piscataway, NJ) size-exclusion chromatography in TBS buffer (20 mM Tris–HCl pH7.5, 150 mM NaCl). Purified αXβ2 was concentrated to 8 mg/ml, and screened for crystallization conditions using the Topaz crystallizer (Fluidigm, South San Francisco, CA). Lead conditions were optimized using hanging drop vapour diffusion.
Three crystal lattices were obtained. The final optimized well solution(s) for lattice A is 0.55 M tri-sodium citrate and 0.1 M imidazole, pH 6.5, for lattice C are 0.915 M sodium/potassium phosphate, pH 7.2 or 32% Tacimate and 0.1 M Bis–Tris Propane, pH 7.0. Crystals with lattice B were obtained by soaking the crystals with lattice A into 0.55 M tri-sodium citrate, 0.05 M MgCl2, 0.1 mM ICAM-1 domain 3 and 0.1 M imidazole, pH 6.5.
To prepare Se-Met αXβ2, cells were washed with phosphate-buffered saline supplemented with 1% dialysed FCS, incubated with methionine-free α-MEM (SAFC Biosciences) supplemented with 50 mg/l L-Se-Met (Sigma) and 10% dialysed FCS, replaced with the same medium after 6 h, and cultured for 3–4 days. Se-Met αXβ2 was purified, deglycosylated, obtained in about 60% yield as native material, and crystallized in lattices A and C as described above.
X-ray crystallography
Diffraction data collected at APS ID-23 (Table 1) were processed with XDS (Kabsch, 2001). [Ta6Br12(H2O)6]2+Br−OH−·5H2O was soaked into crystals with lattice A. Four tantalum bromide clusters were found by SOLVE (Terwilliger and Berendzen, 1999), and initial phases were obtained from Ta SAD (Table 1) data for lattice A. The electron density map of lattice A after density modification by RESOLVE (Terwilliger, 2000) showed a clear boundary between protein and solvent. However, no secondary structural features were revealed. To determine the Se substructure of the Se-derivative crystal, the anomalous signals from the peak wavelength of Se-MAD data for lattice A (Table 1) were combined with the initial phases from Ta SAD data for lattice A to calculate an anomalous Fourier map. The top 12 peaks were picked as the starting Se sites for SOLVE. 78 of 100 Se atoms were identified and a set of phases from Se-MAD data for lattice A was generated by SOLVE. The phases were then transferred to the high-resolution native data for lattice A. Density modification was carried out by RESOLVE to improve the electron density map and extend the phases to higher resolution. The starting model for each domain of integrin αxβ2 was built by homology modelling program MODELLER (Sali and Blundell, 1993) using corresponding domains from integrin αIIbβ3 and αVβ3 structures as templates. Homology models of the β-propeller domain, thigh domain, and βI domain could be placed into the density-modified map by MOLREP (Vagin and Teplyakov, 1997) in CCP4 (Bailey, 1994). The other domains were placed into the map manually. A crude model of integrin αxβ2 was obtained after rigid body refinement by PHENIX.REFINE (Adams et al, 2002) with each domain as one rigid body. Molecular replacement solutions for lattice B and C were solved by PHASER (McCoy et al, 2007) using the crude model of integrin αxβ2 in lattice A as the searching model. The molecular replacement phases for lattice C combined with the anomalous signals from the peak wavelength of Se-MAD data for lattice C (Table 1) were used to determine the Se substructure of Se-derivative crystal with lattice C; 32 of 50 Se atoms were identified and a new set of phases for lattice C was generated by SOLVE. Phases for lattice C were then transferred to the high-resolution native data for lattice C. To improve the phases and extend them to higher resolution, multi-crystal averaging (three crystals in total: lattice A, B, C), multi-domain averaging (with a separate mask for each of the 12 domains resolved in 8 molecules and 13 domains resolved in 2 molecules) along with solvent flattening and histogram matching were performed by DMMULTI (Cowtan, 1994) from the CCP4 suite. The mask for each domain was calculated by NCSMASK in CCP4. As the domain–domain angles are quite different in each molecule (Figure 2H), the NCS matrices for each domain between molecules and crystals were computed by LSQKAB in CCP4. Rigid body refinements were carried out by PHENIX.REFINE for each lattice based on the averaged maps. The new models for each lattice were then used to calculate a set of new NCS matrices for the next cycle of DMMULTI. These steps were cycled three times. Model building in COOT (Emsley and Cowtan, 2004) was based on multi-crystal, multi-domain averaging electron density maps. The phases for lattice A and C after averaging were subsequently used in MLHL refinement of lattice A and C using PHENIX.REFINE. Refinement of lattice B was carried out with no experimental phases. NCS restraints and TLS groups were used in refinement. To stabilize the refinement at such low resolution, hydrogen-bond restraints for secondary structural features were applied in the early stage of the refinement. The secondary structure assignment was based on integrin sequence and structural alignment. These restraints were completely omitted later in refinement. The sequence to structure register was confirmed or adjusted using Se anomalous maps. The sequence register at the two junctions between the β-propeller domain and α-I domain was confirmed by several methods. First, the disulfide bond between Cys-97 and Cys-126 was clear in electron density maps (Figure 1E). Second, the Phe-328 sidechain had clear density, and the anomalous signal for SeMet-332 was clear (Figure 1E). Third, the anomalous signals from four SeMet residues in the αxI domain confirmed its orientation (Figure 1A and B). Validation and Ramachandran statistics used MOPROBITY (Davis et al, 2007).
Interdomain angle calculation
Every tandem domain pair from molecules being compared was superimposed on the first tandem domain using SUPER command in Pymol. The matrix generated by a second superposition on the second tandem domain was obtained with GET_OBJECT_MATRIX command in Pymol and used to calculate the κ angle in polar angles.
Negative stain EM of αXβ2
αXβ2 constructs treated with or without TEV protease were purified on Superdex 200 HR equilibrated with TBS, 1 mM CaCl2, 1 mM MgCl2. Peak fractions were adsorbed to glow discharged carbon-coated copper grids, stained with uranyl formate, and images acquired with an FEI Tecnai 12 electron microscope at 120 kV at a nominal magnification of × 67 000; 2000–6000 particles were interactively collected, and subjected to 10 cycles of multi-reference alignment and k-means classification with SPIDER (Frank et al, 1996) as described (Nishida et al, 2006).
Supplementary Material
Supplementary Figures S1–S7
Review Process File
Acknowledgments
The work is supported by NIH grant AI 72765.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 [DOI] [PubMed] [Google Scholar]
- Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA (2002) Does the integrin αA domain act as a ligand for its βA domain? Curr Biol 12: R340–R342 [DOI] [PubMed] [Google Scholar]
- Astrof NS, Salas A, Shimaoka M, Chen JF, Springer TA (2006) Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry 45: 15020–15028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S (1994) The CCP4 suite-programs for protein crystallography. Acta Crystallogr 50: 760–763 [DOI] [PubMed] [Google Scholar]
- Beglova N, Blacklow SC, Takagi J, Springer TA (2002) Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol 9: 282–287 [DOI] [PubMed] [Google Scholar]
- Cowtan K (1994) An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM. Newsl Protein Crystallogr 31: 34–38 [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB III, Snoeyink J, Richardson JS, Richardson DC (2007) MOLPROBITY: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res 35: W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A (1996) SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol 116: 190–199 [DOI] [PubMed] [Google Scholar]
- Gupta V, Gylling A, Alonso JL, Sugimori T, Ianakiev P, Xiong JP, Arnaout MA (2007) The beta-tail domain (betaTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood 109: 3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth JR, Olejniczak ET, Mendoza R, Liang H, Harris EA, Lupher ML Jr, Wilson AE, Fesik SW, Staunton DE (2000) NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc Natl Acad Sci USA 97: 5231–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Andricioaei I, Springer TA (2004) Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure 12: 2137–2147 [DOI] [PubMed] [Google Scholar]
- Kabsch W (2001) Chapter 25.2.9 XDS. In: Rossmann MG, Arnold E (eds). International Tables for Crystallography. Kluwer Academic Publishers: Dordrecht: 730–734 [Google Scholar]
- Kalomiris EL, Coller BS (1985) Thiol-specific probes indicate that the beta-chain of platelet glycoprotein Ib is a transmembrane protein with a reactive endofacial sulfhydryl group. Biochemistry 24: 5430–5436 [DOI] [PubMed] [Google Scholar]
- Kamata T, Tieu KK, Tarui T, Puzon-McLaughlin W, Hogg N, Takada Y (2002) The role of the CPNKEKEC sequence in the beta(2) subunit I domain in regulation of integrin alpha(L)beta(2) (LFA-1). J Immunol 168: 2296–2301 [DOI] [PubMed] [Google Scholar]
- Lau TL, Kim C, Ginsberg MH, Ulmer TS (2009) The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J 9: 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Conte L, Chothia C, Janin J (1999) The atomic structure of protein-protein recognition sites. J Biol Chem 285: 2177–2198 [DOI] [PubMed] [Google Scholar]
- Lu C, Ferzly M, Takagi J, Springer TA (2001a) Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J Immunol 166: 5629–5637 [DOI] [PubMed] [Google Scholar]
- Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA (2001b) Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci USA 98: 2393–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B-H, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B-H, Takagi J, Springer TA (2004) Locking the β3 integrin I-like domain into high and low affinity conformations with disulfides. J Biol Chem 279: 10215–10221 [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Crystallogr 40: 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA (2006) Activation of leukocyte β2 integrins by conversion from bent to extended conformations. Immunity 25: 583–594 [DOI] [PubMed] [Google Scholar]
- O'Neill S, Robinson A, Deering A, Ryan M, Fitzgerald DJ, Moran N (2000) The platelet integrin αIIbβ3 has an endogenous thiol isomerase activity. J Biol Chem 275: 36984–36990 [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815 [DOI] [PubMed] [Google Scholar]
- Shi M, Foo SY, Tan SM, Mitchell EP, Law SK, Lescar J (2007) A structural hypothesis for the transition between bent and extended conformations of the leukocyte β2 integrins. J Biol Chem 282: 30198–30206 [DOI] [PubMed] [Google Scholar]
- Shi M, Sundramurthy K, Liu B, Tan SM, Law SK, Lescar J (2005) The crystal structure of the plexin-semaphorin-integrin domain/hybrid domain/I-EGF1 segment from the human integrin β2 subunit at 1.8-A resolution. J Biol Chem 280: 30586–30593 [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu J-H, Yang Y, Dong Y, Jun C-D, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang J-h, Springer TA !(2003) Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA, Wang J-h (2004) The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv Protein Chem 68: 29–63 [DOI] [PubMed] [Google Scholar]
- Springer TA, Zhu J, Xiao T (2008) Structural basis for distinctive recognition of fibrinogen by the platelet integrin αIIbβ3. J Cell Biol 182: 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Beglova N, Yalamanchili P, Blacklow SC, Springer TA (2001) Definition of EGF-like, closely interacting modules that bear activation epitopes in integrin β subunits. Proc Natl Acad Sci USA 98: 11175–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RH, Tng E, Law SK, Tan SM (2005) Epitope mapping of monoclonal antibody to integrin αLβ2 hybrid domain suggests different requirements of affinity states for intercellular adhesion molecules (ICAM)-1 and ICAM-3 binding. J Biol Chem 280: 29208–29216 [DOI] [PubMed] [Google Scholar]
- Terwilliger TC (2000) Maximum-likelihood density modification. Acta Crystallogr D Biol Crystallogr 56: 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J (1999) Automated MAD and MIR structure solution. Acta Crystallogr D Biol Crystallogr 55: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tng E, Tan SM, Ranganathan S, Cheng M, Law SK (2004) The integrin αLβ2 hybrid domain serves as a link for the propagation of activation signal from its stalk regions to the I-like domain. J Biol Chem 279: 54334–54339 [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A (1997) MOLREP: an automated program for molecular replacement. J Appl Crystallogr 30: 1022–1025 [Google Scholar]
- Vorup-Jensen T, Carman CV, Shimaoka M, Schuck P, Svitel J, Springer TA (2005) Exposure of acidic residues as a danger signal for recognition of fibrinogen and other macromolecules by integrin αXβ2. Proc Natl Acad Sci USA 102: 1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorup-Jensen T, Ostermeier C, Shimaoka M, Hommel U, Springer TA (2003) Structure and allosteric regulation of the αXβ2 integrin I domain. Proc Natl Acad Sci USA 100: 1873–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Wang J-h, Coller BS, Springer TA (2004) Structural basis for allostery in integrins and binding of fibrinogen-mimetic therapeutics. Nature 432: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Shimaoka M, Xiao T, Schwab P, Klickstein LB, Springer TA (2004) The integrin α subunit leg extends at a Ca2+-dependent epitope in the thigh/genu interface upon activation. Proc Natl Acad Sci USA 101: 15422–15427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J-P, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA (2001) Crystal structure of the extracellular segment of integrin αVβ3. Science (NY) 294: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Mahalingham B, Alonso JL, Borrelli LA, Rui X, Anand S, Hyman BT, Rysiok T, Muller-Pompalla D, Goodman SL, Arnaout MA (2009) Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J Cell Biol 186: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Goodman SL, Arnaout MA (2003) New insights into the structural basis of integrin activation. Blood 102: 1155–1159 [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA (2002) Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science (NY) 296: 151–155 [DOI] [PubMed] [Google Scholar]
- Yan B, Smith JW (2001) Mechanism of integrin activation by disulfide bond reduction. Biochemistry 40: 8861–8867 [DOI] [PubMed] [Google Scholar]
- Yang W, Shimaoka M, Salas A, Takagi J, Springer TA (2004) Inter-subunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc Natl Acad Sci USA 101: 2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Q, Lu C, Huang C, Takagi J, Springer TA (2000) The top of the I-like domain of the integrin LFA-1 β subunit contacts the α subunit β-propeller domain near β-sheet 3. J Biol Chem 275: 22202–22212 [DOI] [PubMed] [Google Scholar]
- Zang Q, Springer TA (2001) Amino acid residues in the PSI domain and cysteine-rich repeats of the integrin β2 subunit that restrain activation of the integrin αXβ2. J Biol Chem 276: 6922–6929 [DOI] [PubMed] [Google Scholar]
- Zhu J, Boylan B, Luo B-H, Newman PJ, Springer TA (2007) Tests of the extension and deadbolt models of integrin activation. J Biol Chem 16: 11914–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Barth P, Schonbrun J, Baker D, Springer TA (2009) The structure of a receptor with two associating transmembrane domains on the cell surface: integrin αIIbβ3. Mol Cell 34: 234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S7
Review Process File