Abstract
During nonconscious perception of facial affect, healthy adults commonly activate a right-lateralized pathway comprising the superior colliculus, pulvinar, and amygdala. Whether this system is fully developed prior to adulthood is unknown. Twenty-three healthy adolescents underwent functional magnetic resonance imaging (fMRI) while viewing fearful, angry, and happy faces, backward masked by neutral faces. Left amygdala activation differed among the three affects, showing reductions to masked anger and increases to masked fear and happy faces. During masked fear, left amygdala activation correlated positively with extrastriate cortex and temporal poles and negatively with precuneus and middle cingulate gyrus. Responses of the left amygdala to masked anger correlated positively with right parahippocampal gyrus and negatively with dorsal anterior cingulate. Amygdala responses to masked happy faces were uncorrelated with other brain regions. Contrary to the right-lateralized pathway seen in adults, adolescents show evidence of a predominantly left-lateralized extrastriate pathway during masked presentations of facial affect.
Keywords: FMRI, Neuroimaging, Masked Affect, Nonconscious, Face Perception, Limbic System, Adolescence, Development
Human survival is vitally dependent on the ability to detect and respond rapidly to biologically relevant stimuli in the environment. The visual processing system comprises two separate neural pathways that facilitate flexible appraisal by permitting emotionally relevant visual information to be evaluated and responded to at multiple levels of perceptual awareness (LeDoux, 1996). Specifically, evidence suggests that conscious visual perception of affective information relies on the geniculostriate system, a relatively slow pathway from the retina to the lateral geniculate nucleus to the primary visual cortex and then to higher level visual association regions (Kimura, Yoshino, Takahashi, & Nomura, 2004). Simultaneously, a second more rapid system, comprising the superior colliculus, pulvinar nucleus, and amygdala appears to operate below the level of conscious awareness to permit near instantaneous appraisal and enhance the preparedness of the individual to respond to emotionally salient stimuli (Kimura et al., 2004; LeDoux, 1996; Ohman, Carlsson, Lundqvist, & Ingvar, 2007). Because this second system bypasses the primary visual cortex and association regions, it is hypothesized to operate automatically by processing visual information at a very crude level of spatial resolution without engaging the direct conscious awareness of the individual (Ohman, 2005). This non-conscious visual perception system has been invoked to explain the phenomenon of blindsight, a condition where cortically blind individuals retain the capacity to respond accurately to some aspects of visual stimuli such as movement or emotional content in facial expressions, despite lack of conscious perception of the stimuli (Morris, DeGelder, Weiskrantz, & Dolan, 2001).
The capacity of the brain to process affective information outside of normal conscious awareness has been explored using a technique known as backward stimulus masking. This technique presents the individual with an emotional stimulus below the normal threshold of conscious perception. For example, when an emotional facial expression is presented for an extremely brief duration (e.g., ≤ 30 msec) and followed immediately by a “mask” image of a face displaying a neutral emotion, the target emotional expression is usually not reliably discriminated above chance levels (Williams et al., 2004). Nonetheless, such masked affective stimuli produce reliable patterns of activation within the amygdala and associated affect processing regions during functional neuroimaging (Killgore & Yurgelun-Todd, 2004a; Morris, Ohman, & Dolan, 1998; Whalen et al., 1998). Masked expressions of fear have been by far the most extensively studied, with the majority of evidence suggesting that nonconscious perception of fearful faces is associated with greater activity within the amygdala relative to other unseen affective expressions (Suslow et al., 2006; Whalen et al., 1998). Under normal circumstances, this responsiveness of the amygdala may enhance survival by increasing vigilance and appropriate threat-related responses (LeDoux, 1996). Conversely, extreme levels of amygdala responsiveness have also been associated with excessive anxiety (Killgore & Yurgelun-Todd, 2005; Rauch et al., 2000). Thus, understanding this circuitry is critical to furthering our knowledge regarding brain function, social responses, and anxiety.
Findings from previous research suggest several predictions regarding the functional neuroanatomy that may be activated by masked presentations of facial affect. Because masked visual stimuli are believed to primarily activate the superior colliculus-pulvinar extrastriate pathway, thus bypassing areas involved conscious visual cortical processing, such stimuli are expected to produce very little activation in primary visual cortex and areas involved in affect regulation such as the prefrontal cortex (Morris, Ohman, & Dolan, 1999; Ohman, 2005). While there is considerable support for this model in healthy adults (Killgore & Yurgelun-Todd, 2004a; Morris et al., 1998; Whalen et al., 1998), it remains unclear whether non-conscious activation of the amygdala and deactivation of cortical regions by masked affective stimuli is evident prior to the age of adulthood, and therefore, consistent across development. In the present study, we examined activation of the amygdala and its functional correlation with other regions of the brain in a sample of adolescent and pre-adolescent children viewing a series of masked affective faces. The masked facial stimuli included expressions from three different affect categories, including fear, anger, and happiness. Based on studies masked affect presentations in adults, we hypothesized that the amygdala would respond most strongly to negatively valenced masked affective presentations, would be lateralized to the right, and that this activation would be positively correlated with activity in subcortical limbic structures and extrastriate visual processing regions but negatively correlated with cortical regions involved in cognitive control and affect regulation (i.e., prefrontal cortex, anterior cingulate gyrus).
Methods
Subjects
Twenty-three healthy children (9 male; 14 female) ranging in age from 8 to 18 years (M = 13.0, SD = 3.3) volunteered to undergo functional magnetic resonance imaging (fMRI). This age range was selected in order to encompass the full range of pre- to post-adolescent development. The age of the participants did not differ between male (M = 12.7, SD = 3.1) and female (M = 13.3, SD = 3.3) groups, t(21) = 0.44, ns. Participants were predominantly right handed (4 non-right handed) by self-report. The children were screened for psychopathology using a structured clinical interview and were included only if they were free from any current or past history of psychiatric illness, neurologic problems, or illicit substance or alcohol use. Children had normal visual acuity (or corrected to normal with contact lenses) and were recruited from the local community of Belmont, MA. All procedures and potential risks were explained and written informed consent/assent was obtained from the children and their parents prior to participation. Families were given a small financial payment for their time.
FMRI Stimulation Paradigm
During fMRI, the children completed three task runs that involved masked presentations of angry, fearful, and happy affective face in a counterbalanced order. Each run comprised five 30-second blocks that alternated between masked neutral and masked affect conditions. Each block consisted of 10 trials each lasting 3 seconds. During each trial, a facial expression “target” image was presented for 30 msec followed immediately by a neutral facial expression “mask” for 170 msec. This duration was chosen based on extensive data from our own laboratory (Killgore & Yurgelun-Todd, 2004a, 2007) as well as detailed psychophysical studies from others (Esteves & Ohman, 1993; Williams et al., 2004). Trials were separated by a 2800 msec period of blank screen. Both stimuli in each target-mask pair were posed by the same actor (Ekman & Friesen, 1976). All trials were identical except for the affect displayed on the “target” expression. Neutral trials presented a neutral target followed by a neutral mask whereas affective trials presented an affective target followed by a neutral mask. Participants were not informed about the backward masking of the stimuli and were told only that they would observe photographs of faces presented briefly and would be required to indicate whether each face was male or female by pressing a button with the right hand. The tasks were programmed in Psyscope 1.2.5 software (Macwhinney, Cohen, & Provost, 1997) running on a Power Macintosh G3 computer. Stimuli were back-projected onto a screen placed at the rear of the scanner and viewed via a mirror mounted on the head coil.
Neuroimaging Methods
Data were collected at 3.0 Tesla on a Siemens Trio MRI scanner and a quadrature RF head coil (TR = 3000 msec, TE = 30 msec, flip angle = 90 degrees). At the outset, three dummy images were acquired and discarded to obtain a steady state. For each run, fifty coronal echoplanar images were acquired over 40 slices (5mm thick, interleaved), using a 20 cm field of view and a 64 × 64 acquisition matrix, providing a resolution of 3.125 × 5 × 3.125 mm. Matched T1-weighted high-resolution images were acquired at the outset of each scanning session.
Image Processing
Data were preprocessed and analyzed in SPM99 (Friston et al., 1995). Functional images were motion corrected, realigned in three axes using the standard algorithms in SPM99, and normalized to the three-dimensional space of the Montreal Neurological Institute (MNI). Data were spatially smoothed with an isotropic Gaussian kernel (full width half maximum [FWHM] = 10 mm), and resliced to 2×2×2 mm voxels using sinc interpolation.
Statistical Analysis
The analysis followed a two-step random-effects approach in SPM99. First, functional data for each participant were convolved to a boxcar waveform based on the block design of the task (i.e., masked affect vs. masked neutral) and the canonical hemodynamic response function. For each individual, the BOLD signal was convolved with the alternating neutral and affect conditions to produce a contrast image evaluating within-subject effects of each masked affect versus masked neutral baseline. These contrast images were then analyzed with random effects models in the second stage of analyses. To identify amygdala regions showing variability across the three affect conditions, a priori search territories were placed at the location of the left and right amygdala. These search territories were defined according to the specifications of the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) as implemented within the WFU PickAtlas utility for SPM (Maldjian, Laurienti, Kraft, & Burdette, 2003). Next, constraining the analysis to these amygdala search territories, we conducted a voxel-wise omnibus analysis of variance (ANOVA) in SPM99 across the three affect conditions (masked anger, fear, happy). Because the purpose of this analysis was to identify voxels of interest within the amygdala for further analysis, activation was considered significant if it exceeded an uncorrected threshold p < .05, and spatial extent of k = 10. This analysis yielded an activation cluster of 37 voxels within the left amygdala which was then used as a region of interest (ROI) in subsequent analyses. Although no activation was found within the right amygdala for the omnibus F-test, for completeness the right amygdala was also interrogated by placing a mirror image ROI at the same MNI coordinates in the right hemisphere (i.e., flipping the x-axis coordinates). Data from these functionally defined ROIs within the amygdala were extracted and entered into a 2 (side) × 3 (anger, fear, happy) repeated measures analysis of variance (ANOVA) in SPSS 12. Furthermore, activation within each of these ROIs was compared among the three masked affects using paired t-tests in SPM99 at p < .05, family-wise error (FWE) corrected, k = 10. The correlation between the activation within each ROI and the age of the participants was also calculated. Finally, to examine the functional relationship between the amygdala and whole brain activation for each affect condition, functional data within the left amygdala ROI was extracted and used as a seed region for subsequent whole brain correlation analysis at p < .001, uncorrected, k = 20. Because of the large age range in the participants, chronological age was entered as a nuisance variable using the multiple regression option in SPM99 to control for age-related variance. This was done separately for masked anger, masked fear, and masked happy conditions. Findings of interest were overlaid onto the canonical high resolution brain image in SPM for visualization.
Results
Omnibus F-Test for Affect Conditions
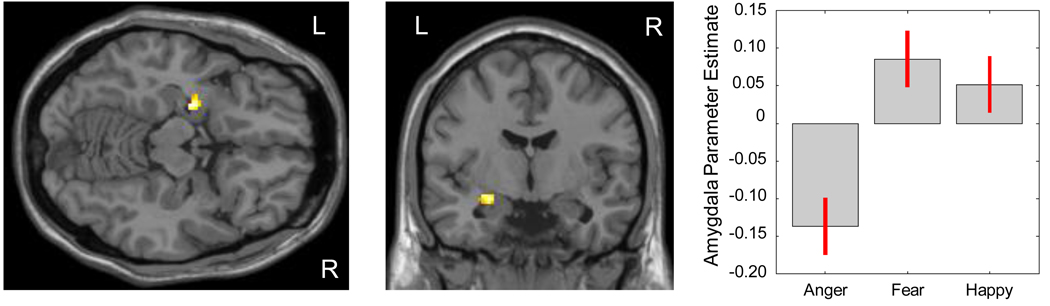
Figure 1 shows that within the bilateral amygdala search territories, a voxelwise analysis of variance across the three masked affect conditions yielded a significant cluster of activation within the left amygdala (37 voxels; F[2,65] = 6.76; MNI coordinates: x= −24, y = −6, z = −14). In contrast, no activation difference was observed in the right amygdala across these three masked affect conditions.
Figure 1.
Within the bilateral amygdala search territories, a voxelwise analysis of variance (ANOVA) yielded a cluster of 37 voxels in the left amygdala that differed significantly across the three masked affect conditions. This cluster can be seen in axial (Left Panel) and coronal slices (Middle Panel). Parameter estimates extracted from the peak voxel (MNI coordinates: x = −24, y = −6, z = −14) are plotted for visualization and show a decline in left amygdala activation for the masked anger vs. neutral contrast relative to the increases shown the fear vs. neutral and happy vs. neutral contrasts (Right Panel).
Lateralized Amygdala Activation by Affect Condition
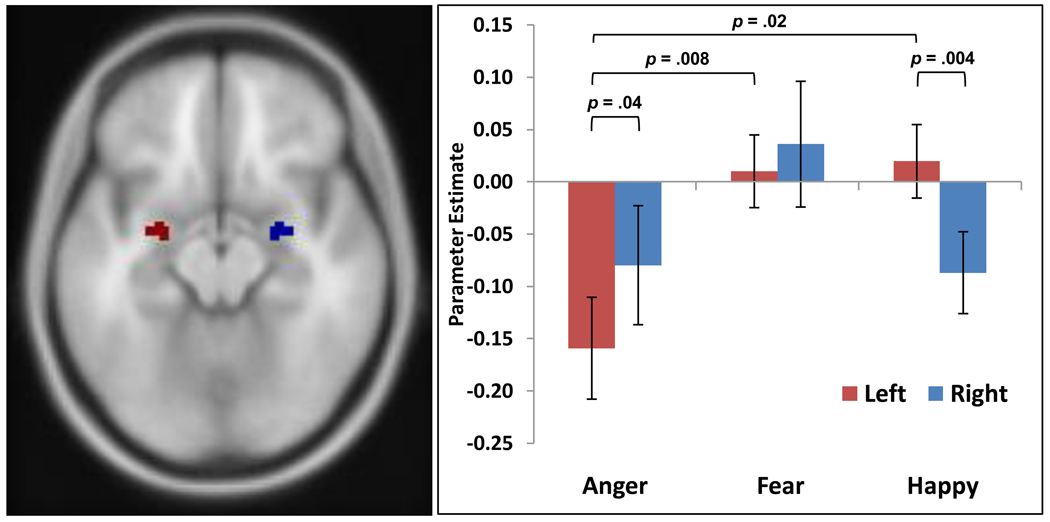
To further clarify the nature of the group differences within the left amygdala, the activation cluster identified in the previous voxelwise omnibus F-test was defined as an ROI for subsequent group comparisons. Although the previous analysis showed no main effect of condition in the right amygdala, for completeness a mirror image ROI for the right amygdala was created by flipping the x-coordinates of first ROI. Functional data were extracted from these bilateral ROIs for each of the affect conditions and subjected to a 2 (hemisphere) × 3 (affect condition) repeated measures analysis of variance (ANOVA). There was no significant main effect of affect or hemisphere, but as evident in Figure 2, there was a significant hemisphere × affect condition interaction, F(2,42) = 6.06, p = .005. Post-hoc comparisons showed that mean BOLD signal intensity within the left amygdala ROI was lower for masked anger than masked fear (p = .008) or masked happy expressions (p = .023). Furthermore, while masked anger showed greater deactivation within the left amygdala than the right (p = .04), masked happy presentations were associated with greater deactivation in the right amygdala relative to the left (p = .004).
Figure 2.
Based on the left amygdala cluster of activation produced by the omnibus ANOVA, identical mirror-image regions of interest (ROIs) were placed within each amygdala. The left panel shows an axial view of the location of the ROIs (Left = Red; Right = Blue). The right panel shows that a repeated measures analysis of variance (ANOVA) on the extracted data from each ROI for each masked affect condition yielded a significant hemisphere by condition interaction (p = .005).
Voxelwise Paired Comparisons Between Affect Conditions
Within the left and right ROIs, voxelwise comparisons were tested among the three masked affect conditions. As evident in Table 1, both masked fear and masked happy conditions showed significantly greater left amygdala activation relative to masked anger expressions, whereas there was no significant difference in left amygdala responses to masked fear and happy face presentations. For the right amygdala, none of the pair-wise comparisons among affect conditions emerged as significantly different.
Table 1.
Regions Showing Differences in Left Amygdala ROI Activation among the Masked Anger, Fear, and Happy Conditions via Paired T-Tests
| Active | MNI Coordinates | FWE | ||||
|---|---|---|---|---|---|---|
| Contrast | Voxels | x | y | z | {t}-score | Corrected P |
| Anger > Fear | -- | -- | -- | -- | -- | -- |
| Fear > Anger | 23 | −28 | −6 | −12 | 3.13 | .014 |
| Anger > Happy | -- | -- | -- | -- | -- | -- |
| Happy > Anger | 11 | −24 | −4 | −14 | 2.80 | .025 |
| Fear > Happy | -- | -- | -- | -- | -- | -- |
| Happy > Fear | -- | -- | -- | -- | -- | -- |
Note. None of the comparisons listed above were significant for the right amygdala. Significance was set at p < .05, Family Wise Error (FWE) corrected within the amygdala search territory, k = 10.
Amygdala Correlations with Age
To examine the relationship between age and amygdala responses, the mean extracted data from each amygdala ROI were entered into a Pearson correlation analysis for each affect condition separately. For the masked anger versus neutral condition, age was not significantly correlated with fMRI signal in the left (r = .14, ns) or right (r = .11, ns) amygdala ROIs. However, for the masked fear versus neutral condition, there was a significant negative correlation between participant age and fMRI signal in the ROI for the left (r = −.48, p = .02) but not the right amygdala (r = −.17, ns). Finally, there was no significant correlation between age and amygdala ROI responses for either the left (r = .10, ns) or right (r = .32, ns) sides during the masked happy versus neutral condition.
Left Amygdala Correlations across Whole Brain
Mean activation from the left amygdala cluster ROI identified by the omnibus F-test was extracted for each subject and used as a seed region for correlation analysis with other regions across the entire brain. After controlling for age effects, partial correlations with whole brain were evaluated for each masked affect condition separately. Figure 3 shows the voxel clusters that correlated with left amygdala responses to masked affect and the coordinates of cluster maxima are listed in Table 2.
Figure 3.
The left amygdala was used as a seed region to determine whole brain correlations with amygdala activation during each affect condition. The three leftmost panels in reach row show maximum intensity projections or “glass brain” views, while the rightmost image shows a representative overlay of activation on the canonical brain template. After controlling for age, activation of the left amygdala during masked anger presentations was associated with a) increased activation within extrastriate visual cortex, cerebellum, and temporal pole regions, and b) reduced activation within posterior medial cortex including the precuneus and middle Adolescent Masked Affect cingulate gyrus. During masked fear presentations, left amygdala activation (controlling for age) was associated with c) increased activation within the right parahippocampal gyrus and d) reduced activation within the dorsal anterior cingulate gyrus, left middle cingulate gyrus, supramarginal gyrus, and middle temporal pole.
Table 2.
Regions Showing Activity Correlations with Left Amygdala Responses to Masked Affect
| Active | MNI Coordinates | ||||
|---|---|---|---|---|---|
| Regions of activation | Voxels | x | y | z | {t}-score |
| Anger: Positive Correlation with Left Amygdala Search Territory | |||||
| L. Inf Occipital Gyrus | 34 | −38 | −82 | −10 | 5.62 |
| L. Amygdala/Sup Temporal Pole | 99 | −28 | −2 | −20 | 4.71 |
| R. Mid/Sup Temporal Pole | 61 | 46 | 14 | −30 | 4.62 |
| R. Cerebellum (area 6) | 195 | 12 | −76 | −14 | 4.56 |
| L. Cerebellum (area 7b) | 85 | −16 | −74 | −44 | 4.42 |
| R. Inf Occipital Gyrus | 38 | 42 | −78 | −6 | 4.39 |
| R. Cerebellar Crus | 47 | 36 | −66 | −30 | 4.25 |
| Anger: Negative Correlation with Left Amygdala Search Territory | |||||
| R. Suppl Motor/Sup Frontal Gyrus | 184 | 18 | −16 | 58 | 5.45 |
| L. Mid Cingulate Gyrus | 241 | −10 | −44 | 40 | 4.89 |
| R. Precuneus | 186 | 16 | −40 | 42 | 4.55 |
| R. Postcentral Gyrus | 51 | 56 | −14 | 40 | 4.46 |
| R. Precentral Gyrus | 20 | 40 | −18 | 54 | 4.25 |
| Fear: Positive Correlation with Left Amygdala Search Territory | |||||
| R. Parahippocampal Gyrus | 44 | 14 | −2 | −14 | 4.28 |
| Fear: Negative Correlation with Left Amygdala Search Territory | |||||
| R. Ant Cingulate Gyrus | 218 | 12 | 22 | 28 | 7.11 |
| L. Mid Cingulate Gyrus | 22 | −10 | −18 | 40 | 5.48 |
| L. Supramarginal Gyrus | 24 | −58 | −38 | 24 | 5.12 |
| L. Mid Temporal Pole | 24 | −42 | 10 | −34 | 4.17 |
| R. Amygdala | 25 | 32 | −4 | −28 | 4.04 |
| Cerebellar Vermis (area 8) | 24 | −2 | −68 | −44 | 3.95 |
| R. Cerebellum (area 6) | 26 | 36 | −56 | −30 | 3.94 |
| Happy: Positive Correlation with Left Amygdala Search Territory | |||||
| None | -- | -- | -- | -- | -- |
| Happy: Negative Correlation with Left Amygdala Search Territory | |||||
| None | -- | -- | -- | -- | -- |
Note. L = left hemisphere, R = right hemisphere. Sup = superior, Mid = middle, Inf = inferior, Ant = anterior, Suppl = supplementary. Atlas coordinates are from the MNI standard atlas, such that x reflects the distance (mm) to the right or left of midline, y reflects the distance anterior or posterior to the anterior commissure, and z reflects the distance superior or inferior to the horizontal plane through the AC-PC line. All values exceed a threshold of p < .001, k = 20.
Masked Anger
During masked anger trials, activation within the left amygdala was positively correlated with activation within secondary visual processing regions, including bilateral inferior occipital gyri, as well as bilateral cerebellum and temporal pole regions (see Figure 3). In contrast, left amygdala responses during masked anger were negatively correlated with activation in regions of the supplementary motor cortex/precentral gyrus, predominantly on the right, and a large cluster of activation encompassing the middle/posterior cingulate gyrus and precuneus (see Figure 3).
Masked Fear
As evident in Figure 3, greater responses within the left amygdala during the masked fear presentations were positively correlated with a single cluster of activation in the contralateral right anterior hippocampus extending inferiorly into the parahippocampal gyrus. Activation in the left amygdala was negatively correlated with responses in a large cluster within the right anterior cingulate gyrus, and a number of smaller and widely distributed regions including the left middle cingulate gyrus, supramarginal gyrus, and middle temporal pole. Interestingly, left amygdala responses to masked fear were also negatively correlated with activation in the right amygdala and cerebellum.
Masked Happy
No regions of the brain showed any significant positive or negative correlations with left amygdala responses to masked happy faces.
Discussion
In the present study of pre-adolescent and adolescent children we found that 1) although amygdala responses were observed, contrary to the predominant findings in adults, activation to masked affective faces was more responsive in the left rather than right amygdala, 2) the left amygdala activation during masked affect presentations showed a differential pattern of lateralized activation depending on the specific affect condition, but this pattern was not systematically related to valence, and 3) the activation of the left amygdala correlated differentially with several brain regions depending on the specific masked affect condition, suggesting that the amygdala may be associated with modulating different aspects of affective processing depending on the specific emotion perceived. A number of implications regarding affective processing during development can be derived from these findings.
The first major finding was that the left amygdala was the most differentially responsive to the three masked affect conditions, whereas there was no evidence of such responsiveness within the right amygdala, regardless of whether voxelwise or ROI analyses were performed. Several studies focusing on adult samples have reported either bilateral (Williams et al., 2006a) or greater right amygdala activation in response to masked affect presentations (Kugel et al., 2008; Morris et al., 1998, 1999; Williams et al., 2006b), a finding supported by a recent meta analysis (Costafreda, Brammer, David, & Fu, 2008). Interestingly, not all studies show right lateralized activation of the amygdala during masked affect presentations in adults. Notably, Whalen and colleagues presented adult subjects with images of “eye-whites” rather than full facial stimuli and found significant responses in the left amygdala for fearful relative to happy eye-whites (Whalen et al., 2004). The similarity between the responses of children viewing masked full faces and adults viewing incomplete (i.e., crude) facial stimuli raises the possibility that the left amygdala response pattern may reflect a more primitive or developmentally immature aspect of nonconscious facial feature processing.
The reason for the reversed lateralization observed in the present child through adolescent sample is unclear but the outcome may reflect a developmental phenomenon. For instance, we have previously found that adolescents show a pattern of reversed lateralized amygdala and prefrontal activation relative to both younger children and adults viewing the overtly presented affective face stimuli (Killgore & Yurgelun-Todd, 2004b). It is well known that the period of adolescence is characterized by dramatic changes in cerebral organization (Hasan et al., 2007; Shaw et al., 2008), neuronal structure (Rabinowicz, Petetot, Khoury, & de Courten-Myers, 2009), and volume changes in the amygdala (Yurgelun-Todd, Killgore, & Cintron, 2003). Our previous work raised the possibility that affective processing during adolescence may be associated with shifts in the lateralization of cortical and limbic processing (Killgore & Yurgelun-Todd, 2004b), similar to that observed for other cognitive and affective capacities (Everts et al., 2009; Killgore, Gruber, & Yurgelun-Todd, 2007). Consistent with that possibility, we found a negative correlation between the age of the participants and responsiveness of the left amygdala to masked fearful faces. The negative correlation of left amygdala activation with age is similar to our previous findings for overtly presented faces (Killgore, Oki, & Yurgelun-Todd, 2001), and may suggest that the amygdala responses may become more right lateralized with maturation through adolescence. While it is possible that the present finding may reflect a similar pattern of reversed lateralization that extends to non-conscious affect processing in adolescents, further research will be needed to establish the reliability of this preliminary finding. Furthermore, there is some evidence that functional activity within the amygdala may be inversely related to prefrontal activation (Whalen et al., 2004), and such prefrontal activation during affective processing may increase with maturation throughout adolescence (Yurgelun-Todd & Killgore, 2006).
The second major finding was that the lateralization of amygdala responses was modulated by the specific affect category presented. For masked angry faces, both the left and right amygdala showed evidence of deactivation relative to masked neutral faces, but the magnitude of deactivation was significantly greater for the left versus the right amygdala. Furthermore, the response observed within the left amygdala was significantly lower for masked anger relative to the masked fear and masked happy conditions. A recent study also reported decreased activation within the right amygdala during presentations of masked angry versus masked neutral faces in healthy children and adolescents but showed that youths with pediatric generalized anxiety disorder showed elevated right amygdala responsiveness during this task (Monk et al., 2008). Together, these studies suggest that reduced amygdala activation to masked angry faces may be a normal response in older children and adolescents, while exaggerated responses to these stimuli may be associated with heightened anxiety in this age group. Interestingly, masked fear, while showing a trend toward greater right amygdala responses relative to the other affect conditions, failed to show significant laterality effects, a pattern that differs from the commonly observed right lateralized effect in adults (Costafreda et al., 2008; Morris et al., 1999). Our previous study with a group of adolescents viewing masked sad faces found significant activation within the right but not left amygdala, a finding that was stronger in adolescents than adults (Killgore & Yurgelun-Todd, 2007). On the other hand, in the present study, masked happy expressions yielded greater activation in the left amygdala and reduced activation within the right, a pattern opposite from that of masked anger. In a previous study of similarly aged adolescents, we failed to observe significant responses in either amygdala during masked happy presentations using a different set of stimuli and faster presentation parameters (Killgore & Yurgelun-Todd, 2007), suggesting considerable variability in how this age group may perceive masked images of happy expressions. Overall, these findings argue against a simple valence effect whereby faces displaying negative affect would be expected to elicit greater amygdala responses than those expressing positive affect, suggesting instead that the amygdala responses may have been affected by some other feature that distinguished among the emotional expression categories (e.g., arousal, dominance, approach/withdrawal). Previous work suggests that the amygdala may be responsive to a broad range of positive and negative facial affect stimuli (Costafreda et al., 2008; Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006), suggesting that amygdala responses may simply reflect visual vigilance for biologically salient information (Suslow et al., 2006). Clearly, further work will be necessary to identify the specific factors that lead to modulation of these responses and whether these are similar for adults and children.
Another primary goal of the present study was to examine the relationship between amygdala activation and responses within other brain regions during perception of masked affective faces. Of note, we found that the correlation between amygdala activation and cerebral responses was modulated by the affective category of the masked expressions. During presentations of masked angry versus neutral faces, left amygdala activation was associated with corresponding increases in bilateral regions of extrastriate cortex (i.e., inferior occipital gyrus; BA 19), bilateral temporal pole and peri-amygdalar cortex, and cerebellum, and decreases in superior frontal regions, middle cingulate gyrus, and precuneus, even after statistically controlling for age. These correlations are generally consistent with the notion that masked affective presentations involve an extrastriate visual pathway (Morris et al., 1999; Ohman, 2005), and further support the operation of such a pathway during the period of adolescence. In particular, with greater amygdala activation in response to masked angry faces, there was also greater activation of areas involved in secondary visual processing, without corresponding increases in primary visual cortex. This suggests that the amygdala responses may be closely associated with a crude visual analysis that bypasses primary visual processing, possibly via the superior colliculus-pulvinar pathway involved in the phenomenon of blindsight (Bittar, Ptito, Faubert, Dumoulin, & Ptito, 1999; Morris et al., 2001; Schoenfeld, Heinze, & Woldorff, 2002). The enhancement of extrastriate visual processing with greater amygdala activation is consistent with a report by Suslow and colleagues who found that amygdala responses were positively correlated with the ability to subsequently identify previously presented masked affect stimuli (Suslow et al., 2006). Interestingly, we also found that amygdala responses to masked anger were negatively correlated with activation within primarily posterior midline regions such as the middle cingulate gyrus and precuneus. These regions are key structures in the “default mode” network, which is activated across a variety of resting states or when an individual is engaged in self-referential introspective cognition or visual imagery, and which is attenuated during the engagement of externally focused cognitive tasks (Cavanna & Trimble, 2006; Raichle et al., 2001). In other words, those children and adolescents showing the greatest amygdala responses to the masked angry faces also tended to have the greatest decline in these posterior midline regions, perhaps reflecting a shift away from self-focused cognition and toward greater awareness of the external environment—a form of enhanced readiness in the face of potential threat.
A different pattern of amygdala correlations emerged for masked fear versus neutral presentations. Signal in the left amygdala ROI was positively correlated with a single cluster of activation localized to the right parahippocampal gyrus, a region of the brain known to be activated by emotional facial expressions (Fusar-Poli et al., 2009), memory consolidation (Janzen, Jansen, & van Turennout, 2008), and successful memory retrieval (Hayes, Nadel, & Ryan, 2007). In contrast, left amygdala activation during the masked fear condition was negatively correlated with a large region of the right dorsal anterior cingulate gyrus, a region involved in error detection (Modirrousta & Fellows, 2008), self-evaluation of decision-making quality (Hewig et al., 2009), and behavioral inhibition (Beaver, Lawrence, Passamonti, & Calder, 2008). Fear related left amygdala activation was also negatively correlated with a number of smaller regions including the right amygdala, left middle cingulate gyrus, left middle temporal pole, left supramarginal gyrus, and cerebellum. Together, these findings suggest that amygdala responses during masked fear presentations were associated with increased activation of systems involved in episodic memory encoding, particularly for emotional information and reduced activation in cognitive monitoring and error detection systems.
Finally, activation within the left amygdala during masked happy versus neutral presentations was not significantly correlated with any other brain regions, consistent with the general notion that the amygdala is most involved in activating a neurobehavioral response to potentially threatening stimuli (Ohrmann et al., 2007). Our previous work using masked affective stimuli with adolescents failed to find significant amygdala responses to masked happy faces (Killgore & Yurgelun-Todd, 2007). Thus, in adolescents, amygdala responses to happy faces seem to be inconsistently associated with a particular pattern of changes in cerebral activation relative to other masked affects.
Overall, the present findings are generally consistent with the hypothesis that masked affect presentations bypass the geniculostriate visual processing system and directly activate limbic and extrastrate regions (LeDoux, 1996), presumably via the superior colliculus-pulvinar-amygdala pathway (Kimura et al., 2004; Morris et al., 1999), and that this system appears to be functional by late childhood or early adolescence, if not earlier. Additionally, the present results further suggest that each affective category is associated with distinct patterns of activation within the amygdala and that the pattern of amygdala lateralization during masked affect processing may be different in adolescent children relative to the eventual right lateralized pattern that is commonly observed in adults. These findings are consistent with a small number of studies suggesting that the adolescent developmental period may be characterized by a shift in lateralized activation within the amygdala. Some evidence suggests that these patterns may differ between males and females. Unfortunately, the sample sizes in the present study precluded adequate power to resolve potential sex differences. Future studies may clarify the extent to which these findings reflect stable response patterns of affective responses to masked stimuli and the extent to which they differ as a function of sex.
References
- Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. Journal of Neuroscience. 2008;28(11):2719–2725. doi: 10.1523/JNEUROSCI.0033-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar RG, Ptito M, Faubert J, Dumoulin SO, Ptito A. Activation of the remaining hemisphere following stimulation of the blind hemifield in hemispherectomized subjects. Neuroimage. 1999;10(3 Pt 1):339–346. doi: 10.1006/nimg.1999.0474. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Esteves F, Ohman A. Masking the face: recognition of emotional facial expressions as a function of the parameters of backward masking. Scandinavian Journal of Psychology. 1993;34(1):1–18. doi: 10.1111/j.1467-9450.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, et al. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human Brain Mapping. 2009;30(2):473–483. doi: 10.1002/hbm.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30(4):1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;5:189–201. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters. 2009;452(3):262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, et al. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18(16):1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17(9):873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewig J, Straube T, Trippe RH, Kretschmer N, Hecht H, Coles MG, et al. Decision-making under Risk: An fMRI Study. Journal of Cognitive Neuroscience. 2009;21(8):1642–1652. doi: 10.1162/jocn.2009.21112. [DOI] [PubMed] [Google Scholar]
- Janzen G, Jansen C, van Turennout M. Memory consolidation of landmarks in good navigators. Hippocampus. 2008;18(1):40–47. doi: 10.1002/hipo.20364. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neuroscience Letters. 2007;416(1):43–48. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004a;21(4):1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Perceptual and Motor Skills. 2004b;99(2):371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Social Neuroscience. 2007;2(1):28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yoshino A, Takahashi Y, Nomura S. Interhemispheric difference in emotional response without awareness. Physiology and Behavior. 2004;82(4):727–731. doi: 10.1016/j.physbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, Ohrmann P, Bauer J, Arolt V, et al. Alexithymic features and automatic amygdala reactivity to facial emotion. Neuroscience Letters. 2008;435(1):40–44. doi: 10.1016/j.neulet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. New York: Simon & Schuster; 1996. [Google Scholar]
- Macwhinney B, Cohen J, Provost J. The PsyScope experiment-building system. Spatial Vision. 1997;11(1):99–101. doi: 10.1163/156856897x00113. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. Journal of Neuroscience. 2008;28(51):14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124(Pt 6):1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating "unseen" fear. Proceedings of the National Academy of Sciences of the U S A. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiology and Behavior. 2007;92(1–2):180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Rauch AV, Bauer J, Kugel H, Arolt V, Heindel W, et al. Threat sensitivity as assessed by automatic amygdala response to fearful faces predicts speed of visual search for facial expression. Experimental Brain Research. 2007;183(1):51–59. doi: 10.1007/s00221-007-1022-0. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Petetot JM, Khoury JC, de Courten-Myers GM. Neocortical maturation during adolescence: change in neuronal soma dimension. Brain and Cognition. 2009;69(2):328–336. doi: 10.1016/j.bandc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Heinze HJ, Woldorff MG. Unmasking motion-processing activity in human brain area V5/MT+ mediated by pathways that bypass primary visual cortex. Neuroimage. 2002;17(2):769–779. [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Ohrmann P, Bauer J, Rauch AV, Schwindt W, Arolt V, et al. Amygdala activation during masked presentation of emotional faces predicts conscious detection of threat-related faces. Brain and Cognition. 2006;61(3):243–248. doi: 10.1016/j.bandc.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp A, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. Journal of Neuroscience. 2006a;26(36):9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, et al. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping. 2006b;27(8):652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, et al. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Human Brain Mapping. 2004;21(2):64–74. doi: 10.1002/hbm.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neuroscience Letters. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WDS, Cintron C. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Perceptual and Motor Skills. 2003;96(1):3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]