Abstract
Adenosine A2A receptor antagonists are psychomotor stimulants that also hold therapeutic promise for movement disorders. However, the molecular mechanisms underlying their stimulant properties are not well understood. Here, we show that the robust increase in locomotor activity induced by an A2A antagonist in vivo is greatly attenuated by antagonizing cannabinoid CB1 receptor signaling or by administration to CB1−/− mice. To determine the locus of increased endocannabinoid signaling, we measured the amount of anandamide [AEA (N-arachidonoylethanolamine)] and 2-arachidonoylglycerol (2-AG) in brain tissue from striatum and cortex. We find that 2-AG is selectively increased in striatum after acute blockade of A2A receptors, which are highly expressed by striatal indirect-pathway medium spiny neurons (MSNs). Using targeted whole-cell recordings from direct- and indirect-pathway MSNs, we demonstrate that A2A receptor antagonists potentiate 2-AG release and induction of long-term depression at indirect-pathway MSNs, but not direct-pathway MSNs. Together, these data outline a molecular mechanism by which A2A antagonists reduce excitatory synaptic drive on the indirect pathway through CB1 receptor signaling, thus leading to increased psychomotor activation.
Introduction
The basal ganglia and its primary input nucleus, the striatum, are critical for motivation and motor control (Graybiel et al., 1994; Hikosaka et al., 2000; Yin and Knowlton, 2006). The striatum integrates information from the cortex, thalamus, and midbrain, and sends projections to downstream basal ganglia nuclei that regulate thalamocortical motor circuits (Bolam et al., 2000). Striatal projection neurons, known as medium spiny neurons (MSNs), are GABAergic and can be divided into two subclasses based on their axonal projections and gene expression patterns. Direct-pathway MSNs, which project directly to basal ganglia output nuclei, express dopamine D1 receptors. Indirect-pathway MSNs, which project to the globus pallidus, express dopamine D2 and adenosine A2A receptors (Gerfen et al., 1990; Schiffmann et al., 1991; Smith et al., 1998). According to classical basal ganglia models, increased direct-pathway activity facilitates movement, whereas increased indirect-pathway activity inhibits movement (Albin et al., 1989; DeLong, 1990).
A2A receptors are Gs-coupled metabotropic receptors that are highly expressed in the striatum and, to a lesser extent, in other brain regions including the globus pallidus, hippocampus, and cortex (Sebastião and Ribeiro, 1996). They are enriched in the postsynaptic density of glutamatergic synapses onto striatal indirect-pathway MSNs (Rosin et al., 2003; Schiffmann et al., 2007), although they are also observed in some presynaptic terminals in the striatum and globus pallidus, where they appear to enhance neurotransmitter release (Shindou et al., 2003, 2008). Behaviorally, A2A receptor agonists decrease movement (Barraco et al., 1993; Hauber and Münkle, 1997) and facilitate the induction of long-term potentiation (Flajolet et al., 2008), suggesting that they increase indirect-pathway activity. In contrast, A2A receptor antagonists increase movement and also cause an increase in immediate-early gene expression in the globus pallidus, suggesting that they decrease indirect-pathway activity (Svenningsson et al., 1997; Hauber et al., 1998; Huang et al., 2005; Mingote et al., 2008; Shen et al., 2008a). A2A and D2 receptors have opposing effects on cAMP accumulation in indirect-pathway neurons, and inhibition of A2A receptors facilitates D2 receptor-mediated processes (Ferré et al., 1997; Strömberg et al., 2000; Tozzi et al., 2007; Kim and Palmiter, 2008). Because of their actions on the indirect pathway, A2A antagonists have been proposed as an adjunct or alternative to dopamine replacement therapy in patients with Parkinson's disease (PD) (Schwarzschild et al., 2006; Simola et al., 2008; Jenner et al., 2009). However, despite extensive characterization of the intracellular signaling pathways involved in A2A receptor signaling (for review, see Fuxe et al., 2007), little is known about the effector pathways mediating their inhibition of the indirect pathway.
Endocannabinoid signaling is prominent in the striatum and represents a major downstream target of D2 receptor activation in indirect-pathway MSNs (Giuffrida et al., 1999; Kreitzer and Malenka, 2007; Shen et al., 2008b), raising the possibility that it could be influenced by A2A receptors (Rossi et al., 2009). Two major endocannabinoids have been identified thus far: anandamide [N-arachidonoylethanolamine (AEA)] and 2-arachidonoylglycerol (2-AG). These endocannabinoids act as retrograde messengers at synapses, where they are released from postsynaptic dendrites and bind to presynaptic CB1 receptors to depress neurotransmitter release (Chevaleyre et al., 2006; Kano et al., 2009). In the striatum, endocannabinoids underlie both short-term and long-term depression (LTD) of excitatory synapses onto indirect-pathway MSNs (Gerdeman et al., 2002; Narushima et al., 2006a). In this study, we tested the hypothesis that A2A antagonists stimulate motor activity by enhancing endocannabinoid-mediated LTD at striatal glutamatergic indirect-pathway afferents.
Materials and Methods
Open-field behavior.
Spontaneous locomotor activity was measured in an automated Flex-Field/Open Field Photobeam Activity System (San Diego Instruments). Male wild-type C57BL/6 mice (Charles River) and CB1−/− mice on the C57BL/6 background (Marsicano et al., 2002), aged 7–11 weeks, were used for behavioral testing. Mice were acclimated to the testing room for at least 30 min. Each mouse was injected with 5 μl/g of either N-(piperidin-1- yl)-5-(4-iodophonyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) (5 mg/kg, i.p.) or its vehicle, a solution of 50% polyethylene glycol (PEG) and 50% saline (0.9% NaCl), immediately before being placed in the center of the test chamber. After a 15 min habituation period, baseline locomotor activity was monitored for 15 min. Then, mice were injected with 5 μl/g 2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine (SCH442416) (3 mg/kg, i.p.; also in 50% PEG vehicle) and monitored for another 30 min. Beam breaks were recorded and binned in 3 min intervals. The test chamber was cleaned with 70% ethanol between testing of each mouse. Mice were excluded from analysis if their average number of beam breaks per 3 min period during baseline was <5 or >150 (to eliminate unusually hypoactive or hyperactive mice), or if the SD of baseline values was >80 (to eliminate mice that exhibited highly variable activity). Statistical significance versus baseline within each group was evaluated by a paired t test. Statistical significance between groups was evaluated by one-way ANOVA with Tukey's honestly significant difference (HSD) test.
Chemical ionization/gas chromatography/mass spectrometry.
Mice (C57BL/6; aged 4–5 weeks) were injected intraperitoneally with 5 μl/g of either SCH442416 (3 mg/kg) or its vehicle (50% PEG). Five to 7 min after injection, mice were killed, and their right and left striatum, as well as their right and left cortex were dissected within 1 min. Each tissue sample (four per mouse) was immediately placed in a 1.5 ml tube, frozen in liquid nitrogen, and stored at −80°C. To reliably quantify the amount of AEA and 2-AG in tissue samples by chemical ionization/gas chromatography/mass spectrometry, three samples of striatum were combined according to each treatment, and their total mass was determined. Because of their larger mass, individual cortical samples were analyzed. All samples were placed in 10 ml of CHCl3 and homogenized for 1 min at 10,000 rpm using a PRO 200 homogenizer (Pro Scientific). The following deuterated standards were added to each homogenate: 150 pmol of d5-2-AG and 50 pmol of [3H]AEA (Cayman Chemical). Lipids were then extracted, purified, and derivatized as described by Muccioli and Stella (2008). Three microliters of each sample (corresponding to 4–8.5 mg tissue/injection) were then injected by a CP-8400 autosampler into a Varian CP3800 Gas Chromatogram. The temperature elution protocol, chemical ionization parameters, and isotope dilution quantification were as described by Muccioli and Stella (2008). The total ion currents were recorded for each sample and the individual endocannabinoids were identified by their diagnostic peaks (2-AG, 433 m/z; AEA, 330 + 420 + 493 m/z) using MSDATA Review (Varian). Statistical significance was evaluated using a two-tailed unpaired t test.
Electrophysiology.
Coronal brain slices (300 μm) were prepared from Drd2-GFP heterozygotic BAC transgenic mice on the C57BL/6 background (postnatal days 21–35). Slices were superfused with an external solution containing the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4-H2O, and 12.5 glucose, bubbled with 95% O2/5% CO2. Slices were allowed to recover for at least 1 h before recording. Whole-cell voltage-clamp recordings were obtained from visually identified green fluorescent protein (GFP)-positive or GFP-negative MSNs in dorsolateral striatum at a temperature of 30–32°C, with picrotoxin (50 μm) present to suppress GABAA-mediated currents. Resistance of the patch pipettes was 2.5–4 MΩ when filled with intracellular solution containing the following (in mm): 120 CsMeSO3, 15 CsCl, 8 NaCl, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, 10 TEA (tetraethylammonium), 5 QX-314 (lidocaine N-ethyl bromide), adjusted to pH 7.3 with CsOH. MSNs were held at −70 mV, and excitatory synaptic currents were evoked by intrastriatal microstimulation with a saline-filled glass pipette placed 50–100 μm dorsolateral of the recorded neuron. Test pulses, which consisted of two stimuli 50 ms apart, were given every 20 s. To evoke LTD, MSNs were stimulated at 20 or 100 Hz for 1 s, paired with postsynaptic depolarization to −10 mV. Tetrahydrolipstatin (THL) was purchased from Sigma-Aldrich, and all other drugs were from Tocris Bioscience. THL was dissolved in DMSO at 10 mm, and used at 10 μm, yielding 0.1% DMSO, a concentration that does not affect striatal LTD (Gerdeman et al., 2002). All data acquisition and analysis were performed online with custom Igor Pro software. Statistical significance was evaluated using a two-tailed unpaired t test.
Results
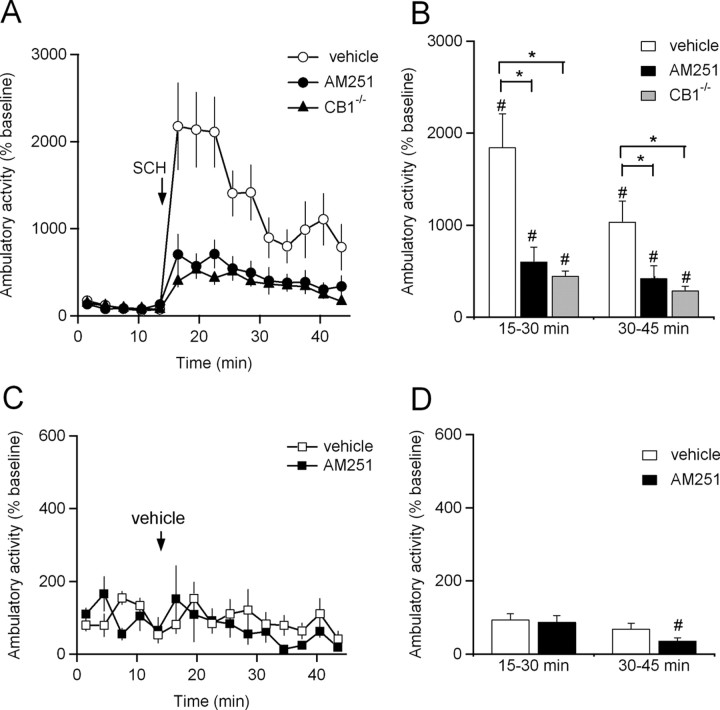
To determine whether psychomotor stimulation by A2A antagonists requires endocannabinoid signaling, the selective A2A antagonist SCH442416 (3 mg/kg, i.p.) was administered to three groups of mice: strain-matched wild-type controls, mice pretreated with the CB1 receptor antagonist AM251 (5 mg/kg, i.p.), and mice lacking CB1 receptors (CB1−/− mice) (Marsicano et al., 2002). A2A antagonist treatment significantly increased ambulatory activity in wild-type mice (Fig. 1A,B, Table 1). Pretreatment of wild-type mice with AM251 (5 mg/kg, i.p.) had little effect on baseline ambulatory activity (105 ± 14% of wild-type baseline; p > 0.05; n = 14) but significantly attenuated the effects of SCH442416 (Fig. 1A,B, Table 1), indicating that functional CB1 receptors are required for mice to fully increase their ambulatory activity in response to an A2A antagonist. Similar results were found when we tested the effects of SCH442416 on CB1−/− mice. CB1−/− mice exhibited slightly higher baseline ambulatory activity compared with strain-matched controls of similar age (161 ± 12% of wild-type baseline; p < 0.05; n = 14). However, similar to AM251-pretreated mice, the effects of SCH442416 treatment on ambulatory activity were greatly attenuated in CB1−/− mice compared with wild-type vehicle-pretreated controls (Fig. 1A,B, Table 1). SCH442416 also induced a small but significant increase in fine movements (Fig. 1C,D, Table 1). However, the increase in fine movements was not altered in AM251-treated mice or CB1−/− mice (Fig. 1C,D, Table 1), indicating that this feature of psychomotor activation by SCH442416 does not require endocannabinoid signaling. To verify that the increases in both ambulatory activity and fine movements that we observed were caused by SCH442416 and not by the injection procedure itself, we injected vehicle instead of SCH442416 into a subset of mice. Injection of vehicle had no effects on either type of locomotor activity (Table 1).
Figure 1.
Stimulation of ambulatory activity by adenosine A2A antagonist treatment is attenuated by loss of cannabinoid CB1 receptor function. A, The A2A receptor antagonist SCH442416 (3 mg/kg, i.p.) was injected into mice pretreated with vehicle (n = 13) or the CB1 receptor antagonist AM251 (n = 14) and into mice lacking CB1 receptors (n = 14). Ambulatory activity is plotted. B, Summary of ambulatory activity at 15–30 and 30–45 min. C, Vehicle was injected into mice pretreated with vehicle (n = 6) or the CB1 receptor antagonist AM251 (n = 6). Ambulatory activity is plotted. D, Summary of ambulatory activity at 15–30 and 30–45 min. In A and C, movements are binned in 3 min intervals. Pretreatment injections were given 15 min before time 0. Data are normalized to baseline activity during the first 15 min of the experiment. *p < 0.05 by one-way ANOVA with Tukey's HSD. #p < 0.05 by two-tailed paired t test. Data are mean ± SEM.
Table 1.
Psychomotor activation in mice treated with the A2A antagonist SCH442416
| Genotype | Pretreatment | Treatment | n | Ambulatory activity |
Fine movements |
||
|---|---|---|---|---|---|---|---|
| % baseline at 15–30 min | % baseline at 30–45 min | % baseline at 15–30 min | % baseline at 30–45 min | ||||
| WT | Vehicle | SCH442416 | 13 | 1849 ± 363* | 1038 ± 226* | 160 ± 14* | 136 ± 13 |
| WT | AM251 | SCH442416 | 14 | 604 ± 158*# | 421 ± 136*# | 183 ± 24* | 172 ± 25 |
| CB1−/− | Vehicle | SCH442416 | 14 | 450 ± 52*# | 290 ± 45*# | 157 ± 17* | 118 ± 14 |
| WT | Vehicle | Vehicle | 6 | 94 ± 16# | 69 ± 16# | 96 ± 2 | 105 ± 4 |
| WT | AM251 | Vehicle | 6 | 88 ± 18# | 41 ± 18*# | 83 ± 15 | 84 ± 16 |
Data are mean ± SEM.
*p < 0.05 versus baseline.
#p < 0.05 versus WT vehicle-pretreated SCH442416 mice.
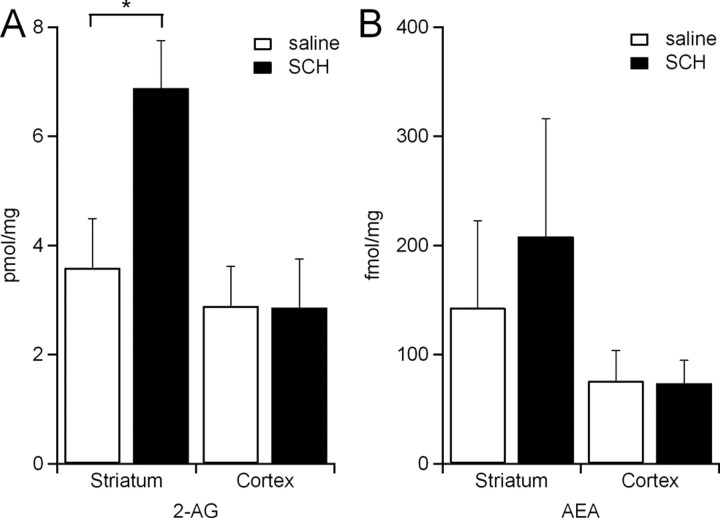
Our behavioral data indicate that the psychomotor effects of A2A receptor antagonists are mediated at least in part by activation of CB1 receptors. To test whether A2A antagonists alter endocannabinoid levels in the striatum or cortex, we measured the amount of 2-AG and AEA in striatal and cortical tissue samples 5–7 min after injection of SCH442416 (3 mg/kg, i.p.) or vehicle solution. Both endocannabinoids were detectable in striatal and cortical samples from mice injected with vehicle (Fig. 2A,B). In mice injected with SCH442416, 2-AG was increased in striatum, but not cortex (Fig. 2A). No significant differences in AEA levels were observed in striatal or cortical samples from mice injected with SCH442416 versus vehicle (Fig. 2B). Together, these results shown that A2A antagonists specifically increase the amount of striatal 2-AG.
Figure 2.
Adenosine A2A receptor blockade increases striatal 2-AG concentration. A, 2-AG concentration in the striatum and cortex in mice injected with SCH442416 (3 mg/kg, i.p.) (6.9 ± 0.9 pmol/mg in striatum, n = 6 mice; 2.8 ± 0.9 pmol/mg in cortex, n = 6 mice) and in saline-injected controls (3.6 ± 0.9 pmol/mg in striatum, n = 6 mice; 2.9 ± 0.7 pmol/mg in cortex, n = 6 mice). B, AEA concentration in the striatum and cortex in mice injected with SCH442416 (208.1 ± 108 fmol/mg in striatum, n = 6 mice; 73.7 ± 21 fmol/mg in cortex, n = 3 mice) and in saline-injected controls (143.2 ± 80 fmol/mg in striatum, n = 6 mice; 76.1 ± 28 fmol/mg in cortex, n = 3 mice). *p < 0.05 by two-tailed unpaired t test. Data are mean ± SEM.
Within the striatum, A2A receptors are highly enriched at excitatory synapses onto indirect-pathway MSNs (Rosin et al., 2003), and decreasing striatal indirect pathway function increases ambulatory activity (Durieux et al., 2009). Because the effects of SCH442416 depend on CB1 receptor activation, we tested whether it induced the release of endocannabinoids from indirect-pathway MSNs. However, application of SCH442416 (1 μm) did not alter baseline excitatory synaptic responses in indirect-pathway MSNs (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). We next tested whether SCH442416 could potentiate endocannabinoid-mediated LTD in indirect-pathway MSNs. First, we elicited LTD using high-frequency stimulation (100 Hz), paired with postsynaptic depolarization. Although this protocol elicited robust LTD, as previously reported (Gerdeman et al., 2002; Kreitzer and Malenka, 2007), the magnitude of LTD was not potentiated by SCH442416 (1 μm) (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). However, a moderate-frequency (20 Hz) stimulation protocol that elicited a small amount of LTD in control conditions gave rise to robust LTD in the presence of SCH442416 (1 μm) (88 ± 7% of baseline at 30–40 min in control conditions; 61 ± 8% of baseline at 30–40 min in SCH442416; p < 0.05) (Fig. 3A). Furthermore, in the presence of SCH442416, this form of LTD was blocked (102 ± 9% of baseline at 30–40 min) (Fig. 3B) by THL (10 μm), an inhibitor of the 2-AG synthetic enzyme diacylglycerol lipase. This enhancement of 2-AG release was pathway specific, because when we delivered 20 Hz stimulation paired with postsynaptic depolarization to direct-pathway MSNs in SCH442416, no enhancement of LTD was observed (86 ± 6% of baseline at 30–40 min in control conditions; 97 ± 10% of baseline at 30–40 min in SCH442416; p > 0.05) (Fig. 3C). Therefore, SCH442416 selectively enhances 2-AG release and LTD induction in indirect-pathway MSNs.
Figure 3.
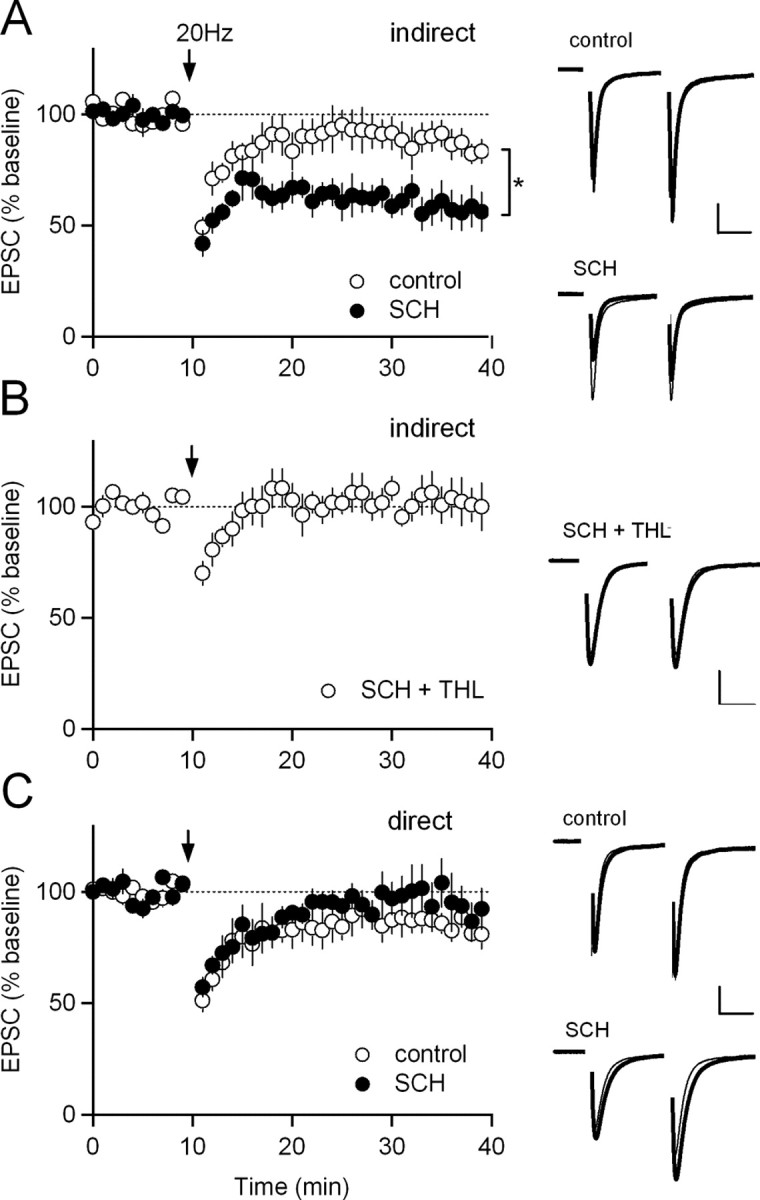
Adenosine A2A receptor blockade increases 2-AG-mediated synaptic depression in the indirect pathway. A, Left, 20 Hz stimulation of afferents to indirect-pathway MSNs in control solution (n = 6) and in 1 μm SCH442416 (SCH) (n = 4). In this and subsequent panels, normalized EPSC amplitudes are plotted over time. The arrow indicates the time of 20 Hz stimulation, which was paired with postsynaptic depolarization to −10 mV. Right, Normalized traces from representative experiments included in A. In all example traces, the thicker gray trace is the average EPSC before 20 Hz stimulation, and the thinner black trace is the average EPSC during the last 10 min of the experiment. Calibration: 100 pA, 20 ms. B, Left, Twenty hertz stimulation of afferents to indirect-pathway MSNs in 1 μm SCH442416 and 10 μm THL (n = 6). Right, Normalized traces from representative experiments included in B. C, Left, Twenty hertz stimulation of afferents to direct-pathway MSNs in control solution (n = 6) and in 1 μm SCH442416 (n = 6). Right, Normalized traces from representative experiments included in C. *p < 0.05 by two-tailed unpaired t test. Data are mean ± SEM.
Discussion
In this study, we identify a molecular mechanism underlying psychomotor activation by A2A antagonists. Specifically, we show that A2A antagonists increase striatal 2-AG and potentiate 2-AG-mediated LTD of excitatory afferents on indirect-pathway MSNs. Furthermore, blocking CB1 receptor function in vivo greatly attenuates the psychomotor stimulating effects of A2A antagonists. Our data are consistent with a model in which endocannabinoid-mediated inhibition of the indirect pathway increases movement.
These findings provide insight into a molecular mechanism for psychomotor stimulation by A2A antagonists. However, A2A receptor antagonists are certain to exhibit complex effects across numerous brain regions. In addition to the striatum, there are other potential sites of interaction between A2A receptors and endocannabinoid signaling, including the cortex and the globus pallidus. However, we do not observe any change in cortical endocannabinoid levels after A2A antagonist treatment, and in the globus pallidus, A2A transcript is not observed postsynaptically (Rosin et al., 2003), where endocannabinoids are produced. Although presynaptic interactions between A2A and CB1 receptors are possible in the globus pallidus, CB1 receptor-mediated inhibition of IPSCs is reportedly mediated by suppression of calcium influx (Engler et al., 2006), whereas A2A receptor-mediated enhancement of IPSCs is independent of calcium (Shindou et al., 2002), suggesting that these pathways act independently of each other. Furthermore, although CB1 receptors were required for the bulk of psychomotor activation by SCH442416, some psychomotor stimulation still occurred when CB1 receptor signaling was blocked (Fig. 1A,B). This remaining stimulation was likely attributable to parallel signaling pathways initiated by A2A receptor inhibition, such as decreased release of GABA in the globus pallidus (Shindou et al., 2003), which would act synergistically with striatal 2-AG-mediated inhibition to reduce the efficacy of the indirect pathway.
We also revealed two interesting features of striatal LTD by using a moderate-frequency (20 Hz) induction protocol. First, we observed a small amount of LTD in both direct- and indirect-pathway MSNs (10–15%), consistent with the idea that endocannabinoids can be produced in both types of MSN under some experimental conditions (Narushima et al., 2006b; Shen et al., 2008b). Second, we found that indirect-pathway LTD in SCH442416 is mediated by 2-AG, whereas LTD elicited by 100 Hz stimulation is reportedly mediated by anandamide (Ade and Lovinger, 2007). Consistent with that study, we also found that striatal LTD elicited by 100 Hz stimulation is not blocked by THL (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material). This suggests that the identity of the endocannabinoid that mediates striatal LTD can vary, depending on the experimental conditions.
A link between A2A antagonists and endocannabinoids has implications for the use of A2A antagonists to treat PD. In mouse models of PD, dopamine depletion causes a loss of endocannabinoid-dependent LTD at excitatory synapses onto indirect-pathway MSNs (Kreitzer and Malenka, 2007; Shen et al., 2008b). Our findings raise the possibility that A2A antagonists can help counter the effects of dopamine depletion by increasing endocannabinoid signaling at these synapses. Although A2A antagonists are already being investigated as PD therapeutics, our findings suggest that the efficacy of these drugs may be increased by developing compounds for human consumption that inhibit endocannabinoid degradation.
Footnotes
This work was supported by National Institutes of Health Grant R01 NS064984, the Pew Biomedical Scholars Program, the W. M. Keck Foundation, and the Wayne and Gladys Valley Foundation. We thank Beat Lutz for kindly providing CB1−/− mice; Nino Devidze, Iris Lo, Tracy Hamto, Lex Kravitz, and Philip Parker for their assistance with the behavioral experiments; and Sergi Ferré for helpful discussion.
References
- Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31:397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d'Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Engler B, Freiman I, Urbanski M, Szabo B. Effects of exogenous and endogenous cannabinoids on GABAergic neurotransmission between the caudate-putamen and the globus pallidus in the mouse. J Pharmacol Exp Ther. 2006;316:608–617. doi: 10.1124/jpet.105.092718. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Genedani S, Agnati L. Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson's disease. Mov Disord. 2007;22:1990–2017. doi: 10.1002/mds.21440. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hauber W, Münkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Nagel J, Sauer R, Müller CE. Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport. 1998;9:1803–1806. doi: 10.1097/00001756-199806010-00024. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson's disease. Parkinsonism Relat Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD. Interaction of dopamine and adenosine receptor function in behavior: studies with dopamine-deficient mice. Front Biosci. 2008;13:2311–2318. doi: 10.2741/2845. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG, Stella N. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology. 2008;54:16–22. doi: 10.1016/j.neuropharm.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima M, Hashimoto K, Kano M. Endocannabinoid-mediated short-term suppression of excitatory synaptic transmission to medium spiny neurons in the striatum. Neurosci Res. 2006a;54:159–164. doi: 10.1016/j.neures.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006b;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Siracusano A, Bernardi G, Usiello A, Centonze D. Caffeine drinking potentiates cannabinoid transmission in the striatum: interaction with stress effects. Neuropharmacology. 2009;56:590–597. doi: 10.1016/j.neuropharm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol. 1996;48:167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008a;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008b;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus. Br J Pharmacol. 2002;136:296–302. doi: 10.1038/sj.bjp.0704702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou T, Richardson PJ, Mori A, Kase H, Ichimura M. Adenosine modulates the striatal GABAergic inputs to the globus pallidus via adenosine A2A receptors in rats. Neurosci Lett. 2003;352:167–170. doi: 10.1016/j.neulet.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Shindou T, Arbuthnott GW, Wickens JR. Actions of adenosine A2A receptors on synaptic connections of spiny projection neurons in the neostriatal inhibitory network. J Neurophysiol. 2008;99:1884–1889. doi: 10.1152/jn.01259.2007. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M, Pinna A. Adenosine A2A receptor antagonists and Parkinson's disease: state of the art and future directions. Curr Pharm Des. 2008;14:1475–1489. doi: 10.2174/138161208784480072. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Strömberg I, Popoli P, Müller CE, Ferré S, Fuxe K. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur J Neurosci. 2000;12:4033–4037. doi: 10.1046/j.1460-9568.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience. 1997;79:753–764. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, Centonze D, Calabresi P, Borsini F. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–789. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]