Abstract
While the number of genetic tests continues to grow, publicly accessible information about the analytic and clinical validity of such tests is lagging. Information gaps impede informed decision making by health care providers and patients. Enhancing the transparency of information about what tests are being offered, for which indications tests are being offered, and the analytic and clinical validity of tests is a key prerequisite to ensuring test quality. A recent government recommendation for a mandatory genetic test registry has received wide stakeholder support but leaves many practical questions unanswered. We propose a ‘blueprint’ for the creation of a genetic test registry in order to expedite its implementation. We describe the goals of a registry, propose criteria for the inclusion of registrants and tests in the registry, and define the categories of information that should be included for such tests. We discuss the sources of legal authority that empower the government to mandate that a registry be established and identify the federal agencies with the relevant expertise and resources to do so. We conclude that establishing a registry is a critical first step in the development of a more transparent, quality-centered system of oversight that will better inform and protect the public.
Keywords: Clinical genetics, Genetic testing, Policy, Registry
As genetic testing becomes an increasingly important part of clinical practice, it is imperative that health care providers, payers, and patients in both the United States and other countries have access to high-quality tests and to information about how to order and interpret them appropriately. However, government oversight does not ensure adequately that tests can provide accurate and meaningful health information to doctors and patients, nor does it require those offering tests to disclose the evidentiary basis for the claims they make about their tests [1–4].
The recent entry into the marketplace of genetic tests sold directly to consumers (DTC) without external scrutiny brings added immediacy to these concerns and has renewed governmental attention to the broader question of genetic testing oversight [3, 5, 6]. The consequences of the current fragmented and anemic oversight system for providers and patients potentially are grave. On the one hand, the premature use of tests that lack adequate validation or proper interpretation may lead to ill-informed treatment decisions and may undermine confidence in personalized medicine [7, 8]. On the other hand, access to new, health-improving tests may be undermined by both lack of market incentives and unduly burdensome regulation [9].
Enhancing the transparency of information about genetic tests is a key prerequisite to improving oversight. Specifically, more publicly accessible information is needed about what tests are being offered, for what indications tests are being offered, and the analytic and clinical validity of tests. Calls for enhancing transparency have come from Congress as well as from advocacy groups and academic institutions [10–13]. In April 2008 the Secretary’s Advisory Committee on Genetics, Health, and Society (SACGHS), which advises the Secretary of the U.S. Department of Health and Human Services (HHS), became the latest body to conclude that there are ‘significant gaps’ that could harm public health in the U.S. system for genetic testing oversight [3]. The Committee recommended that clinical laboratories and others offering testing services be required to submit information about a test’s analytic and clinical validity to a publicly accessible laboratory test registry. Such a registry would address ‘information gaps in the availability of tests and their analytic and clinical validity’ [3] and ‘empower both consumers and providers by arming them with reliable information about what is known and not known about the quality and validity of tests’ [14].
In the United States, many stakeholders, including test manufacturers, laboratories, patient advocacy groups, and associations of health care providers [11, 12, 15–19], support the development of a genetic test registry, but there has been little practical discussion of how such a registry would be implemented, what data should be included, which agency within the federal government has the legal authority and institutional capacity to oversee it, or how compliance would be ensured. Nor has HHS yet acted on SACGHS’s recommendation to appoint and fund a lead agency to develop and maintain the mandatory registry for laboratory tests.
The lack of transparency has implications for both the United States and other countries. The Internet has vastly expanded the ability of doctors and patients to learn about new tests regardless of where the laboratory performing them is located, and the rise of DTC testing has allowed companies offering tests to target consumers directly in the United States and abroad. Thus, a U.S.-based registry would be of great benefit to doctors and patients in other countries as well and would enhance transparency internationally.
This paper offers a concrete ‘blueprint’ for a genetic test registry in order to facilitate its implementation. To that end, this paper describes the need for and benefit of establishing a registry, proposes criteria for the inclusion of registrants and tests in the registry, defines the categories of information that should be included for tests, and recommends the federal agencies that should be tasked with development, implementation, and enforcement of the registry, noting the sources of their legal authority to do so.
Background
The principal goal of a genetic test registry is to provide doctors and patients with information about the analytic and clinical validity of genetic tests so that they can make informed health care decisions. Because most such tests are not subject to review by any government regulatory body, there is no requirement for laboratories or other entities that distribute genetic tests to disclose data supporting the validity of tests they offer. Additionally, there exist few independent information resources to assist providers and patients in evaluating the merits of a test, and few professional guidelines have been developed to guide provider decision-making [20, 21]. In the United States, the University of Washington-based GeneTests website makes available the names of clinical and research laboratories conducting genetic tests as well as the disorders for which they test, based on information voluntarily submitted by these entities [22, 23]. The website also maintains GeneReviews, which contains expert-authored, peer-reviewed descriptions of some of the disorders for which testing is available along with information on diagnosis, management, and counseling of patients and families with these inherited conditions [22, 23]. The laboratories listed in GeneTests primarily offer testing for rare diseases, and GeneTests does not list many of the genetic tests available DTC, pharmacogenetic tests, or genome-scanning tests. Thus, while GeneTests/GeneReviews is a valuable resource to healthcare providers ordering traditional genetic tests, it does not provide sufficient information on who is offering tests or about any quality measures, such as analytic and clinical validity, of tests being offered. Additionally, it collects information only on a voluntary basis.
In Europe, the European Directory of DNA Diagnostic Laboratories (EDDNAL) plays a similar role to Gene-Tests. EDDNAL is a non-profit directory supported mainly by funding from the European Commission [24]. EDDNAL provides and disseminates information concerning the availability of DNA-based diagnostic services for rare genetic conditions in 17 European countries. Another resource in Europe is GENDIA (for Genetic Diagnostics), an international network of laboratories supported by a private diagnostic laboratory in Belgium [25]. EuroGentest has expanded upon GENDIA and EDDNAL to encompass 5 self-identified aspects of genetic testing: quality management, information databases, public health, new technologies, and education [26]. This organization includes genetic testing databases, publications, and professional guidelines and provides workshops as well as fellowship and research funding. However, like in GeneTests, participation in EuroGentest is voluntary, and it does not, at this time, contain data to evaluate the quality of genetic tests beyond laboratory certification.
While these U.S. and European voluntary repositories provide limited information for a subset of genetic tests, none provide data to support analytic and clinical validity claims. In order to document adequately the characteristics and quality of genetic tests and the laboratories providing them, a mandatory, and more expansive, registry is required.
Key Features of a Genetic Test Registry
Mandatory Registration
The overwhelming majority of stakeholders who submitted comments to SACGHS favored a mandatory registry to ensure comprehensive participation [15]. We also support the creation of a mandatory registry, with penalties for noncompliance. As the Food and Drug Administration (FDA) reported regarding the implementation of a database for clinical trials required by the 1997 Food and Drug Administration Modernization Act (FDAMA), in the absence of strong enforcement provisions, compliance with the registry was low [27, 28]. To address this lack of compliance, Congress added penalties for failure to register [29]. Similarly, adequate compliance with a test registry will require both that it is mandatory and that penalties are imposed for noncompliance.
Who Should Be Required to Register
We anticipate that the majority of those required to register will be clinical laboratories. However, it is important to note that while both the laboratory analysis and the reporting and interpretation of test results often are conducted by a single clinical laboratory, these services sometimes are bifurcated. In some cases businesses other than laboratories act as test distributors, providing information about tests and delivering test interpretation but outsourcing the laboratory testing services to a clinical laboratory. To the extent that these test distributors either advertise testing services beyond the laboratory’s stated indications or provide interpretation that is different from or additional to that which is provided by the laboratory, we believe these businesses should be required to register and list their tests, and to submit evidence supporting the clinical validity of the indications for which they are offering testing. Finally, businesses located outside the United States should be required to register if they test specimens from individuals in the United States or distribute testing services to individuals within the U.S. As many DTC companies market their services in the United States even if located ‘off-shore’, requiring them to register regardless of their location would increase the amount of information available to doctors, payers, and patients in both the United States and other countries.
Criteria for Inclusion
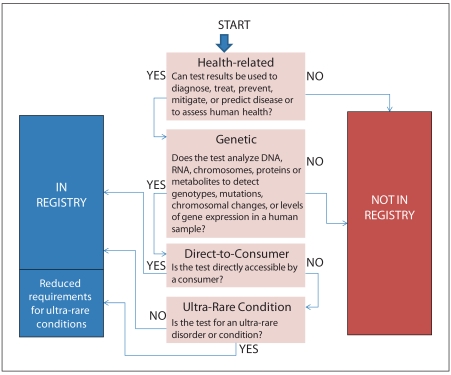
While some have advocated for a comprehensive laboratory test registry, we believe it is more practical, at least initially, to limit the scope of the registry to genetic tests [3]. Genetic tests are among the newest and fastest growing segments of laboratory testing, but as described above, they are subject to inadequate oversight and disclosure requirements. Additionally, in some cases results from these tests are used in the absence of other clinical information in making life-altering decisions. We believe that the potential public health impact of these tests is high and that physicians and patients need access to information about a test’s analytic and clinical validity and clinical utility (see fig. 1). At the same time, we recognize that not all genetic tests have the same potential for public health impact and that the benefit of registration and listing must be balanced against the burden it poses to the laboratory. For this reason, we recommend that providers of genetic tests for ultra-rare disorders be exempt from some information disclosure requirements that could be unduly burdensome and deter them from providing these important services.
Fig. 1.
Decision tree for inclusion of tests in initial registry.
Health-Related
First, we propose that the registry be limited to health-related tests, i.e., tests that are used in diagnosis, treatment, prediction, or prevention of diseases or conditions, or to assess human health. This definition is consistent with the Clinical Laboratory Improvement Act (CLIA) definition of a clinical laboratory and the FDA definition of a medical device [30, 31]. In some instances, it may be difficult to determine whether a test is health-related. For example, variants associated with the ability to perceive bitter taste currently are not known to correlate with health, but such associations may be established in the future. As another example, prenatal fetal gender testing does not typically yield health-related information but could be used to predict whether a male child will be at increased risk of carrying an X-linked disorder. Thus, as discussed below, in some cases it may be necessary to review the claims made by the laboratory or test distributor to determine whether the tests offered are health-related. Consistent with the way the intended use is evaluated for other medical devices, the intended use of a genetic test should be ascertained based on the claims made about the test in the test report or in marketing materials [32].
Genetic Tests
Defining the term genetic test has been a perennial challenge for both scientists and policymakers. For the purpose of this registry, we define a genetic test as the analysis of DNA, RNA, chromosomes, proteins, or metabolites to detect genotypes, mutations, chromosomal changes, or levels of gene expression in a human sample. This definition would include testing for both inherited and acquired mutations (e.g., testing for somatic mutations in tumor cells) as well as tests to detect non-human (e.g., viral or bacterial) DNA in a human specimen for health-related purposes. We propose this definition in an attempt to balance the desire to include genetic tests that have potential for significant public health impact with the need for clear boundaries to prevent the wholesale inclusion of all laboratory tests. However, we recognize that this definition may need further refinement during the implementation phase to expand or refine the scope of the registry.
Offered Direct-to-Consumer
All health-related genetic tests that are available DTC should be included in the registry. As SACGHS noted, these tests ‘have the potential for adverse patient outcomes, social stigmatization, privacy concerns, and cost implications for the health care system’ [3]. Moreover, they can be offered without the involvement of a health care provider or genetic counselor and may be accessed by large numbers of consumers. Thus, inclusion of all health-related DTC tests in a registry is warranted in the interest of promoting transparency and protecting public health.
Reduced Listing Requirements for Laboratories Performing Ultra-Rare Testing
Many genetic tests are for rare disorders with low overall public health impact. Most such tests are offered by only one or a few genetic testing facilities, often located in academic centers. In setting a threshold for rare disorders, we rely on guidance from the Ultra-Rare Diseases Working Group of the American College of Medical Genetics, which defines ultra-rare genetic diseases as those that affect fewer than 2,000 individuals in the United States [33]. We recognize that availability of such tests is important to families whose members suffer from ultra-rare diseases and want to avoid burdening laboratories that offer such tests now. Therefore, we recommend reduced submission requirements for those offering genetic tests for ultra-rare disorders.
Informational Requirements for Included Tests
Since the primary purpose of our proposal is to assist physicians and patients to make informed health care decisions, we believe that the registry must contain information adequate to assess how reliable a test is (analytic validity), how the results relate to current and future disease risk or health status (clinical validity), and how useful the results are in informing patient diagnosis or treatment or in disease prediction, management, or prevention (clinical utility). Table 1 outlines the fields that a registry should use to characterize tests and to allow providers to differentiate tests based on quality measures used by CLIA and New York State’s Clinical Laboratory Evaluation Program (NYS-CLEP) [34]. Given that most laboratories required to submit to the registry will have CLIA certification, the data, for the most part, will be readily available. The sources of data for the requested fields may be developed by the laboratory itself or from published or unpublished sources. As is common in medicine, a test often is available to patients in advance of data on clinical utility, so a laboratory initially may need to fill out the utility field as ‘data not available’. The core aim of the registry is to promote transparency, which includes disclosure of both what is known and what is not known.
Table 1.
Proposed content for a registry
| Issue | |
|---|---|
| Test distributor/laboratory | Name, location, contacts, certification, types of services available (e.g., telephone counseling, DTC). |
| Test description | Test name as per marketing materials and projected turn-around time. Whether testing is performed in-house or outsourced, and where outsourced. Type of sample accepted (e.g., blood, saliva, tumor tissue). Analyte(s) to be detected (e.g., gene name(s), variants, mutations, chromosome(s)) as well as a brief description of the test platform (e.g., full gene sequence, microarray SNP detection). Instrument(s) used in test, and whether the test, test kit, instrument, or critical reagents have been reviewed by FDA. Tests that are FDA-reviewed may have links to the reviewed documents in lieu of some of the content requirements. If the test is not reviewed by FDA, then the test distributor or laboratory should note the status of the test as a laboratory developed test (LDT). |
| Intended use | Purpose (e.g., diagnostic, predictive, prenatal, screening) and for what condition/disorder the test is intended (e.g., ATM gene sequencing for diagnosing ataxia telangiectasia vs. ATM testing to detect mutations associated with breast cancer). |
| Performance evaluation | Description of validation method and reportable range (e.g., variants tested or specific regions of a gene). |
| Analytic validity | A numeric value for analytic sensitivity and specificity; how many samples were used in calculating the analytic sensitivity (n), and references to support analytic validity. |
| Clinical validity | A numeric value for clinical sensitivity and specificity, how many samples were used in calculating the clinical sensitivity (n), what population was used to develop the clinical sensitivity, and references to support clinical validity. |
| Clinical utility | Available data supporting clinical utility or a description of how the test is useful for the public (i.e., whether for information purposes or for decision making). |
| Proficiency testing (PT) | Method of PT (external, internal, or inter-laboratory exchange), PT provider, interval (i.e., how many times per year PT is conducted), and number of samples run for each round of PT. |
| Reporting | Laboratories should upload sample reports. |
In addition to transparency, the registry should facilitate health care decision-making based on timely scientific information. Registrants therefore should be required to update their submitted information promptly in response to changed practices, such as when new tests are added to the test menu, when tests are offered for new indications, or when new information regarding analytic or clinical validity or clinical utility become available.
Legal Basis for Registry
The Federal Government Can Mandate Registries to Protect Public Health
The federal government often has established registries of the kind we propose to achieve a wide variety of public health goals [35], such as evaluating the performance of health care providers, rapidly identifying health threats such as infectious disease or contaminated food, and tracking the performance of high-risk medical products to identify and avoid adverse events at an early stage. For example, in 1992 Congress mandated that clinics providing assisted reproductive technology services report their pregnancy success rates to HHS and that such information be made available to the public [36, 37]. Similarly, in 1997 Congress mandated creation of a public registry of clinical trials of experimental treatments for serious or life-threatening diseases [27]. Congress expanded this registry in 2007 to include information on clinical trial outcomes based on the finding that a ‘uniform, centralized database and registry will help patients, providers, and researchers learn new information and make more informed health care decisions’ [29, 38].
While Congress sometimes mandates registries specifically, in other instances federal agencies have relied on general legislative authority to require the use of registries when necessary to fulfill their broader missions. Thus, FDA can require, and has required, drug and device manufacturers to establish registries as a condition of approval as part of the agency’s broader mandate to ensure that such products are safe and effective [39]. FDA also has employed registries post-market to address newly discovered product hazards [40].
In selecting an agency to implement and mandate submissions to a genetic test registry, 3 essential criteria must be satisfied. First, the agency chosen must have the institutional capacity, meaning the financial and personnel resources, to carry out the task. Second, the agency must have the requisite scientific, technological, and other relevant expertise to establish and maintain the registry. Third, the agency must have the statutory authority to carry out these functions; such authority may be explicit or implicit.
HHS Has Authority to Establish a Genetic Test Registry
In 2 separate but complementary statutes, Congress already has delegated legal authority to the Secretary of HHS sufficient to establish and maintain a registry. The 1st statute, the Federal Food, Drug, and Cosmetics Act (FD&C Act), directs the Secretary to ensure the safety and effectiveness of medical products, including devices used to diagnose, treat, predict, or prevent disease [31]. The 2nd statute, CLIA, directs the Secretary to ensure the accuracy and reliability of laboratory testing [30]. While Congress did not, in either of these statutes, mandate that the Secretary create a test registry, the establishment of a test registry by the Secretary, or by those to whom she has delegated authority, is both authorized and justified by the language and purposes of these pre-existing statutes. Either of these statutes would support the establishment of a registry of the kind we propose.
FD&C Act
The FD&C Act gives the Secretary authority to establish a genetic test registry [31]. The statute directs the Secretary to ensure that drugs and medical devices are safe and effective before they are sold to the public; this authority has been delegated to the FDA Commissioner. FDA defines ‘medical devices’ to include in vitro diagnostic products (IVDs), meaning those used to analyze human specimens to diagnose, treat, predict, or prevent disease [41]. This category includes genetic tests used for these purposes. While FDA historically has limited its oversight to IVDs sold as free-standing ‘kits’ to laboratories by a 3rd-party manufacturer, FDA consistently has asserted that tests developed by laboratories in-house, so-called ‘laboratory developed tests’ (LDTs), are also IVDs subject to the agency’s requirements [42, 43]. FDA’s asserted jurisdiction never has been contradicted by a court, although it has been questioned by some stakeholders [43–47]. While the agency has exercised ‘enforcement discretion’ with respect to most such tests, the agency periodically has sent letters to laboratories providing services using LDTs, informing them that prior FDA review is required in order to offer such tests [2, 42, 43].
The establishment of a registry for genetic tests is fully consistent with the medical device provisions of the FD&C Act. While not all medical devices are regulated in the same way, certain basic requirements apply to all medical device manufacturers. In particular, the FD&C Act requires those involved in the ‘manufacture, preparation, propagation, compounding, or processing’ of medical devices annually to register with FDA and to provide their names and places of business [48]. The registration requirement extends to those who repackage or otherwise change the labeling of a device ‘in furtherance of the distribution of the device from the original place of manufacture to the person who makes final delivery or sale to the ultimate consumer or user’ [48]. In addition, the FD&C Act requires registrants to annually submit a list of the devices they manufacture or distribute. While the registry we propose would include greater detail than currently is required by FDA for other devices, the basic principles of registration and listing of medical devices already are embedded in the FD&C Act. Thus, the Secretary or her delegates have the authority to require these actions from those producing or distributing LDTs.
The FD&C Act also contains specific authorization for establishing a registry for certain types of devices as a condition of marketing. The statute classifies devices according to level of risk, with Class I being lowest risk and Class III being highest. Class III devices always require premarket approval, while Class I never do. Class II devices may or may not require premarket approval depending on FDA’s determination of whether ‘special controls’ can provide adequate assurance of safety and effectiveness. Among the special controls that may be required are post-market surveillance and the establishment of patient registries, as well as ‘other appropriate actions as the Secretary deems necessary to provide’ reasonable assurance of safety and effectiveness. Thus, at least for those producing or distributing LDTs that could be classified as Class II, the establishment of a registry would be an appropriate ‘special control’ under the FD&C Act. For LDTs that could be classified as Class III, the statute permits the establishment of registries as a condition of marketing approval [49]. To date, FDA has classified most genetic test IVDs as Class II [2].
Final support for the establishment of a test registry under the FD&C Act comes from section 701(a) of the statute. This section provides that the ‘authority to promulgate regulations for the efficient enforcement of this Act, except as otherwise provided in this section, is hereby vested in the Secretary’ [50]. This provision has been broadly interpreted by courts to permit FDA to address through regulations situations not specifically contemplated by the statute but clearly necessary to achieve its overarching objectives [51–53]. FDA already has determined it has jurisdiction under the FD&C Act to regulate LDTs, and section 701(a) authorizes the promulgation of regulations necessary to achieve the objectives of the Act with respect to LDTs.
CLIA
CLIA also gives the Secretary authority to establish a genetic test registry. Congress enacted CLIA to ensure the quality of clinical laboratory services after finding that the public was endangered by substandard laboratory performance for certain non-genetic laboratory diagnostic tests [54, 55]. Congress directed the HHS Secretary to take specific steps to strengthen oversight, and the Secretary has delegated implementation and enforcement of CLIA to different agencies at different periods in history. The statute currently is enforced primarily by the Centers for Medicare and Medicaid Services (CMS), although both FDA and the Centers for Disease Control and Prevention (CDC) play important roles as well [56, 57].
Under CLIA, Congress directed the HHS Secretary to certify clinical laboratories before they could test human specimens. Further, Congress required the Secretary to ‘issue standards to assure consistent performance by laboratories issued a certificate’ [30]. Such standards must include requirements for laboratories to engage in quality assurance and quality control, adequate maintenance of records, equipment, and facilities, employment of qualified personnel, performance of proficiency testing, and ‘to meet such other requirements as the Secretary determines necessary to assure consistent performance by such laboratories of accurate and reliable laboratory examinations and procedures’ [30].
Regulations implementing CLIA went into effect in 1992 [58]. These regulations apply different requirements to laboratories based on the complexity of the tests they perform, with laboratories performing high-complexity tests subject to more stringent quality requirements and required to meet specified personnel qualifications [59, 60]. As a condition of certification, all laboratories must submit to CMS information on the name and number of tests performed by the laboratory annually, the methodologies used for each test, and the qualifications of laboratory personnel [61]. Laboratories must notify CMS within 6 months of changes regarding the type of tests performed or the test methodologies used [62]. However, in practice CMS does not possess up-to-date information regarding the number or types of tests performed by laboratories or the methodologies used to perform such tests, nor is the information that CMS does collect accessible to the public (personal communication with Judy Yost, November 18, 2008). The absence of such information leaves both regulators and the public in the dark about test availability and quality.
Although CMS has interpreted its authority under CLIA very narrowly, the statute provides unusually broad authority to the Secretary – or to delegates of her choosing – to implement the statute as she determines is needed to ensure the accuracy and reliability of laboratory examinations [63]. The recent findings of SACGHS and others regarding weaknesses in laboratory oversight and their consequences for test quality provide a compelling case that a test registry would improve the ability of the Secretary to ensure the accuracy and reliability of such services.
Delegation of Authority to Implement and Enforce a Registry
While the authority to develop a test registry can be derived from either CLIA or the FD&C Act, or some combination thereof, this does not mean that CMS or FDA need be the agency charged with primary responsibility for its development or implementation. Congress delegated the authority in CLIA and FD&C to the HHS Secretary, who in turn delegated it to CMS, FDA, and CDC in the case of CLIA; and to FDA in the case of the FD&C Act. There is no legal bar to the Secretary’s reapportioning of authority between these entities or to her redelegating authority to another agency within the Department. Indeed, CLIA specifically directs the Secretary to ‘use the services or facilities of any Federal agency’ to carry out the objectives of the statute [64].
A number of suitable agencies within HHS could be identified to develop, implement, and enforce the registry. In particular, NIH has extensive expertise in registry development and implementation, and FDA has significant enforcement capability. NIH’s mission includes a mandate to ‘develop, maintain, and renew scientific human and physical resources that will assure the Nation’s capability to prevent disease’ [65]. NIH is home to several registries and has the expertise and personnel necessary to create a test registry. For example, through the National Center for Biotechnology Information (NCBI), NIH creates public databases, conducts research in computational biology, develops software tools for analyzing genome data, and disseminates biomedical information [66]. NCBI also manages complex genetics and genomics databases and makes them easily accessible to the public, including the Database of Genotype and Phenotype (dbGaP), which archives results of genotype-phenotype studies, and a catalog of published genome-wide association studies [67–69].
Through the National Library of Medicine, and in collaboration with FDA, NIH administers the website ClinicalTrials.gov. In assigning the responsibility for developing a clinical trials registry under FDAMA, Congress noted that NIH already had similar programs for specific diseases, suggesting that Congress was, in selecting the Director of NIH as the lead, seeking to leverage NIH’s expertise in database development [70]. Similarly, NIH, through the Recombinant DNA Advisory Committee (RAC), developed and administers a public database of human gene transfer research termed the Genetic Modification Clinical Research Information System [71]. Sponsors who receive NIH funding or will conduct their research at institutions receiving NIH funding must submit their protocols for review by the RAC before beginning the research.
FDA also could be a suitable home for the registry. As discussed above, FDA already manages large databases of information, such as manufacturer registration and listing information, and already has significant experience working with drug and device sponsors to establish product registries. Housing the registry at FDA, however, could create unrealistic expectations on the part of the public regarding FDA’s role in genetic test oversight. FDA for the most part does not review the safety and effectiveness of LDTs. While it is possible that FDA may expand its oversight to include certain high-risk LDTs, stakeholders agree that FDA premarket review of all LDTs is neither feasible nor desirable. However, given that FDA does review IVD test kits, the establishment of a test registry could create the misimpression that FDA also oversees the entities submitting the tests as well as the safety and effectiveness of all the tests they perform. Since NIH does not function as a regulatory agency, its management of the database would be less likely to create such false expectations.
Whether the registry is ‘housed’ at NIH or FDA, enforcement to ensure compliance should be delegated to FDA. While NIH has expertise in database development and implementation, the agency does not have robust enforcement capability. For example, as penalty for failing to register an applicable clinical trial with ClinicalTrials.gov, NIH can withhold grant funds [72]. This is a fairly limited enforcement tool, as it does not apply to entities that are not recipients of NIH funds. In contrast, FDA, through the FD&C Act, has robust enforcement capabilities, with the ability to impose both civil and criminal penalties for non-compliance with its requirements, as well as to enjoin distribution of products when manufacturers violate the statute. Most recently, Congress appears to contemplate an enforcement role for FDA with respect to ClinicalTrials.gov. The Food and Drug Administration Amendments Act of 2007 (FDAAA) provides that failure to submit clinical trial information, or the submission of false or misleading information, constitutes a violation of 303(a) of the FD&C Act, which allows the imposition of monetary penalties and/or imprisonment [29].
Some stakeholders have recommended CMS as the appropriate home for the registry. While CMS has the advantage of already overseeing clinical laboratories under CLIA, and while CMS collects some information from laboratories that also would need to be included in a registry, we believe CMS is not the appropriate administrative home for the registry. The CLIA program historically has been a very small component of CMS’s larger mission, which is administering the Medicare and Medicaid programs. Thus, the CLIA program has few personnel or other resources with which to administer a test registry. The data that CMS is required to collect under CLIA are not centrally maintained or accessible to the public. Only recently and under pressure has CMS made a list of CLIA certified laboratories available to the public on the agency’s website. Nor do personnel within CMS have expertise and experience in management of complex information or in making information accessible to the public. For these reasons, CMS administrators have stated publicly that they lack the capacity to establish a registry [6]. Additionally, as we have documented elsewhere, CMS’s implementation of CLIA with respect to genetic tests in particular has been inadequate and has endangered public health [2]. Thus, while efforts should be made to coordinate with CMS to ensure that the data submitted to the registry are consistent in content and format with information required under CLIA to avoid duplication of effort by laboratories, we believe CMS should not be charged with developing or implementing a registry.
Finally, we note that our proposal does not include review of the submitted information for accuracy by the agency or agencies developing and implementing the registry. Thus, one potential weakness of our proposal is that it leaves to the users of the registry to determine whether the submitted information is accurate. However, while there is no official vetting process for the submitted information, some safeguards do exist to ensure that registrants submit accurate information. Registrants would be subject to federal criminal law prohibiting the filing of false information with the government, and also would be subject to scrutiny by competitors with an incentive to highlight unsubstantiated claims or inaccurate data [73].
Concluding Remarks
The establishment of a test registry is critical for informed decision making by health care providers, payers, and patients in both the United States and other countries, all of whom need ready access to truthful information about genetic tests. The Secretary of HHS has the responsibility to ensure a transparent regulatory system and the explicit authority under both the FD&C Act and CLIA to develop a genetic test registry in order to protect public health. NIH’s expertise in informatics as well as genetics and genomics makes it a suitable agency to develop, implement, and maintain the database, and FDA’s existing enforcement capacity makes it the logical agency to enforce compliance with the requirements of registration and listing. Initially, the informational components required under the registry should be modest, in order to minimize burdens on registrants and to allow time to assess the critical informational components. A review of the registry should take place within 2 years of implementation in order to determine that it is meeting its objectives. While a genetic test registry will not address all concerns that have been raised regarding genetic testing oversight, it is a critical first step in the development of a more transparent, quality-centered system of oversight that will better inform and protect the public.
Acknowledgments
The Genetics and Public Policy Center is supported at the Johns Hopkins University by the National Institutes of Health and The Pew Charitable Trusts. The opinions expressed in this report are those of the authors. The authors attest that they hold no conflicts of interest with respect to the manuscript. The authors are grateful to Richard Merrill for his helpful discussions on the issue of legal authority and for his review and comments on several drafts of this paper, and to Stephanie Devaney for her research assistance.
References
- 1.Javitt G, Hudson K. Public health at risk: failures in oversight of genetic testing laboratories. 2006. Available at http://www.dnapolicy.org/images/reportpdfs/PublicHealthAtRiskFinalWithCover.pdf. [DOI] [PubMed]
- 2.Javitt G. In search of a coherent framework: options for FDA oversight of genetic tests. Food Drug Law J. 2007;62:617–652. [PubMed] [Google Scholar]
- 3.Secretary’s Advisory Committee on Genetics, Health, and Society. U.S. system of oversight of genetic testing: a response to the charge of the Secretary of Health and Human Services. 2008. Available at http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf. [DOI] [PMC free article] [PubMed]
- 4.Secretary’s Advisory Committee on Genetic Testing. Enhancing the oversight of genetic tests: recommendations of the SACGT. 2000. Available at http://oba.od.nih.gov/oba/sacgt/reports/oversight_report.pdf.
- 5.U.S. Government Accountability Office. Nutrigenetic testing: tests purchased from four websites mislead consumers. 2006. Available at www.gao.gov/new.items/d06977t.pdf.
- 6.Genetics & Public Policy Center. Senator Smith holds roundtable on genetic testing oversight. 2008. Available at www.dnapolicy.org/news.enews.article.nocategory.php?action=detail&newsletter_id=34&article_id=151.
- 7.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle–will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 8.Offit K. Genomic profiles for disease risk: predictive or premature? JAMA. 2008;299:1353–1355. doi: 10.1001/jama.299.11.1353. [DOI] [PubMed] [Google Scholar]
- 9.Amos J, Grody W. Development and integration of molecular genetic tests into clinical practice: the US experience. Expert Rev Mol Diagn. 2004;4:465–477. doi: 10.1586/14737159.4.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Laboratory Test Improvement Act, S.736, 110th Congress. 2007. Available at http://www.govtrack.us/congress/bill.xpd?bill=s110-736.
- 11.Comments of Eunice Yu, Peter Lurie, Sidney M. Wolfe of Public Citizen’s Health Research Group and Neil A. Holtzman on SACGHS draft report U.S. system of oversight of genetic testing, December 21, 2007, on file with SACGHS.
- 12.Testimony of Sharon F. Terry, Genetic Alliance on SACGHS report on oversight for genetic tests to Secretary’s Advisory Committee on Genetics, Health and Society, February 11, 2008.
- 13.Comments of Stephen M. Modell, University of Michigan School of Public Health, on SACGHS draft report U.S. System of oversight of genetic testing, December 20, 2007, on file with SACGHS.
- 14.Letter from Steven Teutsch to HHS Secretary Levitt. Aug 18, 2008. http://oba.od.nih.gov/oba/sacghs/reports/letter_to_Sec_08-18-08.pdf.
- 15.Genetics & Public Policy Center. Analysis of public comments on the SACGHS genetic testing oversight draft report. 2008. Available at www.dnapolicy.org/resources/SACGHSCommentanalysis02.12.08.pdf.
- 16.Comments of Alan Mertz, President American Clinical Laboratory Association on SACGHS draft report U.S. system of oversight of genetic testing, December 21, 2007, on file with SACGHS.
- 17.Comments of Michael Samoszuk and M.J. Finley Austin, Roche Diagnostics Corporation on SACGHS draft report U.S. system of oversight of genetic testing, December 21, 2007, on file with SACGHS.
- 18.Comments of Robert E. Yocher, Genzyme Corporation on SACGHS draft report U.S. system of oversight of genetic testing, December 21, 2007, on file with SACGHS.
- 19.Comments of Michael D. Maves, American Medical Association on SACGHS draft report U.S. system of oversight of genetic testing, December 21, 2007, on file with SACGHS.
- 20.Khoury MJ, Berg A, Coates R, Evans J, Teutsch SM, Bradley LA. The evidence dilemma in genomic medicine. Health Aff (Millwood) 2008;27:1600–1611. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- 21.Genetics & Public Policy Center. Professional practice guidelines for genetic testing. 2006. Available at www.dnapolicy.org/resources/Professional_Guidelines_Meeting_Summary.pdf.
- 22.GeneTests. Welcome to the GeneTests Website. 2008. www.genetests.com.
- 23.Pagon RA. GeneTests: an online genetic information resource for health care providers. J Med Libr Assoc. 2006;94:343–348. [PMC free article] [PubMed] [Google Scholar]
- 24.EDDNAL. Welcome to EDDNAL: the European Directory of DNA Diagnostic Laboratories. 2008. www.eddnal.com.
- 25.GENDIA. 2008. www.gendia.net.
- 26.EuroGentest. Harmonizing genetic testing across Europe. 2008. www.eurogentest.org.
- 27.Food and Drug Administration Modernization Act of 1997. Public Law No. 105-115, Section 113, 1997.
- 28.Food and Drug Administration. Status report on implementation. 2005. Available at www.fda.gov/oashi/clinicaltrials/section113/113report/executive.html.
- 29.Food and Drug Administration Amendments Act of 2007, Public Law No. 110-85, Section 801, 2007.
- 30.United States Code, Title 42, Section 263a.
- 31.United States Code, Title 21, Section 301 et seq.
- 32.United States Code of Federal Regulations Title 21, Section 801.4.
- 33.Das S, Bale SJ, Ledbetter DH. Molecular genetic testing for ultra rare diseases: models for translation from the research laboratory to the CLIA-certified diagnostic laboratory. Genet Med. 2008;10:332–336. doi: 10.1097/GIM.0b013e318172838d. [DOI] [PubMed] [Google Scholar]
- 34.New York State Department of Health. Clinical Laboratory Evaluation Program, Submission guidelines for test approval. revised July 2008. Available at www.wadsworth.org/labcert/TestApproval/submitguide.pdf.
- 35.Agency for Healthcare Research and Quality. AHRQ Publication Number 07-EHC001-1. 2007. Registries for evaluating patient outcomes: a user’s guide. [Google Scholar]
- 36.Fertility Clinic Success Rate and Certification Act, Public Law No. 102–493, 1992.
- 37.Centers for Disease Control and Prevention. Assisted reproductive technology: home. 2008. Available at www.cdc.gov/art/
- 38.House of Representatives, Report No. 110–225; 110th Congress, 1st Session; 2007. [Google Scholar]
- 39.Food and Drug Administration. Guidance for industry: establishing pregnancy exposure registries. 2002. Available at www.fda.gov/cber/gdlns/pregexp.pdf.
- 40.Food and Drug Administration. FDA public health advisory: strengthened risk management program for isotretinoin. 2005. Available at www.fda.gov/cder/drug/advisory/isotretinoin2005.htm.
- 41.United States Code of Federal Regulations, Title 21, Section 809.3.
- 42.Food and Drug Administration. Draft guidance for industry, clinical laboratories, and FDA staff: in vitro diagnostic multivariate index assays. 2007. Available at www.fda.gov/cdrh/oivd/guidance/1610.pdf.
- 43.Federal Register. 1997 Nov 21;62:62243–62260. [PubMed] [Google Scholar]
- 44.Testimony of Steve Gutman on Regulation of IVDs before Senate Special Committee on Aging; July 27; 2006. [Google Scholar]
- 45.Clinical Reference Laboratory v. Sullivan. 791 F. Supp. 1499 (D. Kan. 1992) (holding CLIA does not preempt FDA authority to regulate clinical laboratories).
- 46.Comments of Washington Legal Foundation on Draft guidance for industry, clinical laboratories, and FDA staff: in vitro diagnostic multivariate index assays, September 2006, on file with FDA.
- 47.Comments of Association of American Physicians and Surgeons on Draft guidance for industry, clinical laboratories, and FDA staff: in vitro diagnostic multivariate index assays, September 2006, on file with FDA.
- 48.United States Code, Title 21, Section 360(a), (b).
- 49.Food and Drug Administration. Guidance for industry and FDA staff – procedures for handling post-approval studies imposed by PMA order. 2007. Available at www.fda.gov/cdrh/osb/guidance/1561.html.
- 50.United States Code, Title 21, Section 371(a).
- 51.U.S. v. Nova Scotia Food Products Corp., 568 F.2d 240 (E.D.N.Y. 1977).
- 52.National Nutritional Foods Assn v. Weinberger, 512 F.2d 688 (2d Cir. 1975).
- 53.Pharmaceutical Manufacturers Assn v. Am. College of Obstetricians and Gynecologists, 484 F. Supp. 1179 (D. Del. 1980).
- 54.Rivers PA, Dobalian A, Germinario FA. A review and analysis of the clinical laboratory improvement amendment of 1988: compliance plans and enforcement policy. Health Care Manage Rev. 2005;30:93–102. doi: 10.1097/00004010-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Clinical Laboratory Improvement Amendments of 1988, H. Rep. 100-899; 100th Congress, 2nd Session. [Google Scholar]
- 56.Federal Register. 1999 Dec 30;64:73561. [Google Scholar]
- 57.Clinical Laboratory Improvement Advisory Committee (CLIAC) 2008. Available at wwwn.cdc.gov/cliac/default.aspx.
- 58.United States Code of Federal Regulations Title 42, Section 493.
- 59.United States Code of Federal Regulations Title 42, Section 493.801.
- 60.United States Code of Federal Regulations Title 42, Section 493.1351.
- 61.United States Code of Federal Regulations Title 42, Section 493.55(c)(3)
- 62.United States Code of Federal Regulations Title 42, Section 493.51.
- 63.Testimony of Thomas Hamilton on CLIA and Genetic Testing Before the Senate Special Committee on Aging; 2008. [Google Scholar]
- 64.United States Code, Title 42, Section 263a(q).
- 65.National Institutes of Health. About NIH. 2007. www.nih.gov/about/
- 66.National Center for Biotechnology Information. 2008. www.ncbi.nlm.nih.gov/
- 67.NCBI. dbGaP GENOTYPE and PHENOTYPE. 2008. Available at www.ncbi.nlm.nih.gov/gap.
- 68.Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, Hao L, Kiang A, Paschall J, Phan L, Popova N, Pretel S, Ziyabari L, Lee M, Shao Y, Wang ZY, Sirotkin K, Ward M, Kholodov M, Zbicz K, Beck J, Kimelman M, Shevelev S, Preuss D, Yaschenko E, Graeff A, Ostell J, Sherry ST. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39:1181–1186. doi: 10.1038/ng1007-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NCBI. A catalog of published genome-wide association studies. 2008. Available at www.genome.gov/gwastudies/
- 70.Food and Drug Administration Modernization and Accountability Act of 1997, S. Rep. 105–43. 105th Congress, 2nd Session. [Google Scholar]
- 71.Recombinant DNA Advisory Committee. NIH/FDA genetic modification clinical research information system. 2008. http://oba.od.nih.gov/rdna/adverse_event_oba.html.
- 72.United States Code, Title 42, Section 282(j).
- 73.United States Code, Title 18, Section 1001.