Abstract
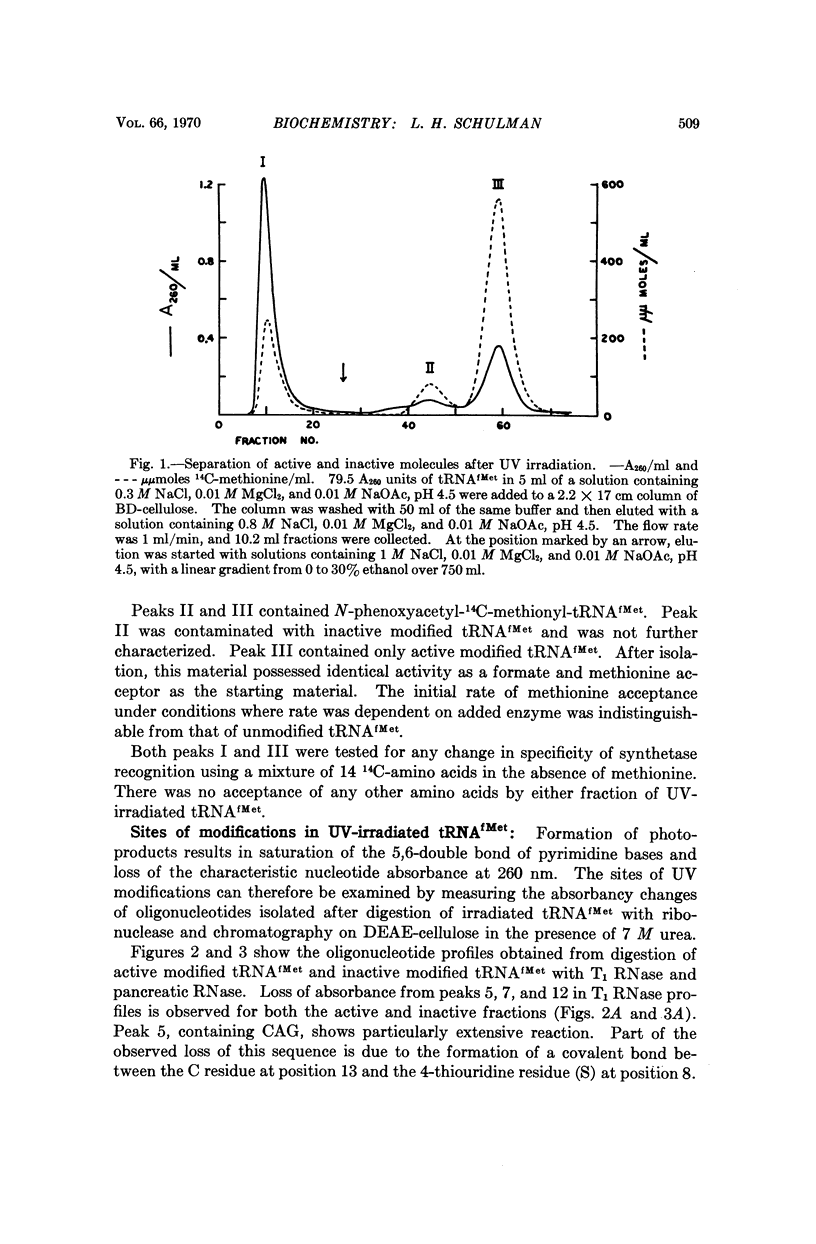
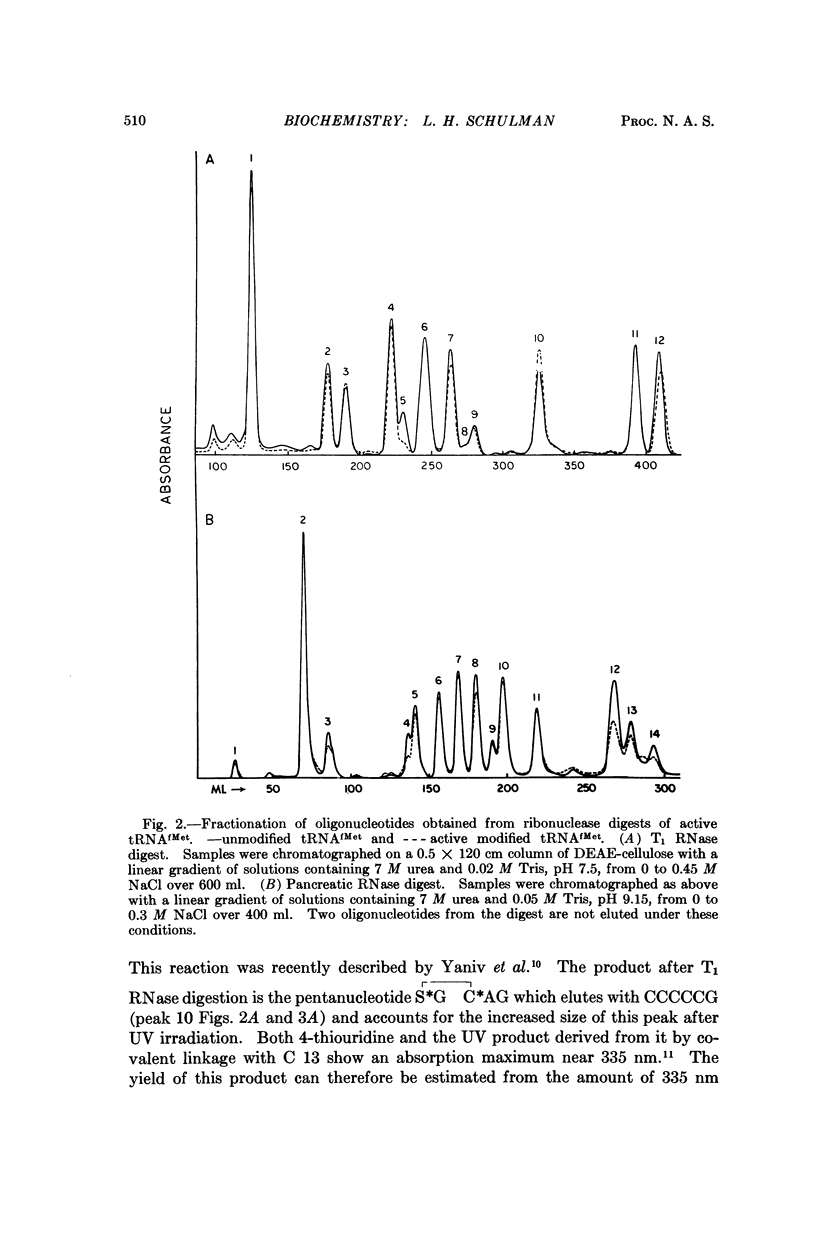
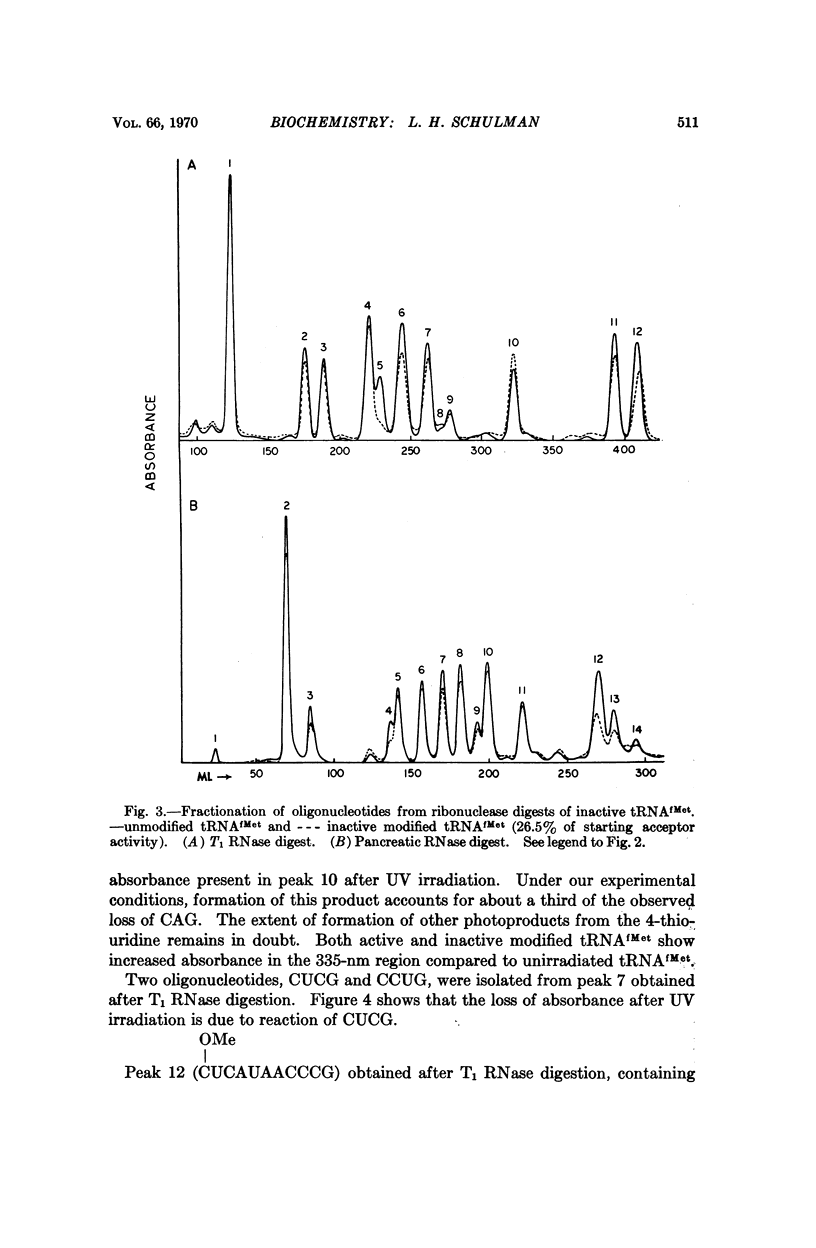
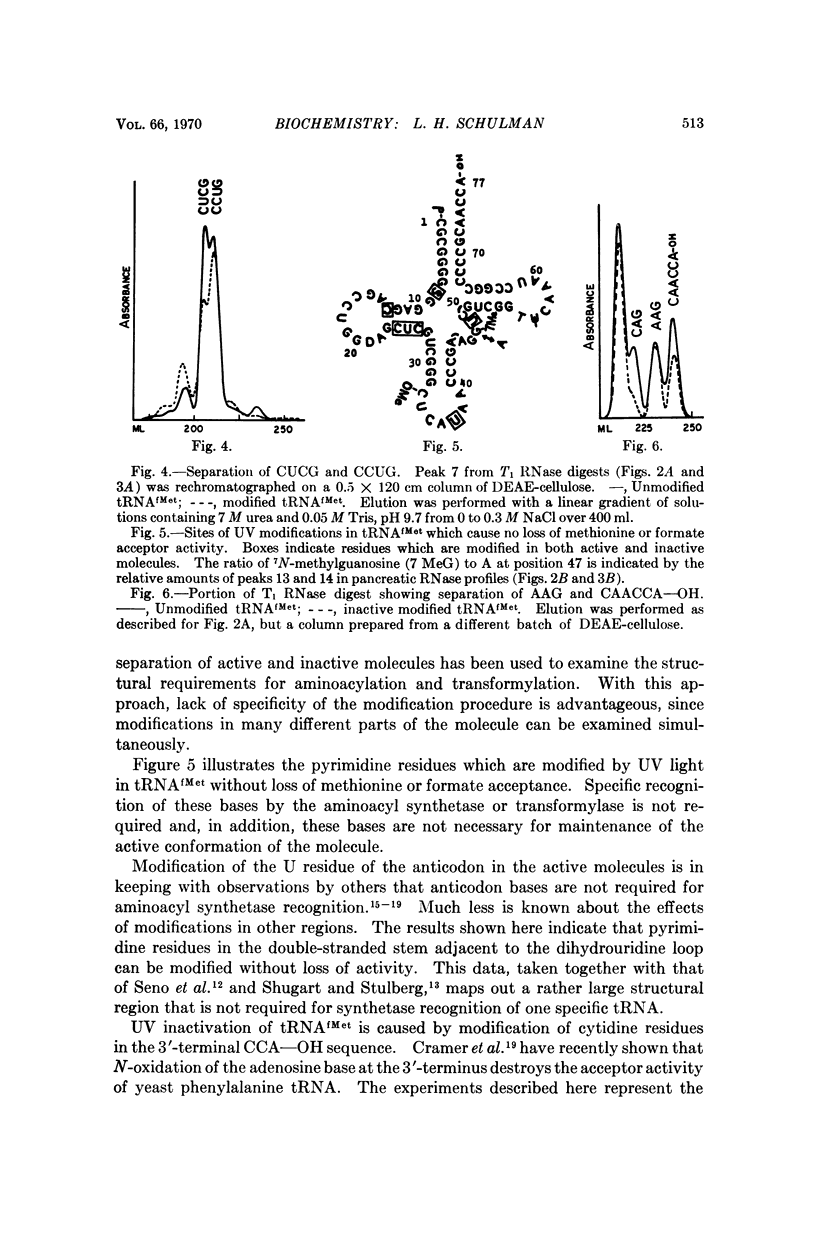
E. coli formylmethionyl tRNA (tRNAfMet) has been irradiated with ultraviolet light in the presence of Mg2+ to the extent of 50 per cent inactivation of amino acid acceptance. Separation of active and inactive molecules after irradiation has shown that ultraviolet light modification of the uridine in the anticodon, the uridine in the small loop, the 4-thiouridine, and the pyrimidines in the double-stranded stem adjacent to the dihydrouridine loop has no effect on aminoacylation or transformylation. The ultraviolet light-induced inactivation of methionine acceptance by tRNAfMet is due almost entirely to modification of the cytidine residues in the 3′-terminal CCA—OH sequence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuguev I. I., Axelrod V. D., Bayev A. A. The role of anticodon in the acceptor function of tRNA. Biochem Biophys Res Commun. 1969 Feb 7;34(3):348–353. doi: 10.1016/0006-291x(69)90839-0. [DOI] [PubMed] [Google Scholar]
- Cramer F., Erdmann V. A., von der Haar F., Schlimme E. Structure and reactivity of tRNA. J Cell Physiol. 1969 Oct;74(2 Suppl):163+–163+. doi: 10.1002/jcp.1040740416. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Wayman B. J., Cory S., Rudland P. S., Clark B. F. Studies on the Escherichia coli Methionine transfer ribonucleic acids. Eur J Biochem. 1969 Mar;8(1):93–100. doi: 10.1111/j.1432-1033.1969.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Favre A., Yaniv M., Michelson A. M. The photochemistry of 4-thiouridine in Escherichia coli t-RNA Vał1. Biochem Biophys Res Commun. 1969 Oct 8;37(2):266–271. doi: 10.1016/0006-291x(69)90729-3. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Kawata M., Takemura S. Recovery of tyrosine acceptor activity by combining 3'-half molecule with stepwise degradation products of 5'-half molecule obtained from tyrosine tRNA. Biochem Biophys Res Commun. 1969 Nov 20;37(5):777–784. doi: 10.1016/0006-291x(69)90959-0. [DOI] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- Reeves R. H., Imura N., Schwam H., Weiss G. B., Schulman L. H., Chambers R. W. Transfer RNA, I. Isolation and characterization of a new yeast alanine transfer RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1450–1457. doi: 10.1073/pnas.60.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K. L., Tener G. M. Inhibition of aminoacyl transfer ribonucleic acid synthetases by modified transfer ribonucleic acids. Biochemistry. 1967 Sep;6(9):2847–2852. doi: 10.1021/bi00861a027. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Chambers R. W. Transfer RNA, II. A structural basis for alanine acceptor activity. Proc Natl Acad Sci U S A. 1968 Sep;61(1):308–315. doi: 10.1073/pnas.61.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Purification of Excherichia coli methionine tRNAF and methionine tRNAM and studies on their biophysical and biochemical properties. Biochim Biophys Acta. 1968 Nov 20;169(1):80–94. doi: 10.1016/0005-2787(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Recovery of transfer RNA functions by combining fragmented Escherichia coli formylmethionine transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):285–303. doi: 10.1016/0005-2787(69)90080-x. [DOI] [PubMed] [Google Scholar]
- Shugart L., Stulberg M. P. Borohydride reduction of phenylalanine transfer ribonucleic acid. Effect on enzyme recognition. J Biol Chem. 1969 May 25;244(10):2806–2808. [PubMed] [Google Scholar]
- Uziel M., Koh C. K., Cohn W. E. Rapid ion-exchange chromatographic microanalysis of ultraviolet-absorbing materials and its application to nucleosides. Anal Biochem. 1968 Oct 24;25(1):77–98. doi: 10.1016/0003-2697(68)90083-3. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Favre A., Barrell B. G. Structure of transfer RNA. Evidence for interaction between two non-adjacent nucleotide residues in tRNA from Escherichia coli. Nature. 1969 Sep 27;223(5213):1331–1333. doi: 10.1038/2231331a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kaziro Y., Ukita T. The modification of nucleosides and nucleotides. X. Evidence for the important role of inosine residue in codon recognition of yeast alanine tRNA. Biochim Biophys Acta. 1968 Oct 29;166(3):646–655. [PubMed] [Google Scholar]



