Abstract
Cystic fibrosis (CF) is a life-shortening disease with significant morbidity. Despite overall improvements in survival, patients with CF experience frequent pulmonary exacerbations and declining lung function, which often accelerates during adolescence. New treatments target steps in the pathogenesis of lung disease, such as the basic defect in CF (CF Transmembrane Conductance Regulator [CFTR]), pulmonary infections, inflammation, and mucociliary clearance. These treatments offer hope but also present challenges to patients, clinicians, and researchers. Comprehensive assessment of efficacy is critical to identify potentially beneficial treatments. Lung function and pulmonary exacerbation are the most commonly used outcome measures in CF clinical research. Other outcome measures under investigation include measures of CFTR function; biomarkers of infection, inflammation, lung injury and repair; and patient-reported outcomes. Molecular diagnostics may help elucidate the complex CF airway microbiome. As new treatments are developed for patients with CF, efforts should be made to balance treatment burden with quality of life. This review highlights emerging treatments, obstacles to optimizing outcomes, and key future directions for research.
Keywords: Cystic fibrosis, Emerging treatments, Improved outcomes
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive genetic condition with an incidence of approximately 1:3500 in most European and North American countries and 1:5000 to 1:20,000 in Latin America, the Middle East, and South Africa (1). Progressive obstructive lung disease causes over 90% of deaths in patients with CF (2). The pathophysiology of CF is outlined in Table 1. Mutations in the gene coding for the CF Transmembrane Conductance Regulator (CFTR) result in an absent or dysfunctional protein at the surface of certain epithelia (3). CFTR normally regulates ion (chloride and sodium) flux across the cell membrane. In the lungs, the mechanism by which CFTR dysfunction leads to the characteristic lung disease of CF is complex. The predominant theory is that CFTR dysfunction causes dehydration of the airway surface liquid and impairment of mucociliary clearance (4). Chronic respiratory infections follow, leading to inflammation and progressive lung destruction. Other potential effects of CFTR dysfunction include activation of host inflammatory response, defects in bacterial phagocytosis by airway macrophages, changes in antimicrobial properties of airway mucus, and alterations of sodium transport across the epithelial sodium channel (ENaC) (5–7). Patients with CF are susceptible to opportunistic bacteria, most notably Pseudomonas aeruginosa (8). Chronic infection with the mucoid phenotype of P. aeruginosa is associated with shortened survival (9). A wide variety of other microbes has also been found in the airways of patients with CF (10).
Table 1.
Pathogenesis, treatments, and outcome measures for cystic fibrosis (CF) lung disease
| Step in pathogenesis | Treatment | Biomarker/outcome measure |
|---|---|---|
| CFTR mutation | Gene therapy | Transgene/protein expression |
| CFTR protein absence/dysfunction |
Read-through of stop mutations (PTC 124) Correctors (Vertex 809) Potentiators (Vertex 770) |
Sweat chloride Nasal potential difference Rectal potential difference Tissue expression of mature CFTR |
| Abnormal ASL/mucociliary clearance |
Mannitol dry powder Dornase alfa Hypertonic saline Airway clearance treatments |
Mucociliary clearance |
| Infection colonization–exacerbation |
Antimicrobials: IV, inhaled PA eradication |
Number of exacerbations Bacterial quantification Molecular diagnostics/ microbiome shifts Antimicrobial susceptibility Serology for pathogens |
| Inflammation | Macrolides (azithromycin) NSAIDs (ibuprofen) |
Serologic/sputum inflammatory markers |
| Lung destruction Lung Structural Abnormalities |
All of the above | FEV1, lung volumes and other measures of lung function High resolution CT scan Biomarkers of lung tissue breakdown Lung clearance index |
| Morbidity/mortality | All of above | Patient-reported outcomes Hospitalizations Measures of treatment burden |
ASL = airway surface liquid; CFTR = Cystic Fibrosis Transmembrane Conductance Regulator; CT = computed tomography; FEV1 = forced expiratory volume in 1 second; IV = intravenous; NSAIDs = nonsteroidal anti-inflammatory drugs; PA = Pseudomonas aeruginosa.
Current CF treatments target respiratory infections, inflammation, mucociliary clearance, and nutritional status. Many new therapies are in development, including molecules aimed at the basic defect in CF (11). One of the biggest challenges in CF research is identifying valid and sensitive, age appropriate biomarkers that assess response to therapies (12). Forced expiratory volume in 1 second (FEV1) and pulmonary exacerbation rate are the most commonly used outcomes in CF clinical trials. As improvements in treatment slow pulmonary function decline, the utility of FEV1 and exacerbation rate as outcome measures may decrease. CF-specific patient-reported outcomes have been validated for use in clinical trials and are currently being included as either primary or secondary outcomes if they meet regulatory approval (13;14). Other outcome measures that will likely be important in future studies include measures of CFTR function (sweat chloride concentration and transepithelial potential difference), chest imaging (standard chest radiography and high-resolution computed tomography [CT]), inflammatory markers, and microbial biomarkers (15).
Patients with CF and their care providers face many obstacles including uncertainty or lack of information about best therapies, high treatment burden and substantial medical expenses (16). As new treatments become available, these challenges will only increase. The best application of all potential therapies needs to be determined while balancing treatment burden and quality of life. We convened an expert panel to review current outcomes, goals of therapy, key barriers to treatment, and research opportunities. This manuscript, resulting from the expert panel meeting, is not meant to be a comprehensive review of the treatment of pulmonary disease in patients with CF. Rather it is focused on key areas of research and opportunities for improving the treatment of CF patients.
2. Current outcomes
2.1. Survival
The Cystic Fibrosis Foundation (CFF) Patient Registry includes data on approximately 24,500 patients with CF in the United States (17). Median predicted survival in 2007 calculated via a standard life table analysis was 37.4 years. However, the median age at death, a metric that has not increased to the same extent as the median predicted survival, was 25.9 years. Approximately 25% of deaths occurred in children, adolescents, and young adults ≤ 21 years of age. Data from the German Cystic Fibrosis registry in 2005 showed similar survival figures with median age at death of 23.7 years and the median cumulative survival 37.4 years (18). In 2005, 17.6% of deaths recorded in the German registry occurred in patients less than 18 years of age. Similar survival data have also been reported in France (19). Further research into the causes and potential preventive measures for early deaths is needed.
2.2. Lung function and Body Mass Index
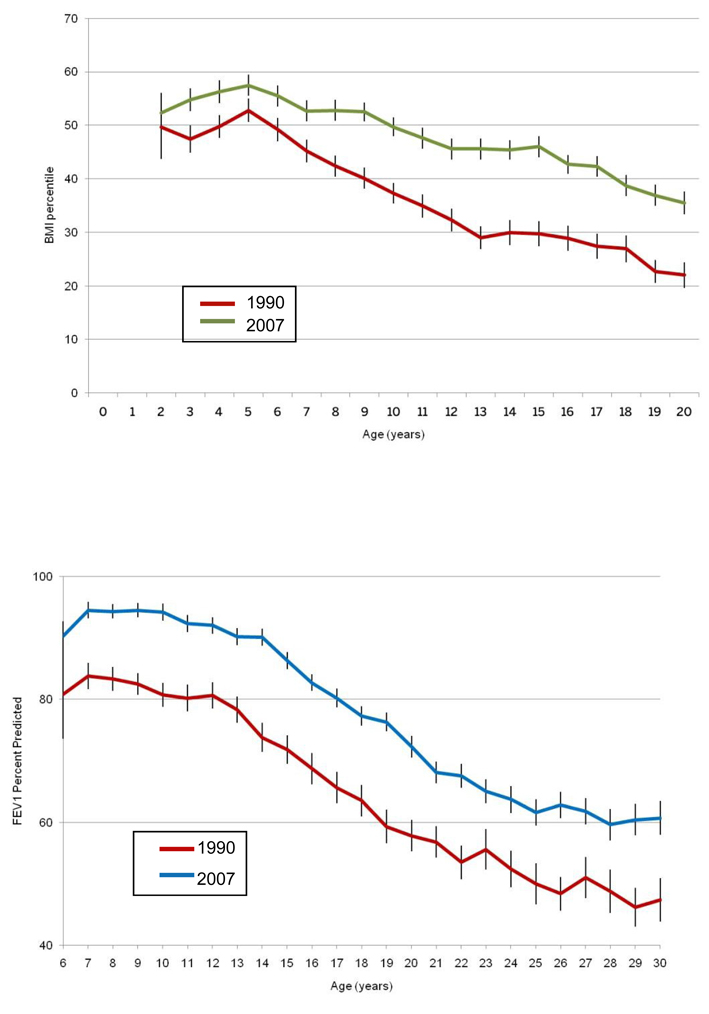
As noted from an analysis of the CFF Patient Registry in 2007, improvements were seen in several markers of disease severity, including mean body mass index (BMI) and mean FEV1 (% predicted), compared with 1990 (Fig. 1a, 1b) (17). In this analysis, lung function and BMI were higher at each age. Nonetheless, BMI (percent predicted) peaks during early childhood followed by a steady decline in school-age children and adolescents. Lung function (percent predicted) is maintained relatively well during the first decade of life and then declines steeply beginning around the age of 12 years.
Fig. 1.
Fig. 1a. Mean Centers for Disease Control (CDC) Body Mass Index (BMI) percentiles versus age with 95% confidence intervals. BMI was higher at each age in 2007 compared to 1990. CFF Patient Registry Data (16).
Fig. 1b. Mean forced expiratory volume in 1 second (FEV1) percent predicted versus age with 95% confidence intervals. Lung function was higher at each age in 2007 compared to 1990. Data from CFF Patient Registry, 1990 and 2007 (16).
The association of better nutritional status with improved lung function is well documented (20–23), and poor nutrition is a risk factor for accelerated decline in lung function (24). Data from 6,835 CF patients in the German CF Registry from 1995–2005 found that low BMI (< 19 kg/m2) and low FEV1 (<80%) correlated with mortality (18). It is not known whether the decline in BMI predates the FEV1 decline in individual patients. There may be periods of time that children are at particular risk for decline (e.g., BMI decline in school-age children and FEV1 decline in adolescents). Identifying these high-risk periods may help focus interventions.
Studies of lung function decline provide essential information for clinical care and research. Understanding the mechanisms of lung injury and risk factors for lung function decline may guide choices in clinical care and development of new therapies. Defining the rate of lung function decline in patients with CF is also important as a reference for assessing therapies and designing clinical trials (25). A recent retrospective study by McPhail et al compared lung function and nutritional outcomes in 144 patients with CF divided into two birth cohorts (26). The authors found substantial improvements in lung function and nutritional outcomes (weight, height, and BMI percent predicted) and a significant decrease in the rate of FEV1 decline from the ages of 6 to 12 years in the latter cohort. Although the study was retrospective, it is the first to demonstrate a change in the rate of lung function decline between birth cohorts. The decreased rate of lung function decline was associated with a higher baseline BMI (percent predicted) and slower rate of BMI decline.
Although lung function and nutritional status have shown steady improvements, the frequency of pulmonary exacerbations remained unchanged from 1990 to 2007 (B. Marshall, MD, oral communication, 2009). Whether this finding is due to lack of impact from current interventions on exacerbation frequency, changes in the definition of exacerbation, or a tendency toward more aggressive therapy is not known. Emerging research suggests that exacerbations play a large role in the overall decline in lung function, which may not return to baseline following exacerbation (27). Understanding practice patterns for exacerbation treatment and outcomes following therapy will guide future clinical care guidelines. Applying a consistent definition of exacerbation is also important for use as an outcome measure in clinical trials.
2.3. Newborn screening
Newborn screening for CF began in the 1980s in several countries including Australia, several European countries, and parts of the United States (28;29). The recommendation in 2004 by the US Centers for Disease Control and Prevention in favor of newborn screening led to the rapid implementation of testing across the United States. Forty-eight states now screen for CF and the remaining two states will begin screening by 2010 (30). The European CF Society recently published best practice guidelines for newborn screening in anticipation of increased screening programs across Europe (31). Between 2004 and 2008 the number of infants screened in European countries increased from 1.6 to 3 million, and this increase is expected to continue. Many of the screening programs in Europe are regional rather than national, reflecting geographic and ethnic variations (32;33). Screening protocols vary between programs necessitating guidelines for future program implementation. Widespread implementation of newborn screening in the US is reflected in the proportion of newborn screen diagnoses recorded by the CFF registry . Prior to 2005, less than 15% of all new cases were diagnosed by newborn screening. By 2007, more than 30% of new cases were diagnosed by newborn screening (17), and this number is expected to increase over the next few years. Because screening detects infants with CF who are asymptomatic, changes to current infant care guidelines are needed. Rather than focusing on the treatment of problems such as failure to thrive and pulmonary exacerbation, care of infants with CF should reflect more preventive treatments such as nutritional intervention with pancreatic enzyme replacement therapy and fat soluble vitamin supplements to achieve and maintain normal growth and development (34). Studies from the Wisconsin newborn screening program clearly show that early intervention improves nutritional outcomes, and there is some evidence of improved overall survival in newborns diagnosed before one month of age compared with infants diagnosed later (35–38). Important questions remain regarding optimal treatment to prevent lung disease and to ensure normal growth and nutrition. Studies are now under-way to assess pulmonary therapies and nutritional interventions in infants and toddlers; patient-reported outcomes in this young population are also currently being validated (39).
2.4. Adolescents
Lung function decline appears to accelerate during adolescence. Understanding factors that contribute to this decline is important for developing effective therapeutic approaches. Konstan et al examined a subset of data collected for the Epidemiologic Study of Cystic Fibrosis (ESCF), a prospective observational study designed to characterize the natural history of CF (24). Data from 4,923 patients aged 6 to 17 years were examined to characterize the change in FEV1 percent predicted over 3 to 6 years and to identify risk factors for FEV1 decline among patients in three age groups (6 to 8 years, 9 to 12 years, and 13 to 17 years). Children 6- to 8-years-old had a slower rate of lung function decline compared with patients in the other two groups. Risk factors for decline in lung function for all three age groups were high baseline FEV1, female gender, and crackles on auscultation. P. aeruginosa was a risk factor for decline in the 6- to 8-year-old and 9- to 12-year-old groups.
Other possible contributors to lung function decline in adolescence are the onset of CF-related diabetes and infection with pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA), P. aeruginosa and other gram-negative bacteria, fungi and nontuberculous mycobacterium (40;41). In addition, adherence to treatments has been shown to decline in this age group (42;43). Adolescents are often balancing academic demands and pressure to fit in with peers with time-consuming pulmonary treatments such as inhaled tobramycin, dornase alfa, and hypertonic saline. Rates of depression and anxiety are also high during adolescence, contributing to worse compliance (44). Compared with younger children, adolescents often receive less parental supervision of their treatment regimen, which has been shown to reduce the frequency and duration of therapies (45). Understanding which factors are most strongly related to lung function decline would allow patients and caregivers to focus on the most important therapies for this age group, and thus preserve lung function through this critical life stage.
2.5. Adults
Just as the landscape for infant care in CF is changing, so is the care of adults with CF. As survival has improved, the number of patients with CF reaching adulthood has expanded dramatically. Between 1985 and 2007, the number of patients ≥18 years of age in the CFF Patient Registry increased from 4000 to more than 10,000 (17). The number of adult CF programs has also expanded, although more adult CF physicians and programs are needed. Importantly, a shift in the clinical phenotype of these patients has also occurred. In 1985, 32% of 18-year-old patients were classified as having mild or no lung disease (FEV1 ≥70% of predicted) and 33% were classified as having severe lung disease (FEV1 <40% of predicted). In 2007, 67% of 18-year-old patients had mild or no lung disease, and only 7.6% of patients had disease classified as severe. Clinical approaches to adults with mild lung disease are needed to preserve lung function while maximizing quality of life.
3. Barriers to Care
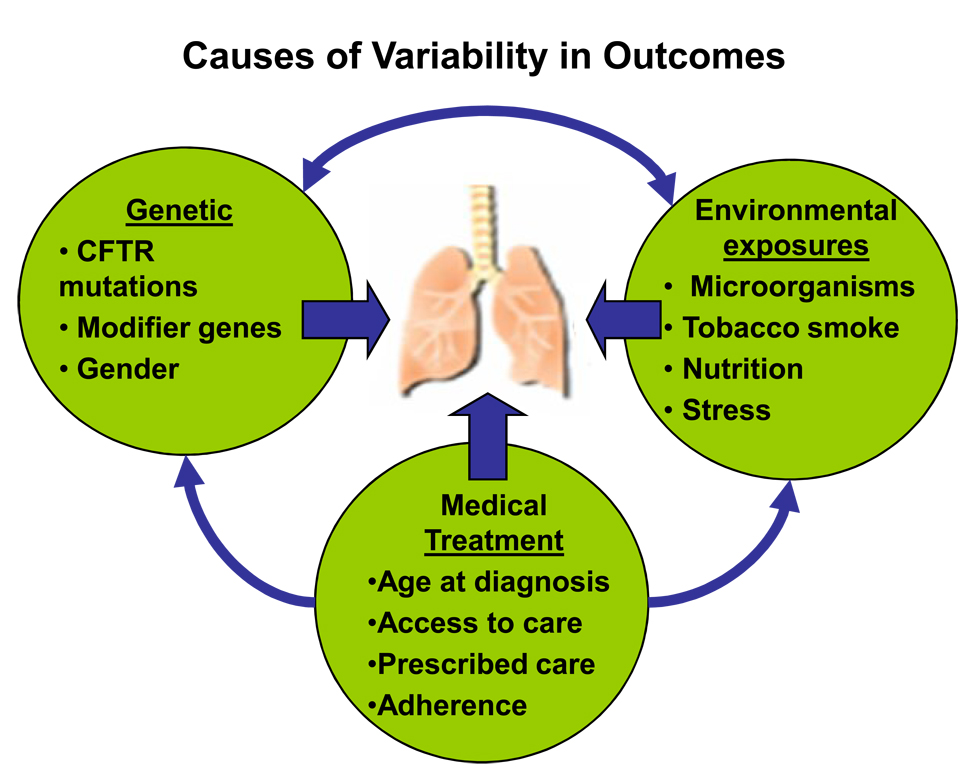
The treatment goals for patients with CF lung disease are to halt or slow disease progression by maintaining FEV1 and preventing pulmonary exacerbations, to provide symptom relief, and to improve quality of life. As discussed above, despite the availability of proven therapies, significant variability exists in these outcomes and the use of medications. Many issues likely contribute to this variability, including genetic, medical, and environmental factors (Fig. 2). Understanding how these factors affect outcomes and addressing the barriers to care is a critically important task. In an attempt to identify existing barriers, we considered specific obstacles facing patients, clinicians, and researchers (Table 4). By targeting barriers in each group for intervention, we hope to advance the development and delivery of CF care.
Fig. 2.
Multifactorial causes of variability in outcomes. CFTR = Cystic Fibrosis Transmembrane Conductance Regulator. Adapted from original provided by Michael Schechter, MD, Emory University, Atlanta, GA, USA.
Table 4.
Barriers to optimal therapy
| Patients/families | Medical care providers | Researchers |
|---|---|---|
| Adherence Treatment burden |
Lack of resources/time Lack of time |
Identifying appropriate outcome measures |
| Mental health/depression Lack of insurance, underinsurance |
Implementation of guidelines/ evidence-based medicine Drug interactions |
Need for large sample size Appropriate selection of controls |
| Lack of understanding (disease seriousness, treatment benefits, etc) |
Relaying hope vs seriousness of disease |
Navigating regulatory guidelines |
| Heterogeneity of response/ pharmacogenetics |
Managing treatment failures, adverse events |
Balancing need for new therapies with patient safety Lack of resources/time Competing clinical trials |
3.1. Barriers for Patients
Patients with CF and their families face many barriers including (i) large treatment burden that may make adherence difficult, (ii) financial challenges, (iii) lack of education around illness or limited understanding of disease process and (iv) depression and/or anxiety (16;44;46). CF places a large treatment burden on patients and their families with complex, time-intensive therapies and multiple medications administered throughout the day. Patients with chronic P. aeruginosa infection who are receiving inhaled tobramycin and dornase alfa likely spend several hours on treatments just to maintain health during times of clinical stability. A recent study of adults with CF found that perceived treatment burden was highly associated with the number of nebulized medications prescribed (47). Subjects reported spending an average of 108 minutes (SD ± 58 minutes) on daily CF treatments. In addition, lengthy hospitalizations, which are commonplace in treatment plans, are disruptive to families, schooling, work, and extracurricular activities (48) .
Poor adherence to therapy is very common and the likeliest cause of treatment failure (49). In addition to the high treatment burdens that limit adherence, families may have a poor understanding of how specific medications work or how to correctly administer them. As CF care is complex, with multiple treatments targeting different pathophysiologic processes, it is easy for families to feel overwhelmed. With improved survival in CF, families may not fully understand the seriousness of the disease, the need for daily preventive therapies, and the risks associated with poor adherence. Consequences of poor adherence are often seen more over the long term than immediately (i.e., exacerbation after months of noncompliance is more common than an immediate increase in symptoms), so the cause-and-effect relationship may not be obvious. Furthermore, adolescents often face peer- pressure and may resist any intervention that differentiates them from their peers. New adherence interventions targeting these issues have been developed and are being evaluated for use in clinical settings (50;51).
Adherence is also strongly affected by the cost of therapy. Although most patients with CF in the US have some form of health insurance, many have inadequate coverage. The increasing cost of co-payments and coinsurance make clinic visits, hospitalizations, and procuring medications difficult or impossible for some families. Patients classified as underinsured often do not fulfill the CFF Clinical Care Guidelines’ recommendation for quarterly clinic visits and may not be hospitalized for pulmonary exacerbations even when needed (52). Patients with low socioeconomic status are at a particularly high risk for inadequate resources and poor adherence. In many other countries, access to health insurance is much better than in the US; thus CF patients in these countries may not face these same barriers to treatment (53). The consequences of poor adherence include increased symptoms, accelerated decline in lung function, more frequent hospitalizations, increased family stress and conflict, and higher healthcare costs (43). A new clinical study now underway will implement an adherence intervention using prescription refill histories as the primary outcome. (A. Quittner, MD, oral communication, 2009)
Symptoms of depression and anxiety are common among patients with CF and parent caregivers, as has been found in many chronic illnesses (44). Rates of depression in adults with CF range from 29% to 46%, and similar rates have been documented in parent caregivers, although at least one study of adult CF subjects found no increase in anxiety and depression (30% and 13% respectively) compared to published normative data (54). Other studies however have found that adolescents with CF have higher rates of depression than the general population (44). Importantly, patients with depression are less likely to adhere to their therapies, more likely to miss clinic visits, engage in more risky behaviors, including smoking and illicit drug use, and report worse quality of life. A recent study by Quittner et al showed a link between depression in parent caregivers of children with CF, decreased compliance with enzyme therapy, and decreased weight in these children at the next clinic visit (55). The International Depression/Anxiety Epidemiological Study (TIDES) is an ongoing, international epidemiologic study, recruiting 3,600 patients and caregivers, aimed at establishing the prevalence of depression and anxiety in adolescents and adults with CF and in caregivers of children with CF.
3.2. Barriers for Clinicians
CF clinical care providers face barriers to providing optimal care. A lack of resources can significantly affect the clinician’s ability to adhere to best care practices. Resources needed to ensure optimal care for patients with CF include adequate clinic space and a dedicated CF team with nurses, a nutritionist, social workers, pharmacists, and respiratory therapists, as well as ancillary support from psychology, clinical laboratories (microbiology, chemistry, molecular diagnostics), radiology, and pulmonary function testing (56;57). Smaller CF centers may have particular difficulty supporting a CF team and ancillary staff. Providers also face many time constraints. Confronted with complex medical care, it may not be possible to adequately address all issues in the time allotted, and assessing patient compliance is difficult, as self-reported adherence typically represents an overestimation (58).
With the expansion of care guidelines and new therapies, staying current with new developments and recommendations may be difficult. Many disciplines, including pulmonary and critical care medicine utilize protocols to improve delivery of care (59;60). Protocols lead to less variability in care and remind clinicians of all therapies that may apply to particular patients (61). Quality improvement projects in CF are standardizing clinician education and adherence to practice guidelines with positive results. Data from these protocols and projects demonstrate that clinical improvements can occur without losing individualization of medical care(62–64).
As new therapies are approved, clinicians will have to decide how to incorporate these treatments into existing regimens. Whether new treatments should be used as additive or as replacements for existing therapies is unknown. Patients who develop adverse effects from medications or who do not respond to particular treatments are particularly challenging. Identifying biomarkers that distinguish patients who may not respond to particular treatments will allow better individualization of therapies. Whether biomarkers similar to those used in research studies will be useful in managing the care of individual patients needs to be examined. Adverse events and toxicities from medications are frequent challenges for clinicians. Risk of renal injury from aminoglycosides, for example, is increased with higher doses, high cumulative lifetime exposure, concurrent use of other nephrotoxic medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), and preexisting kidney disease from CF-related diabetes, dehydration, or immunosuppression after lung transplantation (65;66). Once-daily dosing of intravenous tobramycin appears to have equal efficacy but less nephrotoxicity in children with CF compared with TID dosing (67). Vestibular toxicity and ototoxicity are also common, but likely under appreciated, complications of aminoglycoside use (68;69). As survival improves, monitoring and early detection of these complications will become increasingly important.
Finally, clinicians may struggle to communicate hope along with the serious nature of CF. Families should be encouraged by the major advances in CF care and the great improvements made in life expectancy. However, these advances come with a high cost in daily treatment burden and toxicities. Communicating the sense of urgency for preventive and aggressive care along with an attitude of optimism can be challenging. Open, frequent, and clear communication with families about risks and benefits of both treatment and lack of treatment is essential.
3.3. Barriers for Researchers
Research into new, improved therapies in CF is needed to build on current advances. CF researchers face obstacles similar to those of clinicians in terms of the lack of time and resources. With so many treatment options currently in or approaching clinical trials, a lack of eligible study subjects and qualified CF research teams may be a barrier. Clinical research must balance the urgent need for new therapies with deliberate research studies that allow careful, reproducible and reliable assessment of patient safety and an adequate understanding of benefits.
One of the biggest challenges facing CF clinical research is the need for sensitive biomarkers and outcome measures for use in clinical trials (70). Outcome measures can be categorized as (1) clinical efficacy measures (pulmonary exacerbations, hospitalizations, antibiotic use, growth parameters, patient-reported outcomes, and quality of life measures), (2) surrogate endpoints (FEV1, chest x-ray or chest computed tomography (CT) scan, infant and preschool lung functions, lung clearance index), and (3) biomarkers (microbial cultures, inflammatory markers, CFTR functional measures such as nasal potential difference and sweat chloride) (12). Clinical efficacy endpoints are defined by the FDA as “a characteristic or variable that reflects how a patient feels, functions, or survives” and are generally required for trials seeking FDA approval (71). Previous phase III CF clinical trials used lung function (FEV1) and pulmonary exacerbations as key outcome measures. As new, approved therapies result in improved outcomes, these measures may become less sensitive in detecting a difference between treatment groups (12). Appropriate selection of outcome measures for clinical trials depends on the population under study (infants versus adolescents or adults), severity of disease, and therapeutic target under investigation (CFTR modulation, airway infection, mucociliary clearance). With the expansion of newborn screening and emphasis on early, preventative care in CF, finding sensitive, precise and feasible clinical outcome measures for young children (< 6 years) is critical (72). Current outcome measures under investigation include infant pulmonary function testing (plethysmography, gas dilution and raised volume rapid thorocoabdominal compression techniques), preschool pulmonary function testing (spirometry, force oscillometry, lung clearance index measured by multiple-breath washout, and measures of airway resistance), chest CT, and bronchoalveolar lavage for microbial culture and inflammatory biomarkers. Chest CT imaging may be more sensitive than lung function in detecting early structural changes in the lung and peripheral airway disease (73).
In older children and adolescents outcome measures include clinical efficacy endpoints such as pulmonary exacerbations, hospitalizations, patient reported outcomes (PRO’s) and quality of life measures (13). Older patients are more likely to spontaneously expectorate sputum or tolerate sputum induction, allowing analysis of microbiologic and inflammatory biomarkers (74). Given concerns about adherence to therapies, measures of compliance including patient diaries, pharmacy refill reports and electronic monitors may also provide useful data from clinical trials (58). For outcome measures in all age groups, standardization of operating procedures and equipment is vital, especially when performing multi-center clinical trials. The CFF is now working with researchers to bank sputum and plasma specimens from participants in clinical trials. Such specimens would be available to researchers for future microbiologic and protein biomarker studies.
Patient-reported outcomes (PRO) are increasingly important (13) and have been approved by the FDA for use in clinical trials. PROs evaluate changes in symptoms, perceptions of health, and daily functioning as perceived by the patient. Measures range from a single-item rating of pain to multidimensional, health-related quality-of-life instruments. These measures improve our understanding of how treatments impact CF symptoms and daily functioning, e.g.: school attendance, and may be more sensitive than physiologic measures such as FEV1. Over the past decade, four CF-specific PRO measures have been developed, including CFQ-R (14), the CF Respiratory Diary (75), CF QoL (76), and Questions on Life Satisfaction (77). Several of these measures are validated and the minimal clinical important difference for the CFQ-R has now been determined. Validation studies are also under way for a preschool version of the CFQ-R.
3.4. Quality improvement and practice patterns
Major advances in CF care over the past 15 years include the introduction of dornase alfa (DNase), tobramycin solution for inhalation, high dose ibuprofen, chronic oral azithromycin, and hypertonic saline therapy. Randomized placebo-controlled trials for each of these therapies demonstrated significant improvements in FEV1 and/or reduced pulmonary exacerbation or hospitalization rates for treated patients compared with controls (78–80). Despite clear benefits, some patients are still not receiving all appropriate therapies. The CFF Patient Registry tracks chronic respiratory medication use in eligible patients. Patients are considered eligible for treatment with dornase alfa and hypertonic saline if they are aged ≥6 years and for treatment with inhaled tobramycin if they are P. aeruginosa positive and aged ≥6 years. Patients considered eligible for chronic azithromycin therapy are those aged ≥6 years with chronic P. aeruginosa, weight ≥25 kg, FEV1 (percent predicted) ≥30% and no contraindications to treatment. In 2007, of those eligible for therapy, 74% received dornase alfa, 34% received hypertonic saline, 67% received inhaled tobramycin, and 63% received chronic macrolides, with notable differences in use between CF centers (17). Potential reasons for not receiving indicated therapies include (i) overall complexity of medical care, (ii) a lack of provider education and patient understanding regarding clinical indications for treatment, (iii) difficulty adhering to these time-consuming treatments, (iv) poor tolerance of certain medications and (v) financial burden in covering co-payments or lack of insurance. Quality improvement initiatives at many CF centers have sought to improve provider consistency with standards of care. Challenges to implementing guideline recommendations vary by center. Smaller centers may struggle with a lack of resources, personnel, and time. Larger centers may struggle with the bureaucracy often required to change practice patterns. The CFF estimates that by providing currently available, appropriate therapy to all patients with CF, significant improvements in life expectancy could be made. Interventions to improve patient adherence and tolerance of particular medications are needed (58;81;82).
4. CF Lung Disease: Therapeutic Targets
4.1. Early P. aeruginosa eradication
One of the most important advances in CF care is early management of P. aeruginosa infection with the goal of bacterial eradication from airway secretions (83). Chronic P. aeruginosa infection is associated with impaired lung function and decreased survival in CF (84). Multiple studies have shown that aggressive antibiotic treatment at the time of initial P. aeruginosa detection delays the time to chronic infection (85). Hansen et al. recently reviewed their 15 year experience using inhaled colistin and oral ciprofloxacin for P. aeruginosa eradication (86). At their center, 146 CF patients were followed for a median observation time of 7 years. Of those treated for a first ever P. aeruginosa isolate during the study period, 80% remained free of chronic colonization at the end of the study. Additional studies or early P. aeruginosa eradication were reviewed in detail in a recent article (87). Although early treatment strategies are now used by most CF centers, there is no agreement on optimal treatment (88–91). Published studies from the United States, the European Union, Australia, and Canada have used various combinations of intravenous antibiotics, oral ciprofloxacin, inhaled tobramycin, and inhaled colistin for differing durations. However, comparing approaches is complicated by the use of different study populations and outcome measures.
Two large multi-center studies warrant mention. One study, the Early Pseudomonas Infection Control (EPIC) study, is a randomized trial in the United States assessing the efficacy and safely of inhaled and oral anti-pseudomonal antibiotics following the initial isolation of P. aeruginosa (92). The second study, EarLy Inhaled Tobramycin for Eradication (ELITE), is a multi-center European study that assessed the duration of P. aeruginosa eradication following a 28- or 56-day course of inhaled tobramycin. Preliminary results from this study show a high rate of successful and durable eradication with no difference in time to recurrence between treatment groups (93).
The increasing use of eradication strategies for P. aeruginosa has raised the concern that overuse of antibiotics may lead to multidrug-resistant P. aeruginosa infection (87). A recent retrospective study by Ho et al compared the antibiotic susceptibility of first and subsequent P. aeruginosa isolates from patients with CF treated with eradication therapy (94). The study included patients treated with varied regimens of anti-pseudomonal therapy over 10 years. They found no change in susceptibility patterns of isolates following intensive treatment and no difference between isolates that were successfully eradicated and those that failed eradication. Microbial sensitivity indices will be important outcome measures in future studies of eradication protocols. Other challenges in eradication therapy that need to be studied include differentiating true eradication from bacterial suppression, and assessing reinfection from environmental sources.
4.2 Chronic Pseudomonas aeruginosa therapy
Both the overall and age-specific prevalence of P. aeruginosa appear to have decreased over the past decade, possibly secondary to aggressive early eradication strategies (95). However in 2007, more than 50% of patients with CF had a positive culture for P. aeruginosa, and this number approached 80% for patients over the age of 25 years (17). Currently, in the US, there is only one Food and Drug Administration (FDA)-approved therapy for patients with CF and chronic P. aeruginosa infection: tobramycin solution for inhalation. Chronic, alternate month treatment with inhaled tobramycin has been shown to improve lung function, decrease sputum P. aeruginosa bacterial counts, and reduce hospitalizations in CF subjects with chronic P. aeruginosa infection (79;96).
Chronic macrolide therapy is also beneficial in patients with chronic P. aeruginosa infection. In a randomized placebo-controlled trial, oral azithromycin (dosed three times a week for 24 weeks) improved lung function and reduced pulmonary exacerbations in CF subjects with chronic P. aeruginosa infection (78). The mechanism of action of chronic macrolides is more likely related to anti-inflammatory properties than to antimicrobial effects (97). A randomized, placebo-controlled, multi-center trial in France studied azithromycin in subjects regardless of chronic P. aeruginosa status (98). A significant reduction in exacerbations and antibiotic use was demonstrated in the treated group compared to placebo regardless of infection status.
Inhaled colistin is a frequently used alterative to inhaled tobramycin and is considered first-line therapy for chronic P. aeruginosa infection in the United Kingdom (99). A randomized one-month trial comparing tobramycin solution for inhalation with inhaled colistin found no difference in the reduction of P. aeruginosa in culture, but significant improvement in lung function in the inhaled tobramycin group (100). The latter result may have reflected previous exposure to the drug in the colistin group and treatment with a 1 MU rather than a 2 MU dose. An open-label follow-up to this study showed a persistent advantage in lung function for the inhaled tobramycin group (101).
New antibiotic treatment options are needed for patients with CF and chronic P. aeruginosa infection for several reasons. First, some patients are unable to tolerate current inhaled antibiotics due to allergic reactions or adverse effects (102;103). Second, when administered by standard jet nebulization, both inhaled tobramycin and colistin require long daily nebulizer treatments, which may reduce patient compliance. Finally, some patients do not show expected or sustained benefit from particular medications. For example, studies have shown a heterogeneous response to azithromycin with some patients improving significantly but others worsening, as measured by FEV1 (104). In addition to inhaled antibiotics for chronic use, advances have been made in intravenous anti-pseudomonal therapies, and several new treatments are now available. New therapeutic options for P. aeruginosa, Burkholderia cepacia complex, and other common CF pathogens are in clinical trials (Table 2). Aztreonam lysine solution for inhalation (AZLI), tobramycin powder for inhalation (TIP), and dry powder colistin are currently in phase III trials and are discussed below.
Table 2.
New antimicrobial treatments in cystic fibrosis (CF)
| Treatment | Potential target | Research phase |
|---|---|---|
| PA eradication | Early PA infection | Multicenter trials (EPIC,a ELITE) |
| Azithromycin in PA-negative patients |
Airway inflammation, bronchiectasis |
Phase IVa,b |
| Fosfomycin | MDR-PA | FDA approved: Non-CF indication |
| Tigecycline | MRSA, MSSA, BCC, MA, MC | FDA approved: Non-CF indication |
| Aztreonam lysine for inhalation | PA, possibly BCC | Phase IIIa,b Extended-access program |
| Tobramycin powder for inhalation |
PA | Phase IIIa,b |
| Fosfomycin: tobramycin inhalation |
PA, MRSA, MSSA, BCC, SM | Phase IIa |
| Liposomal ciprofloxacin | PA | Phase II |
| Levofloxacin solution for inhalation |
PA | Phase IIb |
| Liposomal amikacin | PA, biofilm penetration, BCC | Phase Ib/IIaa,b |
| Dry powder colistimethate | PA | Phase III |
| Ciprofloxacin PulmoSphere® inhalation powder |
PA | Phase IIa,b |
Information derived from www.clinicaltrials.gov.
Information derived from Cystic Fibrosis Foundation Therapeutics.
AZLI = aztreonam lysine solution for inhalation; BCC = Burkholderia cepacia complex; ELITE = EarLy Inhaled Tobramycin for Eradication Study; EPIC = Early Pseudomonas Infection Control Study; FDA = Food and Drug Administration; IV = intravenous; MA = Mycobacterium abscessus; MC = Mycobacterium chelonae; MDR-PA = multidrug-resistant Pseudomonas aeruginosa; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus; PA = Pseudomonas aeruginosa; SM = Stenotrophomonas maltophilia.
4.3 Inhaled antibiotics: Phase III trials
4.3.1 Aztreonam lysine for inhalation (AZLI)
Aztreonam is a monobactam antibiotic with activity against a broad spectrum of Gram-negative bacteria, including P. aeruginosa, currently approved for intravenous administration. Aztreonam lysine (AZLI) was formulated specifically for inhalational use to improve airway tolerability. The eFlow© electronic nebulizer was designed to efficiently deliver AZLI in a relatively monodispersed particle spectrum (average delivery rate 2.5 ml/min). Phase Ia and Ib pharmacokinetic and safety studies in healthy adults and patients aged >12 years with CF demonstrated that doses up to 225 mg were safe and well tolerated (105). A multi-center phase II randomized, placebo-controlled trial studied safety and efficacy of 2 weeks of AZLI therapy in 105 subjects with CF (age ≥ 13 years), chronic P. aeruginosa infection, and FEV1 ≥ 40% predicted (106). Change in FEV1 did not reach statistical significance, although there was a significant improvement in FEV1 in a subset of patients with moderate lung disease (FEV1 ≤75% of predicted).
On the basis of phase II data, three phase III studies were performed. Patients with CF ≥6 years of age with baseline FEV1 levels 25% to 75% of predicted and P. aeruginosa detected by sputum culture were eligible for enrollment. In the first study (AI-007), 164 patients were randomized to receive placebo or AZLI 75 mg TID for 28 days (107). Results demonstrated a significant improvement in respiratory symptoms as measured by a patient-reported outcome, the CF Questionnaire-Revised (CFQ-R), and improvement in FEV1 (10.3%) in the treatment group compared with the placebo group. A second phase III study (AI-005) was designed to assess the safety and efficacy of a 28-day treatment with AZLI and the ability of AZLI to maintain or improve clinical status following a 28-day course of tobramycin solution for inhalation in patients with CF (108). A total of 211 patients received a 28-day course of tobramycin solution for inhalation, followed by randomization to placebo or AZLI 75 mg BID or TID for 28 days. Results from this study showed significant improvement for AZLI-treated patients compared with placebo-treated controls in time to anti-Pseudomonas antibiotic need, FEV1, and CFQ-R score. Respiratory symptoms were the most commonly reported adverse events, and occurred in similar rates in placebo and treatment groups. A reduction in P. aeruginosa colony-forming units from microbial culture was observed following AZLI treatment in both studies.
An open-label follow-on study (AI-006) for patients who participated in AI-007 or AI-005 evaluated the safety of repeated 28-day alternate-month courses of AZLI over 18 months. 274 patients with CF were enrolled and received AZLI 75 mg BID or TID. There was no evidence of increased adverse events over repeated courses compared to a 28 day course of AZLI. Patients receiving repeated courses of AZLI demonstrated improvements in pulmonary function, CFQ-R score, and decreased P. aeruginosa density in sputum compared with baseline (109). Several additional phase III studies are underway in the European Union and the United States along with an expanded access program for patients with severe lung disease (FEV1 <50% predicted) (110)
4.3.2 Tobramycin inhalation powder (TIP)
TIP is a dry powder that was developed as an alternative to nebulized tobramycin. Potential advantages over the nebulized formulation include an administration time of <3 minutes, an ability to store medication at room temperature, and no need to clean or disinfect the delivery device. A study using scintography demonstrated improved lung deposition with dry powder compared with nebulized medication (111). A phase I, multi-center, single-dose, dose-escalation study compared pharmacokinetics and safety of TIP to tobramycin solution for inhalation (TSI) in 90 CF subjects (112). Four capsules (112 mg) of TIP produced comparable pharmacokinetics to 300 mg TSI. Adverse events were more common in the TIP group and primarily consisted of increased cough and dysgeusia. Most adverse events were mild and self-limited and the authors speculated that if used chronically, subjects may acclimate to the high inhaled powder load. Two phase III studies to determine the efficacy and safety of TIP are now completed and data analysis is ongoing.
4.3.3 Dry Powder Colistin
Colistin is a polymyxin antibiotic with activity against P. aeruginosa and low rates of resistance (113). Nebulized colistin sulphomethate has been used since the 1980’s as inhaled therapy for CF patients chronically and intermittently colonized with P. aeruginosa (114;115). In order to make delivery of the medication more efficient and less time-consuming for the patient, dry powder formulations of colistin sulfate and colistin sulphomethate have been studied (116–118). (118) A feasibility study of 25 mg dry powder colistin sulfate in healthy volunteers and CF patients found moderate to severe cough and decrease in lung functions in some CF subjects after dry powder inhalation (119). Pharmacokinetic data suggested that the dry powder dose could be decreased to 10 mg while maintaining equivalence to 160 mg nebulized colistin sulphomethate, possibly reducing adverse events. More recently, Westerman et al. performed a single dose pilot study comparing 25 mg dry powder colistin sulphomethate to 158 mg nebulized colistin in 10 CF subjects (120). The dry powder colistin was well tolerated with only mild cough in some subjects and no decrease in lung functions. However, the dry powder colistin had a lower AUC, maximum concentration (Cm) and time to maximum concentration (Tm) compared to nebulized colistin. The authors speculated that optimization of the particle size and inspiratory flow rate may improve pulmonary deposition. A multi-center phase III trial of dry powder colistin is currently underway in Europe.
4.4 CFTR protein rescue
CFTR mutations may be categorized into 5 classes based on the mechanism of abnormal function or trafficking (121). Class I mutations are nonsense mutations that result from premature stop codons that terminate ribosomal translation of mRNA into protein. Class II mutations impair protein processing, resulting in CFTR degradation and lack of transport to the cell membrane. Class III mutations disrupt functioning of the regulatory domain. CFTR is transported to the cell membrane, but remains closed with no chloride transport. Class IV mutations result in decreased chloride transport through cell membrane proteins, and Class V mutations are splicing mutations that result in variable amounts of functioning protein (122). Therapies targeting the basic defect of CFTR protein function or processing are now in development, and ultimately may allow genotype-specific treatment. These therapies can be divided into 3 general types: (i) molecules that read through premature stop codons (class I), (ii) correctors that facilitate movement of CFTR to the cell membrane (class II) and (iii) potentiators that increase chloride conductance through CFTR (class III and possibly other classes). Correctors and potentiators may have an additive effect, and both mechanisms may be needed to achieve adequate levels of CFTR function for some mutations (123). Small-molecule CFTR potentiators and correctors are currently in early clinical trials. One phase II trials of PTC-124, a small molecule that induces ribosomes to read through premature stop codons, was recently completed which demonstrated a statistically significant difference in nasal potential difference following treatment compared to before treatment (124).
4.5 Mucociliary clearance
CFTR dysfunction impedes chloride ion transport into the airway lumen, which leads to dehydration of the airway surface liquid lining the airway epithelium. Increased transepithelial sodium absorption caused by loss of CFTR's regulatory effect compounds this factor. Cilia are unable to function properly, and mucociliary clearance is reduced (4). In addition, neutrophilic inflammation leads to high levels of DNA released from degenerating leukocytes and increased mucus viscosity (6). Current therapies include hypertonic saline, which may improve airway surface hydration (125), and dornase alfa, which breaks down DNA, and decreases mucus viscosity (126). Two new treatments currently in clinical trials, denufosol and inhaled mannitol, are discussed below.
4.5.1. Denufosol
Denufosol tetrasodium is a P2Y2 receptor agonist that stimulates chloride secretion by activating an alternative chloride channel in airway epithelium. Denufosol also inhibits sodium absorption, enhances mucin secretion from goblet cells, increases ciliary beat frequency, and stimulates surfactant release from type II alveolar cells (127). The result is increased hydration of the airway surface liquid, improved ciliary function, and more rapid clearance of airway mucus. Phase I and early phase II studies demonstrated safety and tolerability of nebulized denufosol in healthy nonsmokers, smokers, and patients with CF, although some increase in adverse events and intolerability was noted among CF subjects with lower lung function (<75%) (128). A phase II placebo-controlled trial evaluated the safety and efficacy of denufosol in 89 patients with CF and mild lung disease (FEV1 ≥75% of predicted) (129). Patients were randomized to received placebo or 1 of 3 doses of denufosol (20, 40, or 60 mg) 3 times daily for 28 days. Denufosol was found to be well tolerated at all doses. The most common adverse event was cough, which occurred in similar numbers of control and treated patients. Patients who received denufosol demonstrated a significant increase from baseline in forced vital capacity (FVC), FEV1, and forced mid-expiratory flow rate (FEF25–75) after 28 days compared with controls. Two, randomized, placebo-controlled phase III studies are now under way to evaluate denufosol 60 mg TID in 350 patients with CF and mild lung disease.
4.5.2. Inhaled mannitol
Inhaled mannitol is an osmotic agent delivered as a dry powder that may improve mucociliary clearance by drawing water into the airways. Daviskas et al demonstrated improved lung clearance at 2 and 24 hours after a single dose of inhaled mannitol in patients with bronchiectasis (130). A 2-week, randomized, double-blind, placebo-controlled crossover study in 39 patients with CF showed a significant improvement in FEV1 (change in FEV1, 7.0% vs. 0.3%) and CFQ-R Respiratory Symptoms in patients given mannitol compared with those who received placebo (131). No significant adverse events were reported. Phase III, multinational randomized trials are ongoing to determine the safety and efficacy of inhaled mannitol in patients with CF.
5. CF Lung Infections: Research Opportunities
Many research opportunities in CF care are currently available. Table 3 highlights some of these emerging issues. Below we discuss in more detail issues specific to CF microbiology research.
Table 3.
Research questions in cystic fibrosis (CF)
| How can we use early diagnosis through newborn screening to improve outcomes? |
| What are the appropriate outcome measures in infants and toddlers? |
| What is the contribution of pulmonary exacerbation to lung function decline? |
| What causes the gender difference in outcomes? |
| What is the clinical impact of polymicrobial infection in CF lung disease? |
| What is the optimal management of methicillin-resistant Staphylococcus aureus infection in patients with CF? |
| Are there biochemical markers of lung injury/damage and destruction? |
| How can we integrate new therapies into existing management strategies? |
5.1. Polymicrobial infections in CF
The most common bacterial pathogens associated with chronic lung infections in CF are P. aeruginosa, S. aureus, and Haemophilus influenzae (132). Burkholderia cepacia complex, now known to include ≥17 distinct species, are found in a small percentage of patients with CF, and these pathogens can have devastating effects on mortality and options for lung transplantation (133). Stenotrophomonas maltophilia and Achromobacter xylosoxidans are emerging Gram-negative bacterial pathogens that may have a deleterious effect on the CF-compromised lung (134;135). Careful microbial culture of airway samples detects these pathogens and can guide antimicrobial therapy. In addition to routinely cultured bacteria, other potential pathogens include fungi, viruses, mixed anaerobic bacteria, and nontuberculous mycobacterium (136–138). The most common fungus detected in CF airway samples, Aspergillus fumigatus, causes allergic bronchopulmonary Aspergillosis (ABPA) in 1% to 15% of patients with CF (139). Patients with A. fumigatus without ABPA may also develop a fungal bronchitis responsive to antifungal therapy (140).
Increasingly research demonstrates that CF lung infection is polymicrobial (10). Patients frequently harbor more than one pathogen. One prospective study of sputum cultures from 595 patients with CF found 2.9 bacterial pathogens per sample among 1753 specimens (132). Fungi and nontuberculous mycobacterium are also frequently isolated and may interact with bacterial pathogens. Culture-independent molecular techniques are now available to identify difficult-to-culture bacteria (141). Using a molecular approach, Harris et al examined bronchoalveolar lavage (BAL) samples from 42 patients, including 28 with CF (142). BAL from 13 patients with CF contained bacteria not routinely assessed by culture, including anaerobes. Future studies are needed to improve our understanding of microbial communities, virulence, and interactions in CF lung disease.
5.2. Methicillin-resistant Staphylococcus aureus (MRSA)
The prevalence of MRSA has increased over the past decade from 0.1% in 1995 to 17.2% in 2005 (95). The impact of MRSA infection on CF lung disease is still debated (143). Recently, two large epidemiologic studies sought to determine the impact of MRSA infection on lung function decline. In a study of data collected for the ESCF, researchers examined data from 5,090 patients with CF over two years (144). During that time, 12% of patients had an incident MRSA infection. Patients with MRSA had lower lung function and more rapid decline in lung function than patients without MRSA. However, the rate of lung function decline compared before and after MRSA detection did not differ. Therefore, although patients with MRSA had lower lung function, a causative effect from MRSA was not demonstrated. The second study used CFF Patient Registry data to study 17,357 patients with CF between 1996–2005 (average follow-up, 5.3 years) (145). This study found a significant increase in rate of lung function decline in patients with persistent MRSA infection (defined as ≥3 MRSA-positive cultures) compared with those without MRSA. Contrary to the ESCF data, this study found that the rate of lung function decline increased after MRSA detection compared to the rate of decline before detection. The longer study period, larger group and use of “persistent” MRSA infection definition may explain some of the difference in findings between studies. Further research on the impact and optimal management of MRSA in CF is needed.
5.3. Pseudomonas aeruginosa serology
As treatment for P. aeruginosa eradication becomes standard care, outcome measures to define infection and eradication states for this pathogen are needed. Eradication strategies rely on sensitive and accurate detection of P. aeruginosa infections, for both determining who needs treatment and assessing efficacy of therapy. Serum antibodies may be helpful, especially if they are elevated prior to a positive culture and decrease with successful eradication. However, while some studies have found an increase in serum antibodies before first detection by airway culture, other studies have not confirmed this finding (146–148). Ratjen et al recently determined cutoff values for three anti-pseudomonal antibodies, alkaline protease, exotoxin A, and elastase by studying serum samples from 375 patients with CF and known P. aeruginosa status (negative, chronic, or intermittently infected) (149). In a second cohort of 56 patients with CF who had new-onset infection undergoing eradication therapy, it was found that 42% had positive antibodies at the time of initial positive culture. Following eradication treatment, patients who were successfully treated had a significant decrease in mean antibody titers, while those who failed eradication showed an increase in titer levels. Thus, although serum antibodies had a low sensitivity for detecting early infection with P. aeruginosa, they may be useful for monitoring response to eradication therapy. Future studies are planned, using the combination of alkaline protease, exotoxin A, and elastase antibodies as part on ongoing eradication intervention trials. Likely a combination of culture, molecular diagnostics, and serology may provide the best sensitivity for early P. aeruginosa detection (150).
6. Conclusion
Although CF remains a life-shortening condition associated with significant morbidity, recent advances and new treatments offer great hope and promise. Expanded newborn screening programs and improvements in early disease management may delay disease progression in future cohorts; an increasing number of adults with CF are living with more mild disease and are engaged in normal work and family roles. Treatment of chronic lung infection will likely improve with new antimicrobials, better delivery mechanisms for existing antibiotics, and better microbial detection. Patient-reported outcome measures provide data on the effects of new medications, as well as an increased understanding of how CF affects daily functioning.
Many barriers still remain for patients, clinicians, and researchers. Poor adherence is a significant contributor to treatment failures and will require implementation of practical-, clinic-and family-based interventions. Quality improvement initiatives are expanding to improve outcomes with currently available treatments. Continued research is needed to identify optimal outcome measures for clinical trials.
Acknowledgements
MGD Development Group, Basking Ridge, NJ assisted with editing.
Funding: 1 U01 HL081335-01, Cystic Fibrosis Foundation. Independent grant from Gilead Sciences, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript arose from proceedings at the following meeting:
Cystic Fibrosis Expert Meeting
Supported by an independent grant from Gilead Sciences, Inc.
October 3, 2008
Washington, D.C.
Conflict of interest statements
Edith Zemanick has nothing to disclose relevant to this manuscript.
J. Kirk Harris has nothing to disclose relevant to this manuscript.
Steven Conway is a member of the advisory board for Phillips Medizinsysteme Böblingen (Germany) and has recently served on an advisory board for Gilead Sciences, Inc. He has previously served on advisory boards for Roche and Novartis Pharmaceuticals.
Michael W. Konstan has served as a paid consultant to Axcan Pharma, Digestive Care Inc, Genentech, Gilead Sciences, Inc., Novartis Pharmaceuticals, PTC Therpaeutics, Solvay, Transave, Inc., and Vertex Pharmaceuticals, Inc.
Bruce Marshall has nothing to disclose relevant to this manuscript.
Alexandra L. Quittner has received consulting income from Gilead Sciences, Inc., Novartis Pharmaceuticals, Vertex Pharmaceuticals, Inc., and Transave, Inc. She serves on the North American Advisory Group for Genentech (NASAG) and has an investigator-initiated grant from Novartis Pharmaceuticals.
George Retsch-Bogart received support as a site principal investigator to conduct clinical trials in the Cystic Fibrosis Foundation Therapeutics Development Network from Gilead Sciences, Inc. and Inspire Pharmaceuticals.
Lisa Saiman has served on advisory boards for Aridis Pharmaceuticals, Gilead Sciences, Inc., Novartis Pharmaceuticals, and Transave, Inc. She has received research funding from Bayer and Chiesi Pharmaceuticals and has served on the Cystic Fibrosis Foundations Data Safety Monitoring Board.
Frank Accurso has no personal conflict of interest. Dr. Accurso’s institution received funding for clinical trials for agents mentioned in this manuscript from Gilead Sciences, Inc., Vertex Pharmaceuticals, Inspire Pharmaceuticals and PTC Therapeutics.
Contributor Information
Edith T. Zemanick, Department of Pediatrics, University of Colorado Denver, 13123 E. 16th Avenue, Aurora, Colorado 80045.
J. Kirk Harris, Department of Pediatrics, University of Colorado Denver, 13123 E. 16th Avenue, Aurora, Colorado 80045.
Steven Conway, St. James University Hospital, Beckett Street, Leeds, UK LS97TF.
Michael W. Konstan, Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children’s Hospital, 11100 Euclid Avenue, Cleveland, Ohio 44108.
Bruce Marshall, Cystic Fibrosis Foundation, 6931 Arlington Drive, Bethesda, Maryland 20814.
Alexandra L. Quittner, Department of Psychology and Pediatrics, University of Miami, 5665 Ponce de Leon Boulevard, Miami, Florida 33146.
George Retsch-Bogart, Department of Pediatrics, University of North Carolina, Chapel Hill, 5126 Bioinformatics Building, CB# 7217, 130 Mason Farm Road, Chapel Hill, North Carolina 21599-7217.
Lisa Saiman, Department of Pediatrics, Columbia University, 350 W. 168th Street, New York, New York 10032.
Frank J. Accurso, Department of Pediatrics, University of Colorado Denver, 13123 E. 16th Avenue, Aurora, Colorado 80045.
Reference List
- 1.World Health Organization and Cystic Fibrosis Worldwide. The molecular genetic epidemiology of cystic fibrosis. 2002 Report of a joint meeting of WHO/ECFTN/ICF(M)A/ ECFS. [Google Scholar]
- 2.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 3.Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 4.Puchelle E, Bajolet O, Abely M. Airway mucus in cystic fibrosis. Paediatr Respir Rev. 2002;3(2):115–119. doi: 10.1016/s1526-0550(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 5.Quinton PM. Too much salt, too little soda: cystic fibrosis. Sheng Li Xue Bao. 2007;59(4):397–415. [PubMed] [Google Scholar]
- 6.Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros. 2005;(4 Suppl 2):15–23. doi: 10.1016/j.jcf.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132(5):1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 8.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 10.Sibley CD, Rabin H, Surette MG. Cystic fibrosis: a polymicrobial infectious disease. Future Microbiol. 2006;1:53–61. doi: 10.2217/17460913.1.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Kerem E. Pharmacological induction of CFTR function in patients with cystic fibrosis: mutation-specific therapy. Pediatr Pulmonol. 2005;40(3):183–196. doi: 10.1002/ppul.20200. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):370–377. doi: 10.1513/pats.200703-040BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss CH, Quittner AL. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):378–386. doi: 10.1513/pats.200703-039BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld M. An overview of endpoints for cystic fibrosis clinical trials: one size does not fit all. Proc Am Thorac Soc. 2007;4(4):299–301. doi: 10.1513/pats.200611-178HT. [DOI] [PubMed] [Google Scholar]
- 16.Ziaian T, Sawyer MG, Reynolds KE, Carbone JA, Clark JJ, Baghurst PA, et al. Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. J Paediatr Child Health. 2006;42(10):596–600. doi: 10.1111/j.1440-1754.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 17.Cystic Fibrosis Foundation Patient Registry. Annual data report to the center directors. Bethesda, Maryland: 2007. [Google Scholar]
- 18.Stern M, Wiedemann B, Wenzlaff P. From registry to quality management: the German Cystic Fibrosis Quality Assessment project 1995 2006. Eur Respir J. 2008;31(1):29–35. doi: 10.1183/09031936.00056507. [DOI] [PubMed] [Google Scholar]
- 19.Bellis G, Cazes MH, Parant A, Gaimard M, Travers C, Le Roux E, et al. Cystic fibrosis mortality trends in France. J Cyst Fibros. 2007;6(3):179–186. doi: 10.1016/j.jcf.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 21.Pedreira CC, Robert RG, Dalton V, Oliver MR, Carlin JB, Robinson P, et al. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39(3):276–280. doi: 10.1002/ppul.20162. [DOI] [PubMed] [Google Scholar]
- 22.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137(3):374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 23.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax. 2002;57(7):596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstan MW, Morgan WJ, Pasta DJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–139. doi: 10.1016/j.jpeds.2007.03.006. 139. [DOI] [PubMed] [Google Scholar]
- 25.Corey M. Power considerations for studies of lung function in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):334–337. doi: 10.1513/pats.200611-176HT. [DOI] [PubMed] [Google Scholar]
- 26.McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr. 2008;153(6):752–757. doi: 10.1016/j.jpeds.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Wagener J, Rasouliyan L, Pasta DJ, Mabie J, Morgan W, Konstan MW. Practice patterns for treating respiratory exacerbations in cystic fibrosis. Pediatric Pulmonology. 2008;43 Supplement 31:359. [Google Scholar]
- 28.Wilcken B, Towns SJ, Mellis CM. Diagnostic delay in cystic fibrosis: lessons from newborn screening. Arch Dis Child. 1983;58(11):863–866. doi: 10.1136/adc.58.11.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagener JS, Sontag MK, Sagel SD, Accurso FJ. Update on newborn screening for cystic fibrosis. Curr Opin Pulm Med. 2004;10(6):500–504. doi: 10.1097/01.mcp.0000138993.69390.ef. [DOI] [PubMed] [Google Scholar]
- 30.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellani C, Southern KW, Brownlee K, Dankert RJ, Duff A, Farrell M, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8(3):153–173. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6(1):57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Munck A, Dhondt JL, Sahler C, Roussey M. Implementation of the French nationwide cystic fibrosis newborn screening program. J Pediatr. 2008;153(2):228–233. doi: 10.1016/j.jpeds.2008.02.028. 233. [DOI] [PubMed] [Google Scholar]
- 34.Farrell MH, Farrell PM. Newborn screening for cystic fibrosis: ensuring more good than harm. J Pediatr. 2003;143(6):707–712. doi: 10.1016/j.jpeds.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Farrell PM, Lai HJ, Li Z, Kosorok MR, Laxova A, Green CG, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005;147(3 Suppl):S30–S36. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Lai HJ, Cheng Y, Farrell PM. The survival advantage of patients with cystic fibrosis diagnosed through neonatal screening: evidence from the United States Cystic Fibrosis Foundation registry data. J Pediatr. 2005;147(3 Suppl):S57–S63. doi: 10.1016/j.jpeds.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM. Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr. 2006;149(3):362–366. doi: 10.1016/j.jpeds.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 38.Dankert-Roelse JE, Merelle ME. Review of outcomes of neonatal screening for cystic fibrosis versus non-screening in Europe. J Pediatr. 2005;147(3 Suppl):S15–S20. doi: 10.1016/j.jpeds.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Quittner AL, Barker DH, Marciel KK, Grimley ME. Cystic fibrosis: a model for drug discovery and patient care. In: Roberts M, editor. Handbook of Pediatric Psychology. fourth edition. New York: Guilfor Press; 2009. [Google Scholar]
- 40.Beringer PM, Appleman MD. Unusual respiratory bacterial flora in cystic fibrosis: microbiologic and clinical features. Curr Opin Pulm Med. 2000;6(6):545–550. doi: 10.1097/00063198-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol. 2005;43(7):3467–3470. doi: 10.1128/JCM.43.7.3467-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLambo KE, Ievers-Landis CE, Drotar D, Quittner AL. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. J Pediatr Psychol. 2004;29(5):343–353. doi: 10.1093/jpepsy/jsh038. [DOI] [PubMed] [Google Scholar]
- 43.Modi AC, Marciel KK, Slater SK, Drotar D, Quittner AL. The influence of parental supervision on medical adherence in adolescents with cystic fibrosis: developemental skills from early to late adolecence. Children's Health Care. 2008;37:78–92. [Google Scholar]
- 44.Quittner AL, Barker DH, Snell C, Grimley ME, Marciel K, Cruz I. Prevalence and impact of depression in cystic fibrosis. Curr Opin Pulm Med. 2008;14(6):582–588. doi: 10.1097/MCP.0b013e3283121cf1. [DOI] [PubMed] [Google Scholar]
- 45.Holzer FJ, Schnall R, Landau LI. The effect of a home exercise programme in children with cystic fibrosis and asthma. Aust Paediatr J. 1984;20(4):297–301. doi: 10.1111/j.1440-1754.1984.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 46.Krauth C, Jalilvand N, Welte T, Busse R. Cystic fibrosis: cost of illness and considerations for the economic evaluation of potential therapies. Pharmacoeconomics. 2003;21(14):1001–1024. doi: 10.2165/00019053-200321140-00002. [DOI] [PubMed] [Google Scholar]
- 47.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J Cyst Fibros. 2008 doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quittner AL, Drotar D, Levers-Landis C, Seidner D, Slocum N, Jacobsen J. In: Adherence to medical treatments in adolescents with cystic fibrosis: the development and evaluation of family-based interventions. Drotar D, editor. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 383–407. [Google Scholar]
- 49.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2008;33(9):916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruz I, Marciel KK, Cohen I, Shahzeidi S, Quittner AL. Barriers to nutritional adherence and family-based solutions. Pediatric Pulmonology. 2008;31 Supplement:423–424. [Google Scholar]
- 51.McDonald L, Quittner AL, Grimley ME, Botteri M, Barker DH. Effects of a clinic-based intervention on caregiving stress for parents of children with CF. Journal of Cystic Fibrosis. 2007;6 Supplement:S75. [Google Scholar]
- 52.Nathanson I, Ramirez-Garnica G, Wiltrout SA. Decreased attendance at cystic fibrosis centers by children covered by managed care insurance. Am J Public Health. 2005;95(11):1958–1963. doi: 10.2105/AJPH.2004.059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lameire N, Joffe P, Wiedemann M. Healthcare systems--an international review: an overview. Nephrol Dial Transplant. 1999;(14 Suppl 6):3–9. doi: 10.1093/ndt/14.suppl_6.3. [DOI] [PubMed] [Google Scholar]
- 54.Havermans T, Colpaert K, Dupont LJ. Quality of life in patients with Cystic Fibrosis: Association with anxiety and depression. J Cyst Fibros. 2008;7(6):581–584. doi: 10.1016/j.jcf.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Quittner AL, Barker DH, Getter D, Butt S, Gondor M. Effects of maternal depression on electronically monitored enzyme adherence and changes in weight for children with CF. Journal of Cystic Fibrosis. 2007;6 Supplement 1:s78. [Google Scholar]
- 56.Kerem E, Conway S, Elborn S, Heijerman H. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4(1):7–26. doi: 10.1016/j.jcf.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Cystic Fibrosis Foundation guidelines for patient services, evaluation, and monitoring in cystic fibrosis centers. The Cystic Fibrosis Foundation Center Committee and Guidelines Subcommittee. Am J Dis Child. 1990;144(12):1311–1312. doi: 10.1001/archpedi.1990.02150360033014. [DOI] [PubMed] [Google Scholar]
- 58.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5(3):177–185. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Dries DJ, McGonigal MD, Malian MS, Bor BJ, Sullivan C. Protocol-driven ventilator weaning reduces use of mechanical ventilation, rate of early reintubation, and ventilator-associated pneumonia. J Trauma. 2004;56(5):943–951. doi: 10.1097/01.ta.0000124462.61495.45. [DOI] [PubMed] [Google Scholar]
- 60.Kelly CS, Andersen CL, Pestian JP, Wenger AD, Finch AB, Strope GL, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509–516. doi: 10.1016/S1081-1206(10)62514-8. [DOI] [PubMed] [Google Scholar]
- 61.McFadden ER, Jr, Elsanadi N, Dixon L, Takacs M, Deal EC, Boyd KK, et al. Protocol therapy for acute asthma: therapeutic benefits and cost savings. Am J Med. 1995;99(6):651–661. doi: 10.1016/s0002-9343(99)80253-8. [DOI] [PubMed] [Google Scholar]
- 62.Wazeka A, Valacer DJ, Cooper M, Caplan DW, DiMaio M. Impact of a pediatric asthma clinical pathway on hospital cost and length of stay. Pediatr Pulmonol. 2001;32(3):211–216. doi: 10.1002/ppul.1110. [DOI] [PubMed] [Google Scholar]
- 63.Holcomb BW, Wheeler AP, Ely EW. New ways to reduce unnecessary variation and improve outcomes in the intensive care unit. Curr Opin Crit Care. 2001;7(4):304–311. doi: 10.1097/00075198-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Browne GJ, Giles H, McCaskill ME, Fasher BJ, Lam LT. The benefits of using clinical pathways for managing acute paediatric illness in an emergency department. J Qual Clin Pract. 2001;21(3):50–55. doi: 10.1046/j.1440-1762.2001.00405.x. [DOI] [PubMed] [Google Scholar]
- 65.Al Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39(1):15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 66.Smyth A, Lewis S, Bertenshaw C, Choonara I, McGaw J, Watson A. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2008;63(6):532–535. doi: 10.1136/thx.2007.088757. [DOI] [PubMed] [Google Scholar]
- 67.Smyth AR, Tan KH. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD002009.pub2. CD002009. [DOI] [PubMed] [Google Scholar]
- 68.Coulthard KP, Nilsson C, Smith C, Gibki S, Martin J. Hearing loss in patients with cystic fibrosis exposed to tobramycin. Pediatric Pulmonology. 2008;43 Supplement 31:346. [Google Scholar]
- 69.Handlesman JA, King W, Nasr SZ. Vestibular ototoxicity in cystic fibrosis. Pediatric Pulmonology. 2008;43 Supplement 31:414. [Google Scholar]
- 70.Ramsey BW. Outcome measures for development of new therapies in cystic fibrosis: are we making progress and what are the next steps? Proc Am Thorac Soc. 2007;4(4):367–369. doi: 10.1513/pats.200703-038BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- 72.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):418–430. doi: 10.1513/pats.200703-041BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Jong PA, Lindblad A, Rubin L, Hop WC, de Jongste JC, Brink M, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2006;61(1):80–85. doi: 10.1136/thx.2005.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4(4):406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009 doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax. 2000;55(11):946–954. doi: 10.1136/thorax.55.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldbeck L, Schmitz TG, Henrich G, Herschbach P. Questions on life satisfaction for adolescents and adults with cystic fibrosis: development of a disease-specific questionnaire. Chest. 2003;123(1):42–48. doi: 10.1378/chest.123.1.42. [DOI] [PubMed] [Google Scholar]
- 78.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 79.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 80.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 81.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: what gets in the way? J Pediatr Psychol. 2006;31(8):846–858. doi: 10.1093/jpepsy/jsj096. [DOI] [PubMed] [Google Scholar]
- 82.Modi AC, Marciel KK, Slater SK, Drotar D, Quittner AL. The influence of parental supervision on medical adherence in adolescents with cystic fibrosis: developemental skills from early to late adolecence. Children's Health Care. 2008;37:78–92. [Google Scholar]
- 83.Lee TW. Eradication of early Pseudomonas infection in cystic fibrosis. Chron Respir Dis. 2009;6(2):99–107. doi: 10.1177/1479972309104661. [DOI] [PubMed] [Google Scholar]
- 84.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12(3):158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 85.Ratjen F. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis. Curr Opin Pulm Med. 2006;12(6):428–432. doi: 10.1097/01.mcp.0000245712.51514.a1. [DOI] [PubMed] [Google Scholar]
- 86.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008;7(6):523–530. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Treggiari MM, Rosenfeld M, Retsch-Bogart G, Gibson R, Ramsey B. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr Pulmonol. 2007;42(9):751–756. doi: 10.1002/ppul.20665. [DOI] [PubMed] [Google Scholar]
- 88.Gibson RL, Emerson J, Burns JL, Mayer-Hamblett N, McNamara S, Accurso FJ, et al. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol. 2007;42(7):610–623. doi: 10.1002/ppul.20625. [DOI] [PubMed] [Google Scholar]
- 89.Douglas TA, Brennan S, Gard S, Berry L, Gangell C, Stick SM, et al. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur Respir J. 2009;33(2):305–311. doi: 10.1183/09031936.00043108. [DOI] [PubMed] [Google Scholar]
- 90.Lahiri T. Approaches to the treatment of initial Pseudomonas aeruginosa infection in children who have cystic fibrosis. Clin Chest Med. 2007;28(2):307–318. doi: 10.1016/j.ccm.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J. 2005;26(3):458–461. doi: 10.1183/09031936.05.00009605. [DOI] [PubMed] [Google Scholar]
- 92.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, et al. Early Anti-Pseudomonal Acquisition in Young Patients with Cystic Fibrosis: Rationale and Design of the EPIC Clinical Trial and Observational Study. Contemp Clin Trials. 2009 doi: 10.1016/j.cct.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ratjen F, Munck A, Kho P. Short and long-term efficacy of inhaled tobramycin in early P. aeruginosa infection: The ELITE Study. Pediatric Pulmonology. 2008;43 Supplement 31:319–320. [Google Scholar]
- 94.Ho SA, Lee TW, Denton M, Conway SP, Brownlee KG. Regimens for eradicating early Pseudomonas aeruginosa infection in children do not promote antibiotic resistance in this organism. J Cyst Fibros. 2009;8(1):43–46. doi: 10.1016/j.jcf.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory Microbiology of Patients With Cystic Fibrosis in the United States, 1995–2005. Chest. 2009 doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 96.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38(4):314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 97.Dinwiddie R. Anti-inflammatory therapy in cystic fibrosis. J Cyst Fibros. 2005;(4 Suppl 2):45–48. doi: 10.1016/j.jcf.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trial. Thorax. 2006;61(10):895–902. doi: 10.1136/thx.2005.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Report of the UK Cystic Fibrosis Antibiotic Working Group. 3rd Edition 2009. May 1, Antibiotic treatment for CF. [Google Scholar]
- 100.Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 2002;20(3):658–664. doi: 10.1183/09031936.02.00248102. [DOI] [PubMed] [Google Scholar]
- 101.Adeboyeku D, Scott S, Hodson ME. Open follow-up study of tobramycin nebuliser solution and colistin in patients with cystic fibrosis. J Cyst Fibros. 2006;5(4):261–263. doi: 10.1016/j.jcf.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Hoffmann IM, Rubin BK, Rubin BK, Iskandar SS, Schechter MS, Nagaraj SK, Bitzan MM. Acute renal failure in cystic fibrosis: association with inhaled tobramycin therapy. Pediatr Pulmonol. 2002;34(5):375–377. doi: 10.1002/ppul.10185. [DOI] [PubMed] [Google Scholar]
- 103.Edson RS, Brey RH, McDonald TJ, McDonald TJ, Thibert JM, McCarthy JT. Vestibular toxicity due to inhaled tobramycin in a patient with renal insufficiency. Mayo Clin Proc. 2004;79(9):1185–1191. doi: 10.4065/79.9.1185. [DOI] [PubMed] [Google Scholar]
- 104.Saiman L, Mayer-Hamblett N, Campbell P, Marshall BC. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(8):1008–1012. doi: 10.1164/rccm.200502-218OC. [DOI] [PubMed] [Google Scholar]
- 105.Gibson RL, Retsch-Bogart GZ, Milla C, Oermann C, Pilewski J, Daines C, et al. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41(7):656–665. doi: 10.1002/ppul.20429. [DOI] [PubMed] [Google Scholar]
- 106.Retsch-Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, et al. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol. 2008;43(1):47–58. doi: 10.1002/ppul.20736. [DOI] [PubMed] [Google Scholar]
- 107.Retsch-Bogart GZ, Quittner AL, Gibson RL, Oermann CM, McCoy KS, Montgomery AB, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135(5):1223–1232. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(9):921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oermann C, McCoy K, Gibson R, Retsch-Bogart G, Montgomery AB. Effect of multiple courses of aztreonam lysine for inhalation (AZLI) on FEV1 and weight in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa (PA): Analysis of 18-month data from CP-AI-006. Journal of Cystic Fibrosis. 2009;8:S28. [Google Scholar]
- 110.Cystic Fibrosis Foundation Expanded Access Program. 2009 Feb 13; [Google Scholar]
- 111.Newhouse MT, Duddu SP, Walter YH, Tarara TE, Clark AR, et al. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest. 2003;124(1):360–366. doi: 10.1378/chest.124.1.360. [DOI] [PubMed] [Google Scholar]
- 112.Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol. 2007;42(4):307–313. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 113.Littlewood JM, Lambert PA, Hoiby N, Elborn JS, Conway SP, et al. A ten year review of colomycin. Respir Med. 2000;94(7):632–640. doi: 10.1053/rmed.2000.0834. [DOI] [PubMed] [Google Scholar]
- 114.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros. 2005;(4 Suppl 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 115.Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338(8769):725–726. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 116.Westerman EM, De Boer AH, LeBrun PP, Touw DJ, Roldaan AC, Frijlink HW, et al. Dry powder inhalation of colistin in cystic fibrosis patients: a single dose pilot study. J Cyst Fibros. 2007;6(4):284–292. doi: 10.1016/j.jcf.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 117.Westerman EM, De Boer AH, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. Dry powder inhalation of colistin sulphomethate in healthy volunteers: A pilot study. Int J Pharm. 2007;335(1–2):41–45. doi: 10.1016/j.ijpharm.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 118.De Boer AH, Le Brun PP, van der Woude HG, Hagedoorn P, Heijerman HG, Frijlink HW. Dry powder inhalation of antibiotics in cystic fibrosis therapy, part 1: development of a powder formulation with colistin sulfate for a special test inhaler with an air classifier as de-agglomeration principle. Eur J Pharm Biopharm. 2002;54(1):17–24. doi: 10.1016/s0939-6411(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 119.Le Brun PP, De Boer AH, Mannes GP, de Fraiture DM, Brimicombe RW, Touw DJ, et al. Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2. Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur J Pharm Biopharm. 2002;54(1):25–32. doi: 10.1016/s0939-6411(02)00044-9. [DOI] [PubMed] [Google Scholar]
- 120.Westerman EM, Heijerman HG, Frijlink HW. Dry powder inhalation versus wet nebulisation delivery of antibiotics in cystic fibrosis patients. Expert Opin Drug Deliv. 2007;4(2):91–94. doi: 10.1517/17425247.4.2.91. [DOI] [PubMed] [Google Scholar]
- 121.Kerem E. Mutation specific therapy in CF. Paediatr Respir Rev. 2006;(7 Suppl 1):S166–S169. doi: 10.1016/j.prrv.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 122.McAuley DF, Elborn JS. Cystic fibrosis: basic science. Paediatr Respir Rev. 2000;1(2):93–100. doi: 10.1053/prrv.2000.0029. [DOI] [PubMed] [Google Scholar]
- 123.Rubenstein RC. Targeted therapy for cystic fibrosis: cystic fibrosis transmembrane conductance regulator mutation-specific pharmacologic strategies. Mol Diagn Ther. 2006;10(5):293–301. doi: 10.1007/BF03256204. [DOI] [PubMed] [Google Scholar]
- 124.Kerem E, Hirawat S, Armoni S, Yaakov Y, Shoseyov D, Cohen M, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372(9640):719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 125.Tarran R, Donaldson S, Boucher RC. Rationale for hypertonic saline therapy for cystic fibrosis lung disease. Semin Respir Crit Care Med. 2007;28(3):295–302. doi: 10.1055/s-2007-981650. [DOI] [PubMed] [Google Scholar]
- 126.Shah PL, Scott SF, Knight RA, Marriott C, Ranasinha C, Hodson ME. In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax. 1996;51(2):119–125. doi: 10.1136/thx.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kellerman D, Rossi MA, Engels J, Schaberg A, Gorden J, Smiley L. Denufosol: a review of studies with inhaled P2Y(2) agonists that led to Phase 3. Pulm Pharmacol Ther. 2008;21(4):600–607. doi: 10.1016/j.pupt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Deterding R, Retsch-Bogart G, Milgram L, Gibson R, Daines C, Zeitlin PL, et al. Safety and tolerability of denufosol tetrasodium inhalation solution, a novel P2Y2 receptor agonist: results of a phase 1/phase 2 multicenter study in mild to moderate cystic fibrosis. Pediatr Pulmonol. 2005;39(4):339–348. doi: 10.1002/ppul.20192. [DOI] [PubMed] [Google Scholar]
- 129.Deterding RR, Lavange LM, Engels JM, Mathews DW, Coquillette SJ, Brody AS, et al. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am J Respir Crit Care Med. 2007;176(4):362–369. doi: 10.1164/rccm.200608-1238OC. [DOI] [PubMed] [Google Scholar]
- 130.Daviskas E, Anderson SD, Eberl S, Chan HK, Young IH. The 24-h effect of mannitol on the clearance of mucus in patients with bronchiectasis. Chest. 2001;119(2):414–421. doi: 10.1378/chest.119.2.414. [DOI] [PubMed] [Google Scholar]
- 131.Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest. 2008;133(6):1388–1396. doi: 10.1378/chest.07-2294. [DOI] [PubMed] [Google Scholar]
- 132.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27(1):158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 133.Spicuzza L, Vitaliti G, Di Dio G, Leonardi S, La Rosa M. Emerging pathogens in cystic fibrosis: ten years of follow-up in a cohort of patients. Eur J Clin Microbiol Infect Dis. 2009;28(2):191–195. doi: 10.1007/s10096-008-0605-4. [DOI] [PubMed] [Google Scholar]
- 134.Goss CH, Mayer-Hamblett N, Aitken ML, Rubenfeld GD, Ramsey BW. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax. 2004;59(11):955–959. doi: 10.1136/thx.2003.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros. 2007;6(1):75–78. doi: 10.1016/j.jcf.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 136.Tai A, Ranganath S. Anaerobic bacterial infection in patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;178(9):994. doi: 10.1164/ajrccm.178.9.994. [DOI] [PubMed] [Google Scholar]
- 137.Razvi S, Saiman L. Nontuberculous mycobacteria in cystic fibrosis. Pediatr Infect Dis J. 2007;26(3):263–264. doi: 10.1097/01.inf.0000256964.30181.df. [DOI] [PubMed] [Google Scholar]
- 138.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177(9):995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 139.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;(37 Suppl 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 140.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest. 2006;130(1):222–226. doi: 10.1378/chest.130.1.222. [DOI] [PubMed] [Google Scholar]
- 141.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Kehagia V, et al. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J Clin Microbiol. 2006;44(7):2601–2604. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci U S A. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stone A, Saiman L. Update on the epidemiology and management of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, in patients with cystic fibrosis. Curr Opin Pulm Med. 2007;13(6):515–521. doi: 10.1097/MCP.0b013e3282efbbac. [DOI] [PubMed] [Google Scholar]
- 144.Sawicki GS, Rasouliyan L, Pasta DJ, Regelmann WE, Wagener JS, Waltz DA, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol. 2008;43(11):1117–1123. doi: 10.1002/ppul.20914. [DOI] [PubMed] [Google Scholar]
- 145.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(8):814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 146.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183(3):444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 147.West SE, Zeng L, Lee BL, Kosorok MR, Laxova A, Rock MJ, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA. 2002;287(22):2958–2967. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 148.Doring G, Hoiby N. Longitudinal study of immune response to Pseudomonas aeruginosa antigens in cystic fibrosis. Infect Immun. 1983;42(1):197–201. doi: 10.1128/iai.42.1.197-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ratjen F, Walter H, Haug M, Meisner C, Grasemann H, Doring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol. 2007;42(3):249–255. doi: 10.1002/ppul.20562. [DOI] [PubMed] [Google Scholar]
- 150.Silva Filho LV, Tateno AF, Martins KM, Azzuz Chernishev AC, Garcia DO, Haug M, et al. The combination of PCR and serology increases the diagnosis of Pseudomonas aeruginosa colonization/infection in cystic fibrosis. Pediatr Pulmonol. 2007;42(10):938–944. doi: 10.1002/ppul.20686. [DOI] [PubMed] [Google Scholar]