Abstract
Autoantibodies to islet antigen 2 (IA-2A) are important markers for predicting diabetes in children and young adults. Harmonization of IA-2A assay measurement is essential if results from different laboratories are to be compared. We investigated whether sodium azide, a bacteriostatic agent added to some assays, could affect IA-2A binding and thereby contribute to differences in IA-2A measurement between laboratories. Addition of 0.1 % azide to assay buffer was found to reduce median IA-2A binding of 18 selected sera from IA-2A positive patients with type 1 diabetes and their relatives by 41 % (range, 78 to -33 %, p<0.001). The effect on binding was epitope specific; median IA-2A binding by 14 sera with antibodies to the protein tyrosine phosphatase region of IA-2 was reduced by 48% (range, 11 to 78 %, p<0.001), while binding by 4 sera with antibodies specific to only the juxtamembrane region of IA-2 showed no change (median increase 16 % (range 6 to 33 %, p=0.125). When the Tween-20 concentration was reduced from 1 % to 0.15 % the median reduction in IA-2A binding with azide by the 18 sera was only 10 % (range, -12 to 41 %, p<0.001). Tween-20 also exerted an independent effect, since median IA-2A binding increased by 23 % (range 3 % to 86 %, p<0.001) when Tween-20 concentration was reduced from 1 % to 0.15 % in the absence of azide. We conclude that common assay reagents such as azide and Tween-20 can strongly influence IA-2A binding in an epitope-related manner, and their use may explain some of the differences between laboratories in IA-2A measurement.
Keywords: Islet autoantibody assay, epitope, protein tyrosine phosphatase, Tween-20, azide, type 1 diabetes
1. Introduction
Autoantibodies to islet antigen-2 (IA-2A) are found in approximately 70% of children and adolescents with newly diagnosed type 1 diabetes and, in combination with autoantibodies to glutamate decarboxylase (GAD) and insulin (IAA), underpin current methods for predicting the disease. [Verge et al., 1996; Bingley et al., 1999]. Diabetes associated IA-2A bind to two major epitope regions in the cytoplasmic domain of IA-2 (IA-2ic); the juxtamembrane (JM) region and the protein tyrosine phosphatase (PTP)-like regions of IA-2 or the related IA-2β [Hawkes et al., 1996; Xie et al., 1997; Bearzatto et al., 2002].
There have been major efforts to standardise islet autoantibody measurement across the world, to allow comparability between results obtained in different laboratories [Bingley et al., 2003]. With the aim of developing a common assay protocol to harmonise results, laboratories in Bristol, Denver and Munich undertook a series of experiments to analyse the effect of assay differences on antibody binding in IA-2A radiobinding assays, including the possible effects of buffer composition. Two differences identified were the addition of sodium azide and Tween-20 concentration. Sodium azide is a bacteriostatic agent widely used in immunoassays and was added by one laboratory to prevent bacterial growth interfering with antibody measurement. Tween-20 is a non-ionic detergent which is added to buffers in order to reduce non-specific binding of label; one laboratory used a concentration of 0.15 % and the others a concentration of 1 %.
2. Materials and methods
2.1 Subjects
Sera were available from 31 IA-2A positive relatives of patients with type 1 diabetes from the Munich family study [Achenbach et al., 2004], 14 patients with type 1 diabetes diabetes collected within 3 months of diagnosis and 3 IA-2A positive relatives from the Bart's-Oxford (BOX) family study [Gardner et al., 1999] and a pooled standard serum from patients with type 1 diabetes. Sera from two healthy volunteers and a pooled negative serum were used as negative controls in each assay.
2.2 IA-2A assays
Samples were tested in duplicate as previously described [Bingley et al., 1999]; 2μl of serum was incubated in a 96 well deep well plate (Greiner Bio-one, Stonehouse, UK) with 25μl of labelled antigen containing 20,000 cpm for 18-22 hours at 4°C. Antigen labelled with 35S-methionine was produced using a TnT rabbit reticulocyte lysate coupled transcription translation kit (Promega, Southampton, UK) with plasmids encoding IA-2ic (605-979), PTP (687-979), IA-2β (741-1033) and JM (609-631) provided by Dr. V.Lampasona [Bearzatto et al., 2002]. Immunocomplexes were precipitated using protein A sepharose (GE Healthcare Life Sciences, Amersham, UK) for 90 mins, washed 5 times using 50 mmol/l tris buffered saline, pH7.4 with 1% v/v Tween-20 (TBST) and transferred to a microplate. Scintillant was added to each well and samples counted in a Topcount plate scintillation counter (Perkin Elmer Life Sciences, UK).
Epitope specificity was determined by assaying samples with labels made with the different plasmids; samples were considered positive for antibodies to an epitope if they had levels greater than the mean plus 3 SD of 60 healthy schoolchildren.
For pre-treatment experiments, IA-2ic label was incubated with 0.1 % azide or 10 mmol/l N-ethyl maleimide (NEM) in TBST for 1 or 20 hours at 4 °C, followed by removal of azide or NEM using a NAP5 desalting column (GE Healthcare Life Sciences) prior to assay.
2.3 Analysis
Differences in levels or percentage reduction in antibody binding were compared by the Wilcoxon signed rank test, using the Statistics Package for Social Sciences Version 16 (SPSS, Chicago, IL, USA). Results were considered significant at the 5% level.
3. Results
3.1 IA-2A binding
The first experiment investigated whether addition of sodium azide (Sigma-Aldrich, Poole, UK) to TBST could affect IA-2A binding in the 31 IA-2A positive samples from the Munich family study. Addition of 0.1% sodium azide (N3) to the TBST assay buffer resulted in a 16 % median reduction (p=0.02) in binding when IA-2ic was used as label, but there was a marked variability of the effect among the sera (range 84 % to – 23 %) (Figure 1). IA-2A binding of the WHO standard serum (250 WHO Units/ml) [Mire-Sluis et al. 2000] was reduced by 33 % from 7623 cpm to 5106 cpm, suggesting that addition of azide would also affect IA-2A assay standardisation and agreement between laboratories. The effect of azide was found to be specific to the IA-2A assay, since addition of 0.1% azide had no apparent effect on binding of human GAD65, insulin or tissue transglutaminase antibodies (data not shown).
Figure 1.
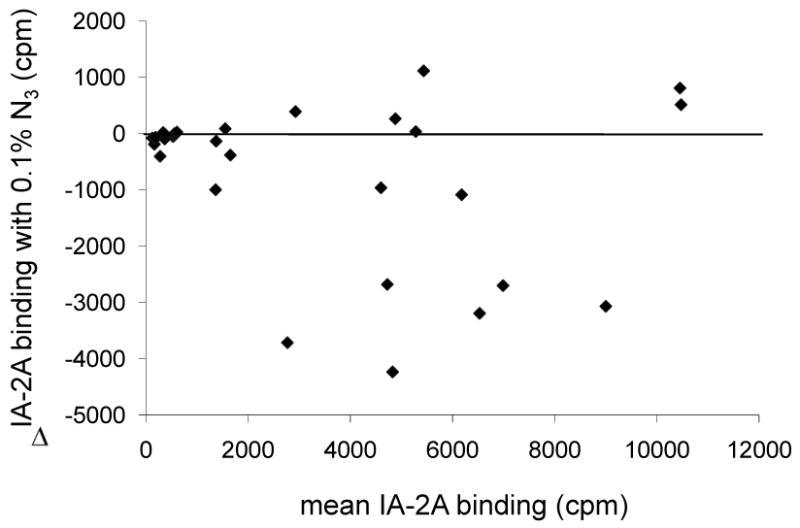
Bland-Altman plot showing difference (Δ) in IA-2A binding for samples from 31 islet autoantibody positive relatives (filled diamonds) participating in the Munich family study when assayed with or without 0.1 % azide (N3) in the buffer, plotted against the mean IA-2A binding obtained with these buffers. Several sera showed more than a 2000 cpm reduction in binding in the presence of azide, but other sera showed similar or even increased binding.
3.2 Binding to IA-2A epitopes
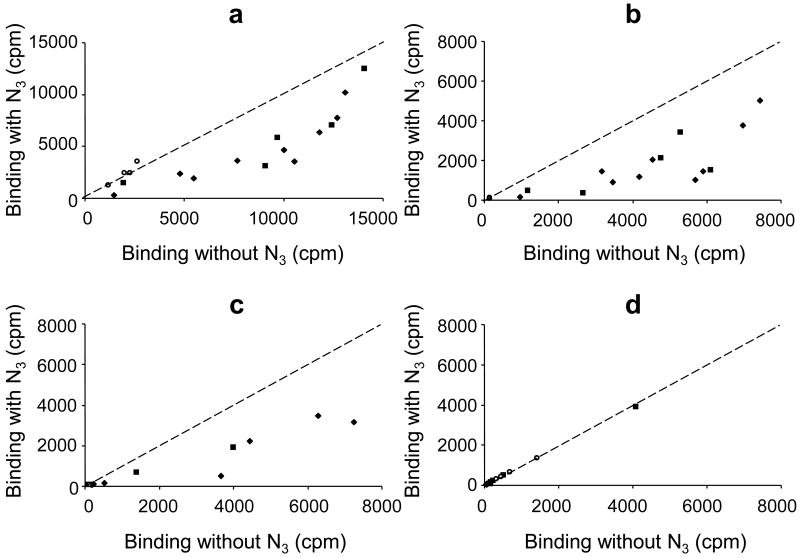
To investigate whether inhibition of binding to particular epitopes of IA-2 could explain the variability of the effect of azide on different sera, we assayed a panel of sera previously shown to bind in different proportions to the PTP and JM regions of IA-2 (Table 1). Binding of the IA-2ic label was reduced overall by a median of 48 % (range 11 to 78 %, p<0.001) for the 9 PTP and 5 PTP/JM specific sera following addition of 0.1 % azide. In contrast the binding of IA-2ic by the 4 JM specific sera showed no change (median increase 16 % (range 6 to 33 %, p=0.125) (Figure 2). Azide reduced binding of the PTP label by a median of 60 % (range 14 to 83 %, p<0.001) in the 14 PTP antibody positive sera and binding of the IA-2β PTP label by the 7 IA-2β PTP antibody positive sera by a median of 53 % (range 45 to 86 %, p=0.016). Binding of JM label by the 9 JM antibody positive sera was only reduced by a median of 4 % (range -1 to 21 %, p=0.012). These results suggest that 0.1 % azide inhibits binding mainly to the PTP region of IA-2 and IA-2β.
Table 1.
Reduction in binding of four IA-2 labels by sera with different epitope specificities under different buffer conditions.
| Antigen epitope specificity (n) |
PTP (9) |
PTP & JM (5) |
JM (4) |
|---|---|---|---|
| IA-2ic (1% Tween, no N3) | 0 | 0 | 0 |
| IA-2ic (1% Tween, 0.1% N3) | 53 (22 to 78) | 40 (11 to 66) | -16 (-33 to -6) |
| PTP (1% Tween, 0.1% N3) | 72 (32 to 83) | 44 (14 to 75) | - |
| PTPβ (1% Tween, 0.1% N3) | 57 (45 to 86) | 57 (48 to 69) | - |
| JM (1% Tween, 0.1% N3) | - | 4 (1 to 21) | 4 (-1 to 5) |
| IA-2ic (0.15% Tween, 0.1% N3) | 19 (2 to 41) | 11 (4 to 27) | 0 (-12 to 3) |
| IA-2ic (0.15% Tween, no N3) | -24 (-72 to -6) | -13 (-33 to -3) | -43 (-86 to -17) |
Values are expressed as median percentage reduction in binding (range). A negative value indicates an increase in binding.
Figure 2.
Binding (cpm) shown by 17 different IA-2A positive BOX sera and a pooled standard serum to labels made using plasmids encoding (a) IA-2ic (605-979), (b) PTP (687-979), (c) IA-2β (741-1033) and (d) JM (609-631) with and without 0.1% azide (N3) added to the TBST assay buffer. The dashed line represents equivalent binding in the presence and absence of azide. PTP specific (filled diamonds) and PTP/JM specific (filled squares) sera show reduced binding in the presence of azide, but JM specific (open circles) sera show no reduction in binding.
3.3 Effect of Tween-20 concentration
The laboratory which added azide to the buffer routinely used a Tween-20 concentration of 0.15% rather than 1%. Inhibition of IA-2A binding by azide was less at this Tween-20 concentration; addition of 0.1 % azide to buffer containing 0.15% Tween-20 led to only a median 13 % (range 2 to 41 %, p<0.001) reduction in binding of IA-2ic label by the 9 PTP specific and 5 PTP/JM specific sera compared to a median reduction of 48 % (range 11 to 78 %, p<0.001) in 1 % Tween (p<0.001).
Binding of the 4 JM specific sera was not altered (median 0 %, range -12 to 3 %, p=1.0). Reducing the Tween-20 concentration from 1 % to 0.15 % increased binding by all 18 sera by a median of 23 % (range 3 to 86 %, p<0.001) (Figure 3). This increased IA-2A binding was not explained by raised non-specific background as median IA-2A binding of three antibody negative control sera increased by only 4 cpm (range, -9 to 21 cpm) in contrast to a median increase of 1331 cpm (range, 62 to 3576 cpm) for the 18 antibody positive sera. Binding of tissue transglutaminase antibodies was also increased at lower Tween-20 concentrations (data not shown).
Figure 3.
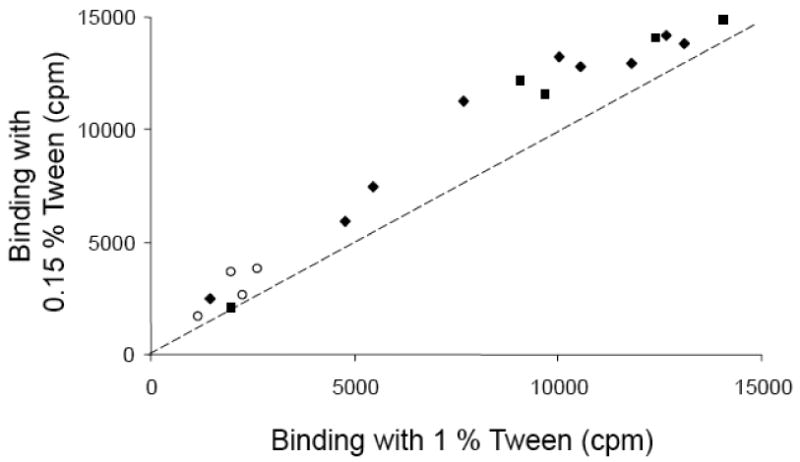
Binding (cpm) shown by 17 different IA-2A positive BOX sera and a pooled standard serum to IA-2ic label when 1 % or 0.15 % Tween-20 was added to the TBST assay buffer. The dashed line represents equivalent binding at either Tween-20 concentration. PTP specific (filled diamonds), PTP/JM specific (filled squares) and JM specific (open circles) sera show increased binding at the lower Tween-20 concentration.
3.4 Pretreatment of IA-2 label with azide
To determine whether azide acts directly on the antigen or interferes with binding in the assay we pre-treated the label for 20 hours with 0.1 % azide, and used the treated label in an assay without addition of azide. This pre-treatment led to a median 65 % reduction in binding (range 4 to 97 %, p<0.001) by the 14 PTP and PTP/JM specific sera compared to those pre-treated with buffer alone. Pre-treatment of label for 1 h led to only a 29% reduction in binding (range 4 to 45%, p<0.001) by these sera. These results suggest that the reduction in IA-2ic binding by PTP specific sera in the presence of azide is caused by time dependent modification of the labelled antigen.
3.5 Pretreatment of IA-2 label with N-ethyl maleimide
To investigate the potential mechanism by which azide modifies the IA-2ic label we compared the effect of pre-treating the label for 20 hours with either 0.1 % azide or 10 mmol/l of the sulphydryl modifying reagent N-ethyl maleimide. Binding by the 14 PTP and PTP/JM specific sera was reduced by a median of 77 % (range 15 to 96 %, p<0.001) and 86 % (range 12 to 92 %, p<0.001) after pre-treatment with azide and NEM respectively, compared to those pre-treated with buffer alone (Figure 4).
Figure 4.
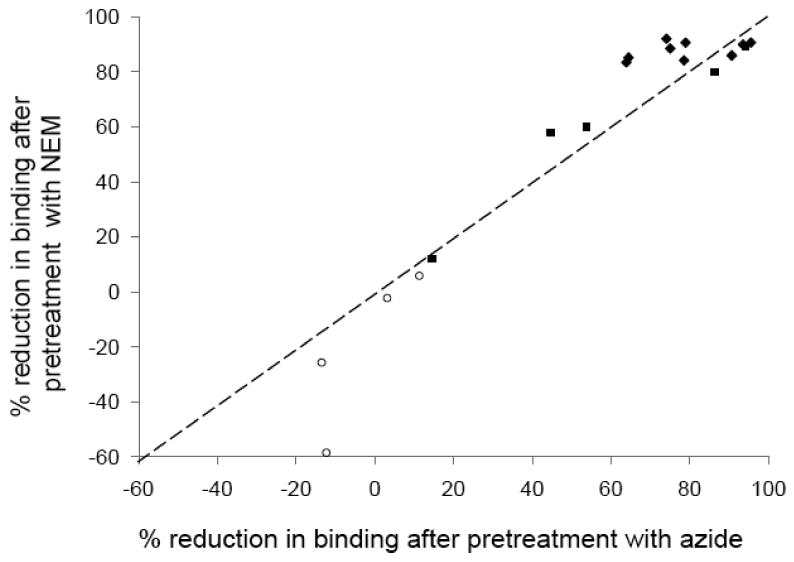
Percentage reduction in binding shown by 17 different IA-2A positive BOX sera and a pooled standard serum to IA-2ic label after pre-treatment of label with 0.1 % azide or 10 mmol/l N-ethyl maleimide. Most PTP specific (filled diamonds) and PTP/JM specific (filled squares) sera show large reductions in binding with both agents while binding of the 4 JM specific (open circles) sera show no reduction in binding. The dashed line represents equivalent changes in binding after pre-treatment with NEM or azide.
4. Discussion
We found that sodium azide, at concentrations commonly used in immunoassays to inhibit bacterial growth can reduce binding of diabetes associated antibodies to IA-2ic. The effect of azide varied between different sera, with antibodies specific for PTP epitopes showing the greatest reduction in binding. Tween-20 was found to modulate the effect of azide, since lowering the Tween-20 concentration from 1 % to 0.15 % attenuated the reduction in binding caused by addition of 0.1 % azide. Further, we found that Tween-20 itself had an independent effect on IA-2A binding, with reduced binding in the majority of sera at the higher Tween-20 concentration.
The mechanism by which azide and Tween modify antibody binding needs further investigation. Pre-treatment of IA-2ic label with azide caused reduced antibody binding, suggesting that azide modifies the PTP epitope of the antigen. We found the effect of azide was very similar to the effect of the sulphydryl modifying agent N-ethyl maleimide. Azide may therefore modify cysteine residues of IA-2 and thereby disrupt the PTP epitopes [Xie et al. 1997]. Further work will be needed to identify the modifications that affect antibody binding, although a recent paper has shown the importance of amino acids within the 831-860 region of IA-2 to antibody binding and T-cell responses [Weenink et al. 2009]. This region contains a cysteine at position 846. Tween-20 may act by a similar mechanism, but has more wide ranging effects which include reducing disease associated tissue transglutaminase antibody binding, an assay we found to be unaffected by azide.
The original aim of our investigations was to improve concordance between laboratories using radiobinding assays for measuring GAD and IA-2 antibodies. The unexpected effects of these common reagents on IA-2A binding are likely to explain some of the differences between laboratories in IA-2A measurement and even some of the variations seen within laboratories over time. Indeed, although the effects were consistent, the magnitude of the effects varied with different label preparations and batches of Tween. We have aimed to optimise signal to noise ratio and have therefore opted to use buffers without azide and with low Tween-20 concentrations to enhance IA-2A binding. The sensitivity of the PTP epitopes to disruption however, suggests that assay conditions will have to be carefully controlled if long-term concordance in IA-2A measurement is to be maintained.
Acknowledgments
This study was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases. The Munich family study is supported by grants from the European Union (EP7-HEALTH-2007, DIAPREPP N202013), the Federal Ministry of Education and Research of Germany (Kompetenznetz Diabetes mellitus, FKZ 01GI0805-07), and the DFG-Center for Regenerative Therapies Dresden, Cluster of Excellence (FZ 111). The BOX family study was funded by Diabetes UK.
Abbreviations
- IA-2
islet antigen 2
- PTP
protein tyrosine phosphatase
- JM
Juxtamembrane
- TBST
Tris buffered saline with Tween-20
- NEM
N-ethyl maleimide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJ, Bingley PJ, Bonifacio E, Ziegler AG. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53(2):384–92. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- Bearzatto M, Naserke H, Piquer S, Koczwara K, Lampasona V, Williams A, Christie MR, Bingley PJ, Ziegler AG, Bonifacio E. Two distinctly HLA-associated contiguous linear epitopes uniquely expressed within the islet antigen 2 molecule are major autoantibody epitopes of the diabetes-specific tyrosine phosphatase-like protein autoantigens. J Immunol. 2002;168(8):4202–8. doi: 10.4049/jimmunol.168.8.4202. [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Williams AJ, Gale EA. Optimized autoantibody-based risk assessment in family members. Implications for future intervention trials. Diabetes Care. 1999;22(11):1796–801. doi: 10.2337/diacare.22.11.1796. [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52(5):1128–36. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- Gardner SG, Gale EA, Williams AJ, Gillespie KM, Lawrence KE, Bottazzo GF, Bingley PJ. Progression to diabetes in relatives with islet autoantibodies. Is it inevitable? Diabetes Care. 1999;22(12):2049–54. doi: 10.2337/diacare.22.12.2049. [DOI] [PubMed] [Google Scholar]
- Hawkes CJ, Wasmeier C, Christie MR, Hutton JC. Identification of the 37-kDa antigen in IDDM as a tyrosine phosphatase-like protein (phogrin) related to IA-2. Diabetes. 1996;45(9):1187–92. doi: 10.2337/diab.45.9.1187. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis AR, Gaines Das R, Lernmark A. The World Health Organization International Collaborative Study for islet cell antibodies. Diabetologia. 2000;43(10):1282–92. doi: 10.1007/s001250051524. [DOI] [PubMed] [Google Scholar]
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45(7):926–33. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- Weenink SM, Lo J, Stephenson CR, McKinney PA, Ananieva-Jordanova R, Rees Smith B, Furmaniak J, Tremble JM, Bodansky HJ, Christie MR. Autoantibodies and associated T-cell responses to determinants within the 831-860 region of the autoantigen IA-2 in Type 1 diabetes. J Autoimmun. 2009;33(2):147–54. doi: 10.1016/j.jaut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Xie H, Zhang B, Matsumoto Y, Li Q, Notkins AL, Lan MS. Autoantibodies to IA-2 and IA-2 beta in insulin-dependent diabetes mellitus recognize conformational epitopes: location of the 37- and 40-kDa fragments determined. J Immunol. 1997;159(7):3662–7. [PubMed] [Google Scholar]