Abstract
During sustained periods of a taxing cognitive workload, humans typically display time-on-task (TOT) effects, in which performance gets steadily worse over the period of task engagement. Arterial spin labeling (ASL) perfusion functional magnetic resonance imaging (fMRI) was used in this study to investigate the neural correlates of TOT effects in a group of 15 subjects as they performed a 20-minute continuous psychomotor vigilance test (PVT). Subjects displayed significant TOT effects, as seen in progressively slower reaction times and significantly increased mental fatigue ratings after the task. Perfusion data showed that the PVT activates a right lateralized fronto-parietal attentional network in addition to the basal ganglia and sensorimotor cortices. The fronto-parietal network was less active during post-task rest compared to pre-task rest, and regional CBF decrease in this network correlated with performance decline. These results demonstrate the persistent effects of cognitive fatigue in the fronto-parietal network after a period of heavy mental work and indicate the critical role of this attentional network in mediating TOT effects. Furthermore, resting regional CBF in the thalamus and right middle frontal gyrus prior to task onset was predictive of subjects' subsequent performance decline, suggesting that resting CBF quantified by ASL perfusion fMRI may be a useful indicator of performance potential and a marker of the level of fatigue in the neural attentional system.
Keywords: Time-on-task effect, psychomotor vigilance test, ASL perfusion fMRI, fronto-parietal network
Introduction
Sustaining attention to a taxing cognitive task often comes at a cost, known in the literature as the “vigilance decrement”, or “time-on-task effect” (Mackworth, 1948, 1968). Behaviorally, this effect manifests itself in failures in target detection (Davies and Parasuraman, 1982), also known as “lapses” (Dinges and Powell, 1989), increasing reaction times (Boksem et al., 2005) and escalating reaction time variability, as well as an increasing sense of subjective fatigue over the course of a mentally challenging task. Although this effect was documented early in the psychological literature, it has not been the subject of much investigation especially in the field of neuroimaging, and the neural correlates of the phenomenon are not well understood.
The real world consequences of fatigue and the time-on-task effect are serious and pervasive. For example, many accidents involving truck drivers and medical personnel have been attributed at least in part to sleepiness, fatigue and lapses in vigilant attention (Landrigan et al., 2004; Arnedt et al., 2005). Because of this, the time-on-task effect has been of special interest to researchers in multiple disciplines who seek to understand the effects of brain function on human work capacity. In particular, the emerging field of neuroergonomics aims to study the neural basis of cognitive and physical work in order to optimize mental functioning (Parasuraman, 2003; Parasuraman and Wilson, 2008). Viewed through the framework of neuroergonomics, the time-on-task effect arises because the workload associated with tasks requiring vigilance is high, and consumes mental resources that cannot immediately be replenished (Warm et al., 2008). This stands in contrast to traditional views of this phenomenon, which attributed the decrement to boredom or motivational decline (Frankmann and Adams, 1962; Mackworth, 1968).
Logically, neural regions associated with time-on-task effects should correspond with areas active during optimal engagement of attention. Studies of attention using positron emission tomography (PET) have implicated a fronto-parietal network associated with successful task performance, including the anterior cingulate cortex (ACC), right middle and inferior prefrontal cortex, right inferior parietal regions, and the thalamus (Kinomura et al., 1996; Paus et al., 1997; Coull et al., 1998; Sturm et al., 1999; Lawrence et al., 2003). This network can be differentiated into areas of “bottom-up” attention, or detecting signals that are intrinsically alerting, and “top-down” attention, including processes such as biasing attention towards specific signal features, inhibiting unwanted distractions from the task, or modulating attention via motivation (Sarter et al., 2001; Corbetta and Shulman, 2002; Sarter et al., 2006). It is thought that the inferior parietal cortex, temporal-parietal junction and the thalamus provide bottom-up input, while dorsolateral prefrontal cortex and ACC perform top-down functions under conscious control (Kinomura et al., 1996; Foucher et al., 2004; Fan et al., 2005).
There have been relatively few studies on neurobiological mechanisms underlying the time-on-task effect. Coull et al. (1998) compared tasks of selective and non-selective attention, and found time-on-task effects exclusively in the latter. These effects were accompanied by decreased regional cerebral blood flow (rCBF) in the right inferior parietal cortex and inferior frontal cortex. Although both selective and non-selective tasks activated this fronto-parietal network, and showed no significant difference when averaged over time, activation to the selective task was preserved across the run, suggesting top-down modulation was involved in maintaining performance. The authors hypothesized that decreasing activation in the non-selective task was due either to habituation or diminished attentional resources. In another experiment, Paus et al. (1997) studied subjects performing a 60-minute continuous auditory vigilance task and reported decreased CBF in the thalamus, which was related to diminishing levels of arousal. Right-lateralized areas of the frontal, parietal and temporal cortex also showed decreasing activity over the course of the task, which were related to shifts in task strategy from controlled to automatic processing. However, both of these studies were analyzed at the group level (over time), and did not examine the relationships between brain activity and behavioral performance or inter-subject variability.
One of the challenges in carrying out neuroimaging studies of sustained attention arises from methodological limitations. Early studies such as those identified above have typically employed PET to measure absolute levels of cerebral blood flow. However, PET has poor temporal and spatial resolution, and cannot provide information about brain activity to individual events within a task block. Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) can overcome some of these problems, yet it is limited by the poor sensitivity to track slow neural activity changes over a time scale longer than a few minutes (Aguirre et al., 1997; 2002). As short tests mostly fail to elicit significant decrements in human performance over time, it is critical to select an imaging method capable of measuring slow varying neural activity with a high-level of spatial and functional resolution.
Using magnetically labelled water in arterial blood as a diffusible tracer, arterial spin labeling (ASL) perfusion fMRI (Detre and Wang, 2002) provides a non-invasive imaging method of quantifying cerebral blood flow (CBF) at task-free resting baselines (Rao et al., 2007a; 2007b) as well as during the performance of cognitive tasks (Wang et al., 2005; Kim et al., 2006; Olson et al., 2006; Rao et al., 2007c). ASL perfusion imaging also offers reliable measures of CBF with excellent reproducibility over long time periods (Aguirre et al., 2002). These features suggest that ASL perfusion fMRI may provide a highly suitable method for imaging the time-on-task effect over long durations. The present study, therefore, used ASL perfusion fMRI to examine the neural correlates of the time-on-task effect during the performance of a highly-demanding attentional task -- the psychomotor vigilance test (PVT). The PVT is a simple reaction time task in which subjects are instructed to respond as rapidly as possible to a single stimulus presented at short, random intervals. We selected this test as our measurement instrument for a few reasons. First, it has been shown that the PVT is a simple, reliable and highly sensitive task for measuring attentional and performance deficits for example due to sleep deprivation and fatigue (Dorrian et al., 2005; Lim and Dinges, 2008). Second, the PVT is free of aptitude and leaning effects which may confound the effects of TOT. Finally, the attentional requirements in the PVT are undiluted by elements of selectivity such as spatial orientation or executive decision-making. As stimulus saliency is held constant throughout the task, the maintenance of optimal performance on the PVT is almost completely mediated by top-down processes. These task features allowed us to isolate the time-on-task effect and minimize the confounding effects of differing visual stimuli, aptitude, strategy shifts or learning. In this current study therefore, we aimed to characterize neural activity associated with time-on-task decline not only during the continuous performance of PVT but also at resting baseline periods before and after the 20-minute task.
Materials and Methods
Participants
Fifteen healthy undergraduate and graduate students (8 male, mean age = 23.2 ± 3.6 yrs) from the University of Pennsylvania participated in this study. Written consent was obtained from all participants according to the University of Pennsylvania Institutional Review Board. Each participant was paid $35 in compensation for their effort and time.
Prior to enrolment in the experiment, all participants were screened to ensure that they had no history of chronic physical or mental illness, were right-handed, and did not habitually consume more than 250 mg of caffeine a day. Participants who qualified for enrolment were instructed to obtain between 6.5 and 8 hours of sleep during the 2 nights prior to the experiment, and not to consume caffeine, alcohol, or any other psychoactive substances during the 24 hours before the study. We took special care to control for baseline levels of sleepiness, as the PVT is typically used in the context of sleep deprivation experiments (Dorrian et al., 2005; Durmer and Dinges, 2005; Lim and Dinges, 2008), and is exquisitely sensitive to the sleep propensity of the test-taker.
Task parameters
The psychomotor vigilance test (Dinges et al., 1997) was used as the sustained attention task in this study. This test is a simple reaction time test with varying and random inter-stimulus intervals (ISI) which range from 2 to 10s (including a 1s delay after each button press for subjects to read their reaction time). A Windows-compatible version of the PVT was used to display the stimuli in this study (Pulsar Informatics, Philadelphia, PA). Before the experiment, subjects were instructed to focus their attention on a red, rectangular box subtending 2 × 1.3 degrees of visual angle in the middle of a black screen, and monitor the space for the appearance of a millisecond counter. They were told to react to and stop the counter with a button press, after which their reaction time would be displayed. Participants were instructed to respond as quickly as possible to the stimuli while avoiding “false starts”, or responses when the counter was not on the screen. The length of a standard PVT administration is 10 minutes; however, in order to elicit a greater time-on-task effect and increase between-subject variability in performance, we imaged subjects as they underwent a 20-minute PVT bout. In order to boost motivation, we emphasized to subjects the importance of giving their best effort “throughout the task”, without any reference to the strenuous nature of the test.
We extracted the following variables from each test as a measure of overall level of performance: median reaction time (RT), standard deviation of reaction times and number of lapses. To assess the time-on-task effect, we divided the PVT bout into 4-minute quintiles and obtained the median RT in each of those time bins, as well as computing the percentage change in reaction times from the first to the last quintile for each subject.
Directly before and after administration of the PVT, subjects were asked to rate their subjective fatigue on a 9-point visual analogue scale. To control for other potential confounds, we also obtained data on recent sleep quality and history using the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), baseline levels of sleepiness using the Epworth Sleepiness Scale (ESS), and a scale containing questions where subjects were asked to provide a subjective rating of their own performance.
Data Acquisition
Functional imaging was conducted on a Siemens 3.0T Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a product 8-channel array coil. All fMRI scans were conducted in the Hospital of the University of Pennsylvania, between 1400 and 1700h. Before entering the magnet, subjects were given a brief 1-minute opportunity to practice performing the PVT. As the PVT is a highly challenging task with negligible learning effects (Dorrian et al., 2005; Lim and Dinges, 2008), we did not allow participants to practice the task for a lengthier period of time.
A pseudo-continuous ASL sequence (Wu et al., 2007; Dai et al., 2008) was used for the perfusion scan. Arterial spin labeling was implemented with series of selective RF pulses (Hanning window, peak/average RF, amplitude B=5.3/1.8μT, duration=500μsec) applied at 9cm beneath the center of the imaging slices. Interleaved images with and without labeling were acquired using a gradient echo-planar imaging (EPI) sequence. The tagging/control duration was 1.77s. A delay of 1s was inserted between the end of the labeling pulse and image acquisition to reduce transit artifact. Acquisition parameters consisted of the following: FOV = 22cm, matrix = 64×64, TR = 4s, TE = 17ms, flip angle = 90°. Sixteen slices (7 mm thickness with 1.4 mm gap) were acquired from inferior to superior in sequential order. The perfusion scanning protocol of PVT performance lasted 20 minutes, and consisted of 300 acquisitions. An additional two 4-minute perfusion scanning protocols consisting of 60 acquisitions were collected while subjects were at rest before and after the PVT to assess resting baseline CBF levels. During these resting times, subjects were asked to relax, keep their eyes open and stay awake. After the functional scans, high-resolution T1-weighted anatomic images were obtained using a 3D-MPRAGE sequence (TR = 1620 ms, TI = 950 ms, TE = 3 ms, flip angle = 15°, 160 contiguous slices of 1.0 mm).
Data Analysis
Functional imaging data processing and analyses were carried out with Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, UK implemented in Matlab 6, Math Works, Natick, MA). In house SPM add-on scripts (online at http://www.cfn.upenn.edu/perfusion/software.htm) developed by two authors (H.R. and J.W.) were used to quantify CBF values and reconstruct CBF maps for perfusion analysis.
For each subject, functional images were first realigned to correct for head motion, co-registered with the anatomical image, and smoothed in space using a three-dimensional, 6-mm full width at half maximum (FWHM) Gaussian kernel. The perfusion weighted image series was then generated by pair-wise subtraction of the label and control images, followed by conversion to an absolute CBF image series based on a single compartment CASL perfusion model (Wang et al., 2004). Thus, the resulting CBF data sets contained 210 acquisitions (30 acquisitions for pre-task resting baseline, 150 acquisitions for PVT, and 30 acquisitions for post-task resting baseline) with an effective TR of 8s. One mean CBF image was generated for every 4-minute perfusion scan for each individual subject. Thus, each subject had one CBF image for pre-task resting baseline, 5 CBF images for the PVT, and one CBF image for post-task resting baseline. These CBF images were normalized to a 2 × 2 × 2 mm3 Montreal Neurological Institute (MNI) template using bilinear interpolation and then entered into the whole brain voxel-wise general linear model (GLM) analysis using the PET model in SPM. The GLM model included an adjustment for global CBF differences. Contrasts were defined to compare the PVT with rest baselines (PVT vs. Rest), the post-task resting baseline with the pre-task resting baseline (Rest2 vs. Rest1), and the last four-minute quintile of PVT with the first four-minute quintile of PVT (PVTq5 vs. PVTq1). For the contrasts of PVT vs. Rest and Rest2 vs. Rest1, activation clusters were identified at a significance level of p < 0.05 (FDR corrected across comparisons for the whole brain) and cluster size larger than 30 voxels. For the contrast of PVTq5 vs. PVTq1, since no area survived the whole-brain correction and a small volume correction (p < 0.05) was applied to regions in the right fronto-parietal network.
Region of interest (ROI) analysis was also conducted to calculate quantitative regional CBF changes and discover whether regional CBF changes were correlated with behavioral time-on-task deficits. These ROIs were determined a priori to be the brain regions comprising the fronto-parietal network that mediates sustained attention, including the thalamus, anterior cingulate cortex (ACC), right middle frontal gyrus (MFG), and right inferior parietal lobe (IPL) (Kinomura et al., 1996; Paus et al., 1997; Coull et al., 1998; Sturm et al., 1999; Lawrence et al., 2003). In addition to the functional ROIs which were defined by the activation clusters from the contrast between the PVT and resting baselines, we also used structure-based ROIs which were independent to the PVT activations to further confirm the relationship between CBF activity and behavioral performance changes and making the findings more generalizable. The structure-based ROIs were defined from an automated anatomical-labeling ROI library (Tzourio-Mazoyer et al., 2002). Quantitative CBF changes in each voxel of each ROI were averaged and read out by the Marsbar toolbox (Brett et al., 2002), which provides routines for region of interest analysis (online at http://marsbar.sourceforge.net). Finally, correlation analyses were performed between performance changes and quantitative regional CBF values (after adjusting for global CBF differences) in these ROIs during the pre-PVT resting baseline.
Results
Behavioral Data
Mean (SD) reaction time (RT) to the PVT across all subjects was 293.5 (28.3) ms. Participants were attentive to the task, as shown by the relatively small number of lapses (reaction times > 500ms) committed overall (mean = 2.1, SD = 3.0). Performing the PVT elicited a clear time-on-task effect, with most participants showing steadily increasing RT over the course of the run (Figure 1a). One-way analysis of variance (within subject repeated measures) of mean RT in the four-minute quintiles revealed a significant effect of time-on-task (Figure 1a, F4,70 = 2.34, p < 0.05). Tukey's post-hoc comparisons showed significant differences between reaction time in the first and last quintile only (p < 0.05). However, there was no significant effect of time-on-task on the standard deviation of RT over the run (F4,70 = 0.50, n.s.), suggesting that this slowing was not confounded with a change in the stability of the attentional system.
Figure 1.
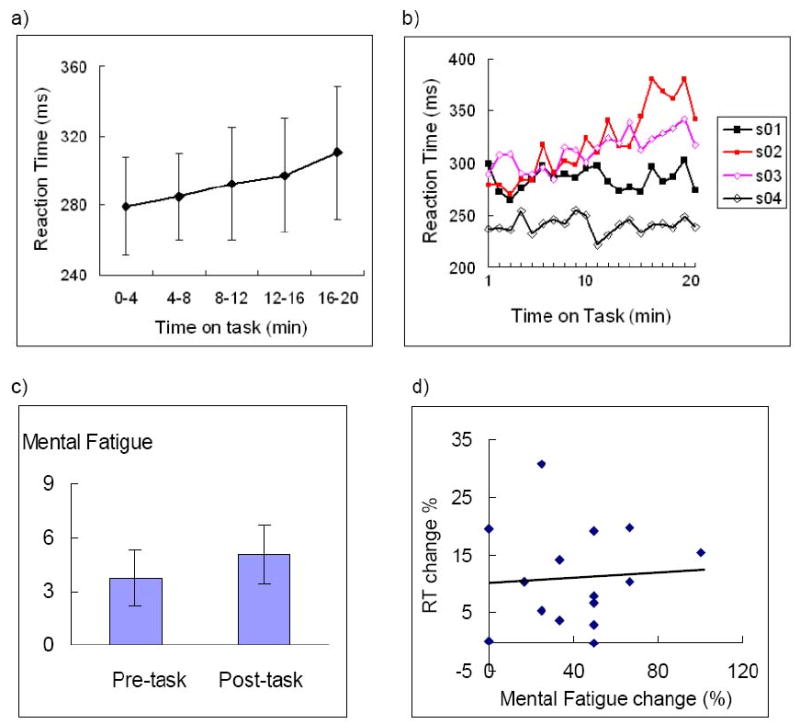
a) Means and standard deviations of reaction time (RT) from the first to the last 4-minute quintiles; b) RT time series of four subjects from the first minute to the last minute of the 20-minute PVT. Note that there were robust individual differences in the rate of RT increases. c) Mean mental fatigue scales reported by subjects before and after the PVT. Note both RT and mental fatigue scales showed significant time-on-task effects. d) Self-reported mental fatigue scale changes showed no correlation with RT changes (r = 0.66, p > 0.9)
Time-on-task vulnerability was quantified by calculating the percentage change in mean reaction times from the first to last quintile of the PVT for each subject. These values ranged from -0.2% to 30.7%. There were noticeable inter-individual differences in the extent of this vulnerability. Figure 1b shows the RT time series of 4 subjects over the 20-minute PVT, with 2 subjects (s1 and s4) demonstrating little increase in reaction times (< 3%) from the first to the last minute of the PVT, and the other 2 subjects (s2 and s3) showing marked performance decrements (> 18%).
Subjects reported being mentally fatigued by performing the PVT, with subjective ratings on the 9-point fatigue scale increasing significantly from pre- to post-task (Figure 1c, p < 0.001). There was no significant correlation between objective performance decline and either increases in self-rated fatigue (Figure 1d, r = 0.06, n.s.), or levels of fatigue prior to engaging in the task (r = 0.33, n.s.). Sleep quality (as measured by the PSQI), subjective performance ratings, and ratings on the ESS were not significant predictors of time-on-task decline (data not shown), and were not subject to any further analysis.
Imaging Results
Quantitative CBF maps
With the exception of a single subject who showed poor labeling and abnormally low global CBF values and were excluded for further analysis, all individual quantitative CBF maps for the task and resting baseline scans were of high quality. As an example, the quantitative CBF image from a representative subject is illustrated in Figure 2a. Perfusion in brain regions was visualized with good sensitivity, and clear contrast between gray and white matter was observed in the perfusion intensity. The mean whole brain (global) CBF values of remaining 14 subjects (mean ± SD, in mL/100g/min) were 67.0 ± 12.0, 65.8 ± 12.2, and 70.3 ± 13.7 for the pre-PVT resting period, the PVT, and the post-PVT resting period, respectively. There were no global CBF differences between the pre-PVT resting baseline and post-PVT resting baseline or between the PVT and the resting baselines, nor correlations between global CBF changes and RT changes (all p > 0.1).
Figure 2.
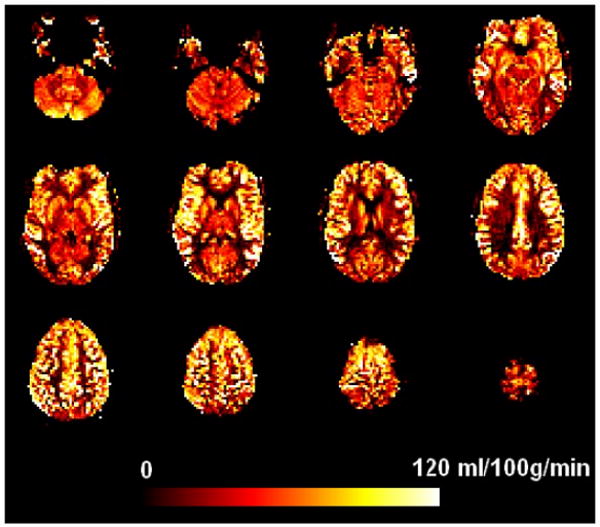
Quantitative CBF maps from a representative subject.
PVT task-related CBF changes
Compared to the resting baseline periods, continuous performance of the PVT increased CBF in right middle frontal gyrus and inferior frontal cortex (MFG/IFC), right, right inferior parietal lobe (IPL), bilateral supplemental motor area/anterior cingulate cortex (SMA/ACC), bilateral basal ganglia/insula, and left sensorimotor cortex (Figure 3a, Table 1). We also observed slight CBF activation in the left MFG/IFG. However, no CBF change was seen in the thalamus. The PVT also induced significant deactivation (CBF decreases) in bilateral occipital cortex and precuneus/posterior cingulate cortex (PCu/PCC).
Figure 3.
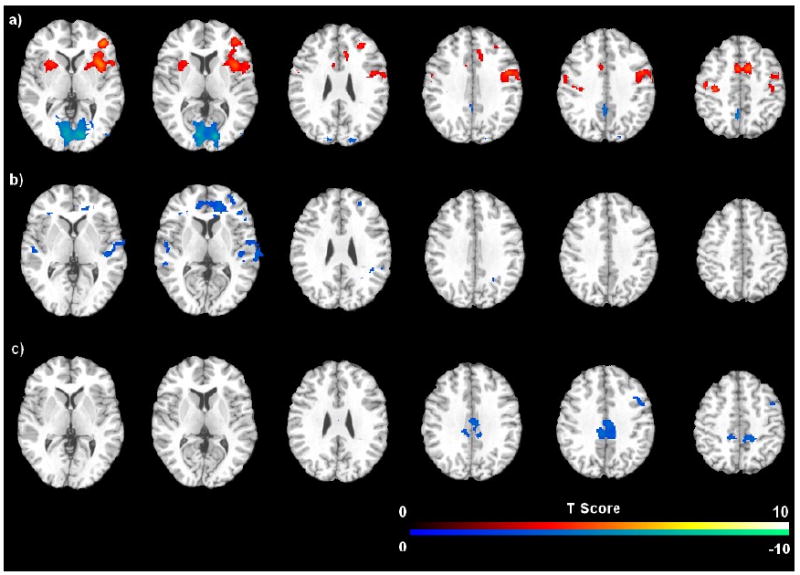
Brain areas associated with a) the comparison of PVT versus resting baselines; b) the comparison of post-task resting baseline versus pre-task resting baseline, and c) the comparison of the last quintile of PVT versus the first quintile of PVT. The threshold of display was set as FDR or small volume corrected p < 0.05.
Table 1.
Brain areas showing significant activation (CBF increases) and deactivation (CBF decreases) to the PVT compared to resting baseline. The threshold was set as whole brain FDR-corrected p < 0.05. L. left, R. right, B. bilateral.
| Region | Cluster Size | MNI Coordinates | Peak Z | Peak p (corrected) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (A) CBF increases | ||||||
| R. middle frontal cortex, inferior frontal cortex, insula, putamen | 3104 | 42 | 44 | 2 | 5.42 | 0.001 |
| 36 | 14 | 2 | 5.25 | 0.001 | ||
| 32 | 36 | 20 | 4.76 | 0.002 | ||
| B. supplementary motor area, anterior cingulate cortex | 655 | 8 | 8 | 48 | 4.71 | 0.002 |
| -4 | 4 | 50 | 4.66 | 0.002 | ||
| L. sensorimotor cortex | 574 | -34 | -22 | 50 | 4.60 | 0.002 |
| -56 | 2 | 18 | 4.46 | 0.003 | ||
| -50 | -16 | 48 | 4.24 | 0.004 | ||
| L. insula, putamen | 325 | -30 | 14 | 6 | 4.25 | 0.004 |
| R. anterior cingulate cortex | 157 | 8 | 26 | 26 | 3.93 | 0.008 |
| L. middle frontal cortex | 66 | -24 | 32 | 22 | 3.61 | 0.014 |
| -34 | 36 | 22 | 3.33 | 0.025 | ||
| R inferior parietal lobe | 127 | 60 | -40 | 42 | 3.42 | 0.021 |
| 54 | -48 | 48 | 3.02 | 0.044 | ||
| (B) CBF decreases | ||||||
| B. occipital | 3234 | 12 | -84 | 6 | 6.56 | <0.001 |
| -12 | -82 | 8 | 6.08 | <0.001 | ||
| L. posterior cingulate cortex | 196 | -6 | -56 | 46 | 4.72 | <0.001 |
Quantitative CBF analysis on the functional ROIs revealed that regional CBF increased 8.4% (SD = 5.1%, p < 0.001) in the right MFG, 6.7 % (SD = 6.4%, p = 0.002) in the right IPL, and 7.3% (SD = 5.7%, p < 0.001) in the ACC during the PVT comparing to the baseline. However, no correlations were found between RT changes and these regional task-induced activations in the attentional network. Quantitative CBF analysis on the independent structural ROIs confirmed that there were no correlations between RT changes and regional CBF activations in the right MFG and right IPL, but showed significant correlations between RT changes and CBF changes in the thalamus and ACC (both p < 0.05). However, these correlations disappeared (both p > 0.1) when the outlier (RT change = 30.7%) was removed.
CBF changes in resting baseline after task
Compared to the pre-task resting baseline, post-task resting baseline scans showed robust deactivation (CBF decreases) in bilateral ACC, MFG, superior temporal cortex (STC), and right IPL/cuneus (Figure 3b; Table 2). The MFG deactivation overlapped with the PVT activated MFG/IFG regions, but the ACC deactivation was more anterior and inferior, and the right IPL deactivation was more posterior and inferior to the PVT activated regions, respectively. Quantitative CBF analysis on the functional ROIs revealed that regional CBF decreased significantly in the right MFG ROI (mean = -5.4%, SD = 6.5%, p = 0.008). Although CBF decreases in the ACC (mean = -1.4%, SD = 10.1%) and right IPL (mean = -2.9%, SD = 11.2%) did not reach significance (both p > 0.1), CBF changes in these three functional ROIs all showed significant correlations with RT changes during the 20-minute PVT (all p < 0.05, Figure 4a). Quantitative CBF analysis on the independent structural ROIs confirmed significant correlations between RT changes and regional CBF deactivations in the ACC, right MFG and right IPL (Figure 4b). These results indicated that better-preserved performance over the 20-minute PVT (smaller RT increases) was associated with smaller CBF decreases in the fronto-parietal network after the task.
Table 2.
Brain areas showing significant CBF decreases for the post-task resting baseline comparing to pre-task resting baseline (Rest2 – Rest1) and for the last quintile of the PVT comparing to first quintile of the PVT (PVTq5 – PVTq1). Thresholds were set as whole brain FDR corrected p < 0.05 and small volume corrected p < 0.05, respectively. L. left, R. right, B. bilateral.
| Regions | Cluster Size | MNI Coordinates | Peak Z | Peak P (corrected) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (A) CBF decreases: Rest2 – Rest1 | ||||||
| R. anterior cingulate cortex, middle frontal gyrus, inferior frontal cortex | 1008 | 18 | 48 | 10 | 5.12 | 0.005 |
| 2 | 42 | 14 | 5.02 | 0.005 | ||
| 18 | 38 | 6 | 4.98 | 0.005 | ||
| L. superior temporal cortex | 313 | -54 | -22 | 0 | 4.56 | 0.006 |
| -52 | -34 | 10 | 4.29 | 0.009 | ||
| R. inferior parietal lobe/cuneus | 82 | 26 | -56 | 28 | 4.48 | 0.007 |
| L. anterior cingulate cortex | 70 | -12 | 44 | 12 | 4.40 | 0.008 |
| R. superior temporal cortex | 477 | 62 | -32 | 8 | 4.12 | 0.011 |
| L. middle frontal gyrus | 46 | -36 | 46 | 6 | 4.05 | 0.013 |
| (B) CBF decreases: PVTq5 – PVTq1 | ||||||
| B. posterior cingulate cortex | 565 | -6 | -32 | 40 | 4.23 | 0.010 |
| 2 | -18 | 36 | 4.12 | 0.011 | ||
| R. middle frontal gyrus | 105 | 44 | 10 | 46 | 3.81 | 0.03 |
Figure 4.
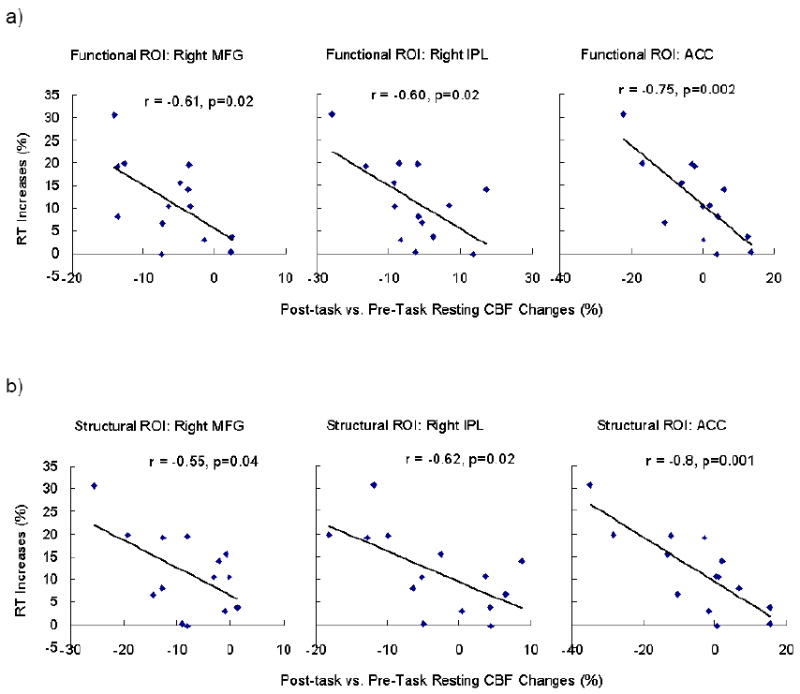
Region of interest (ROI) analysis on the functional (a) and structural ROIs (b) showing that RT increases (%) during the PVT negatively correlated with post-task regional CBF changes in the ACC, right MFG, and right IPC.
CBF changes during the 20-minute PVT
No CBF differences were found when comparing the first quintile to the last quintile of PVT using a whole brain corrected threshold. Using a more liberal threshold of uncorrected p < 0.001 and cluster-size larger than 30 voxels, regional CBF showed significant decreases in bilateral posterior cingulate cortex (PCC), right MFG, right SMA, and right STC from the first to the last quintile of PVT (Figure 3c; Table 2). The right MFG deactivation overlapped with the PVT activated MFG/IFG regions and it survived the small volume correction using a structure-based ROI of MFG. Quantitative CBF analysis on the functional ROIs revealed that regional CBF decreased 12.8 % (SD = 11.1%, p = 0.001) in the right MFG with no significant CBF changes in other functional or structural ROIs, and no correlation between regional CBF changes and RT performance changes.
Correlations between pre-task resting CBF and performance decline
Interestingly, quantitative CBF analysis of pre-task resting CBF in the functional ROIs revealed that resting CBF activity in the right MFG before PVT performance was predictive of the extent to which subjects' performance declined over the course of the 20-minute task. Lower regional CBF in the right MFG at pre-task resting baseline was associated with smaller reaction time increases during the PVT (Figure 5a, r = 0.69, p = 0.007). Quantitative CBF analysis on the independent structural ROIs confirmed significant correlations between performance decline and right MFG resting CBF (Figure 5b, r = 0.64, p = 0.01), and further revealed that pre-task resting CBF activity in the thalamus was also predictive of the reaction time increases during the PVT (Figure 5c, r = -0.59, p = 0.03). Removing the outlier did not change these results.
Figure 5.
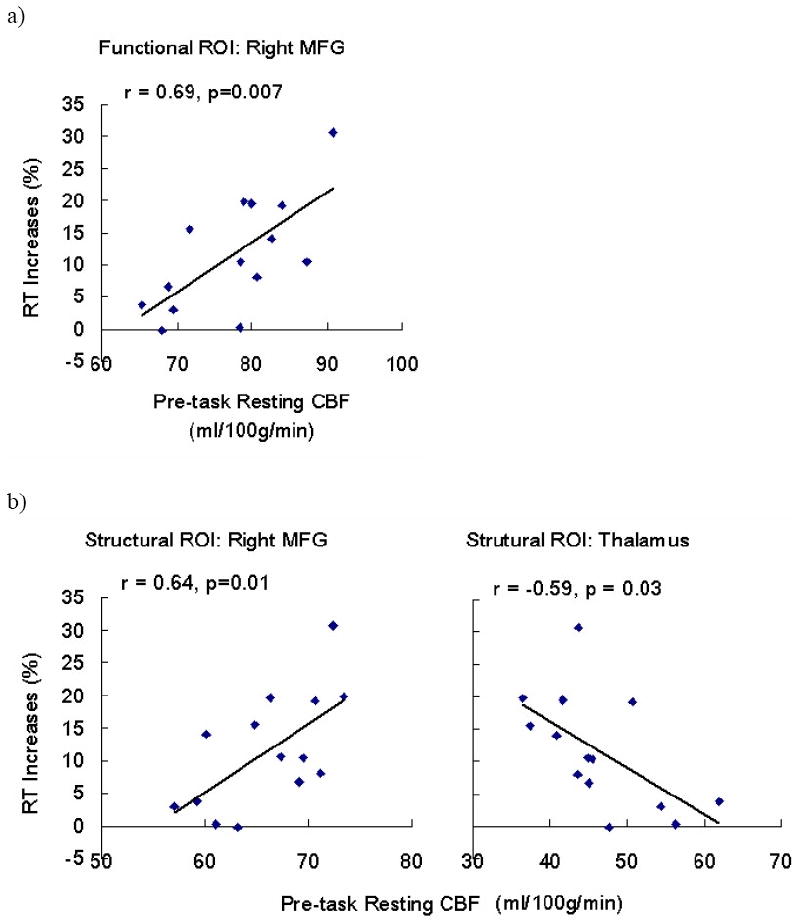
Region of interest (ROI) analysis on the functional (a) and structural ROIs (b) showing that pre-task resting CBF in the right MFG and thalamus predicted RT increases (%) during the PVT.
Discussion
In this experiment, we induced time-on-task deficits by asking subjects to perform the psychomotor vigilance test for 20 minutes. As expected, subjects reacted significantly slower to target stimuli as the task proceeded and reported higher fatigue ratings after the task. Additionally, we observed a substantial amount of inter-individual variation in the degree to which subjects showed this effect. While some participants were able to perform relatively consistently over the course of the 20-minute period, others showed markedly worse performance by the end of the test.
No significant correlation was observed between self-reported mental fatigue changes and objective performance decline. Intuitively, one would imagine that the better-preserved performance is over time, the greater ones subjective sense of fatigue. Our data suggest that this is not the case. This finding is similar to the results from experiments by Leproult et al. (2003) and Van Dongen et al. (2003), in which there was no significant association between subjective and objective measures of alertness in a group of sleep-deprived subjects. Our finding further extended these results by demonstrating a similar dissociation in well-rested subjects, indicating that subjects may not be aware of the degree of their objective impairment after a period of high cognitive workload.
Although the PVT has been widely used for the accurate neurocognitive assessment of performance during sleep loss and the dynamic expression of waking neurobehavioral integrity, there have been relatively few studies on the brain mechanisms mediating PVT performance when compared to other attentional tasks. The only two neuroimaging studies on the PVT to date both employed an event-related BOLD fMRI (Drummond et al., 2005; Schmidt et al., 2009) and contrasted the hemodynamic responses of fast and slow (or average) reaction times to uncover the areas associated with optimal task performance. While this approach yielded some interesting results, it was not a method applicable to studying the process of sustained attention per se. Timely responding on a task of sustained attention may be determined by neural activity antecedent to stimulus onset, something which was not captured by the event-related analysis. Instead of focusing on phasic event-related responses, it is arguable that tonic activity throughout the task block is a more meaningful marker of the level of vigilance displayed by a subject. While low-frequency artifacts in the BOLD spectrum make such a design impossible, ASL perfusion imaging is able to overcome this problem by canceling low-frequency noise through pair-wise subtraction of tagged and control images. Our current study of the PVT therefore overcomes the limitations associated with analysis of phasic events, and gives us greater confidence that regions of activation are associated specifically with sustained attention.
Our ASL fMRI data revealed that performing the PVT engaged a right-lateralized fronto-parietal network of regions, which are reported regularly in tests requiring vigilance and continuous performance (Cabeza and Nyberg, 2000). Similar activation patterns have been reported in a variety of studies employing comparable attention tasks and different neuroimaging techniques, including monkey electrophysiology (Colby et al., 1996), human PET (Corbetta et al., 1993; Nobre et al., 1997; Coull et al., 1998) and fMRI (Fan et al., 2005; Ogg et al., 2008). More interestingly, our results revealed significant associations between behavioral performance decline and regional CBF activity in the sustained attention network. Specifically, an individual's ability to preserve better performance over time (i.e., demonstrate less RT increase over the 20-minute task) was associated with smaller CBF deceases in right MFG, right IPC and ACC from pre-task to post-task baseline and less thalamic and ACC CBF activation during PVT performance. Furthermore, pre-task resting CBF activity in the thalamus and right MFG predicted subsequent performance decline, with better-preserved performance being associated with lower pre-task resting CBF in right MFG and higher resting CBF in the thalamus. These key points of interest are discussed below:
Performance decline correlates with CBF decreases from pre- to post-task rest
The first key finding of this study is that brain fatigue in the right fronto-parietal network persists after a period of heavy mental work and that subjects' ability to maintain baseline levels of performance over time was correlated with CBF decrease from pre- to post-task resting activity in this attentional network. Areas in this network have long been established as important in the effortful maintenance of sustained attention (Lewin et al., 1996; Cabeza and Nyberg, 2000). However, our data extended the classic findings in two important ways:
First, we clearly showed that the residual effects of performing a fatiguing task on brain activity can be detected by ASL perfusion fMRI. Regional activity at rest following task completion decreased in the anterior cingulate cortex, middle prefrontal gyrus and inferior parietal cortex, suggesting that the brain does not immediately “reset” itself following task performance and cognitive resources depleted during demanding tasks can not be restored within a 4-minute post-task window. Second, we showed that larger RT increases were associated with greater post-task CBF decreases, indicating that subjects' ability to better preserve performance may depend on the stability of activity in the attentional system.
The CBF changes associated with time-on-task in our experiment are consistent with those found in previous studies (Paus et al., 1997; Coull et al., 1998), which showed decreases in cortical activation over time. In both of these paradigms, however, decreases in activation were suggested as implying shifts to automaticity. In contrast to oddball paradigms, the PVT demands rapid and timely responding to randomly onset stimuli, and it is not possible for subjects to adapt to the PVT by shifting from controlled to automatic processing and still maintain optimal performance. We thus reject strategy shifts as a theoretical framework for understanding the changes observed in this experiment. Instead, we posit that there is a physiological ceiling to how well one can perform, and that the speed at which these scarce attentional resources get depleted varies widely from person to person, and depends on the demands of the task an individual is engaged in.
One possible alternative account of the data is that declines in performance and brain activity are dictated largely by decreasing motivation. Altering motivation by providing monetary incentives has been shown to modulate the time-on-task effect in college students (Tomporowski and Tinsley, 1996). It seems unlikely, however, that differences in motivation could entirely account for the substantial amount of variability in performance shown in our study. All of our subjects volunteered for the study and were paid and treated identically, and were only required to perform a simple task with no element of motivation and reward. Nevertheless, certain individuals may “try” harder than others on tasks, based (for example) on individual differences in personality traits, and that part of the variance captured in this experiment may reflect effort rather than physiological capacity. Future work is needed to test this hypothesis.
Resting baseline activity predicts subsequent performance decline
Another key finding from the present study was that resting CBF activity in the thalamus and right middle frontal gyrus prior to task onset was predictive of the extent to which subject's performance eventually declined. This result is analogous to various findings in the research on sleep deprivation suggesting that baseline activity is associated with cognitive vulnerability to periods of sleep loss. For example, Mu and colleagues (2005) found in an fMRI study that sleep-deprivation resilient individuals had a significantly greater number of activated voxels globally than sleep-deprivation vulnerable individuals when performing a working memory test. Similarly, Caldwell et al. (2005) found that, in a group of well-rested pilots, global fMRI activation (and activation in the left posterior parietal cortex) to a Sternberg working memory task was correlated with subsequent performance measures on a flight simulator after they were sleep deprived.
Why would pre-task resting activity correlate with the level of vulnerability when the attentional system is challenged? We posit that differing levels of resting thalamic activity reflects individual differences related to the amount of sensory filtering the brain performs while in its baseline state. The thalamus has been implicated in worsening performance due to sleep deprivation (Thomas et al., 2000), and as a mediator of attention between different arousal states (Portas et al., 1998). It is conceivable, therefore, that its default state of activity should have some bearing on its efficiency during tasks that require its engagement. Similarly, the prefrontal cortex, an area with known connections to the thalamus, acts in concert with this region to carry out the early filtering of irrelevant information. Our data suggest that individuals for whom this system operates less efficiently at baseline may be less effective in the early selection of relevant information (reflected by lower baseline thalamic activity), and thus require greater top-down control (reflected by higher prefrontal activity) during later processing stages. Hence, when an additional load is placed on this neural system (as in the case of an attention-demanding task), subjects who are already performing near capacity have no spare neural resources, and thus show the greatest deficits in performance.
Although it may be argued that resting levels of activity could reflect pre-existing levels of sleep debt, we contend that this was not the case, as we controlled for sleep history prior to the scanning session. We found that subjects were regular sleepers as assessed by the PSQI, and neither scores on this questionnaire nor the Epworth Sleepiness Scale correlated with brain activity in any of the attentional regions. We posit therefore, that the observed variability in resting-state activation may reflect a fundamental individual difference trait. Recent perfusion studies showing resting baseline CBF correlating with habitual emotion regulation scores (Abler et al., 2008) and working memory capacity (Beschoner et al., 2008) also support this hypothesis.
Taken together, our main findings highlight the potential importance of resting-state CBF in assessing the degree of taxation on the brain's attentional system, a possibility that has been overlooked by the vast of previous neuroimaging studies for which pre- and post-task activity is taken merely as inseparable “baseline”. Future work is needed to determine the time of course of recovery following a period of sustained activity, that is, how long the brain takes to return to its default resting state.
Task-induced activation did not correlate with performance decline
In contrast to the resting-state data, CBF changes during the task in the attentional network regions did not correlate individual differences in performance decline. This may be due to the mixed CBF signal of both tonic and transient activity changes in this network detected by ASL perfusion while subjects are engaged in a cognitive task. In other words, phasic activity to stimulus responses may have activated regions that overlap with the attentional network (Drummond et al., 2005), thus adding noise to the tonic signal obtained from these areas in our analysis. In general, therefore, the magnitude of activation in perfusion imaging datasets acquired during cognitive task performance should be interpreted with caution, as it may reflect the contribution of multiple sources of variance.
Conclusion
This study demonstrates the utility of ASL perfusion imaging in revealing the neural network associated with tonic activity to a highly demanding sustained attention task. Our results demonstrate the critical role of the right fronto-parietal network in mediating time-on-task effects, and that these effects persist, and can be measured in the immediate aftermath of a period of heavy cognitive workload. Moreover, our findings reveal that differences in neural activity between the resting periods before and after the demanding task can act as markers of cognitive fatigue, and thus contain useful information that has heretofore been neglected in the neuroimaging literature. Most interestingly, pre-task levels of CBF in the thalamus and right middle frontal gyrus appear to be meaningful predictors of subsequent time-on-task decline. These results may help identify neural “risk factors” for accidents and errors due to prolonged task performance and lead to greater safety and work efficiency in populations where fatigue is an ever-present problem.
Acknowledgments
This research was supported by NSF Grants BCS 0224007 and 0517935, NIH Grants P30 NS045839, R01 NR04281, National Space Biomedical Research Institute through NASA NCC 9-58, and Air Force Office of Scientific Research Grant FA9550-05-1-0293.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Hofer C, Viviani R. Habitual emotion regulation strategies and baseline brain perfusion. Neuroreport. 2008;19:21–24. doi: 10.1097/WNR.0b013e3282f3adeb. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15:488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. Jama. 2005;294:1025–1033. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- Beschoner P, Richter S, Lo H, Sim EJ, Baron K, Osterfeld N, Horn AB, Viviani R. Baseline brain perfusion and working memory capacity: a neuroimaging study. Neuroreport. 2008;19:1803–1807. doi: 10.1097/WNR.0b013e32831997f1. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM. Effects of mental fatigue on attention: an ERP study. Brain Res Cogn Brain Res. 2005;25:107–116. doi: 10.1016/j.cogbrainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an spm toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Mu Q, Smith JK, Mishory A, Caldwell JL, Peters G, Brown DL, George MS. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci. 2005;119:694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RS, Frith CD. Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–1334. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Davies D, Parasumaran R. The Psychology of Vigilance. London: Academic; 1982. [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621–634. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Sleepiness impairs optimum response capability. Sleep Res. 1989;18:366. [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: A neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep Deprivation: Clinical Issues, Pharmacology and Sleep Loss Effects. New York, NY: Marcel Dekker, Inc.; 2005. pp. 39–70. [Google Scholar]
- Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D. Where arousal meets attention: a simultaneous fMRI and EEG recording study. NeuroImage. 2004;22:688–697. doi: 10.1016/j.neuroimage.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Frankmann JP, Adams JA. Psychological bulletin. Psychol Bull. 1962;59:257–272. [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. NeuroImage. 2006;31:376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, Lilly CM, Stone PH, Lockley SW, Bates DW, Czeisler CA. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- Lewin JS, Friedman L, Wu D, Miller DA, Thompson LA, Klein SK, Wise AL, Hedera P, Buckley P, Meltzer H, Friedland RP, Duerk JL. Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm. J Comput Assist Tomogr. 1996;20:695–701. doi: 10.1097/00004728-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Mackworth JF. The breakdown of vigilance during prolonged visual search. Quarterly Journal of Experimental Psychology. 1948;1:6–21. [Google Scholar]
- Mackworth JF. Vigilance, arousal, and habituation. Psychol Rev. 1968;75:308–322. doi: 10.1037/h0025896. [DOI] [PubMed] [Google Scholar]
- Mu Q, Mishory A, Johnson KA, Nahas Z, Kozel FA, Yamanaka K, Bohning DE, George MS. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–446. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120(Pt 3):515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magn Reson Imaging. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Olson IR, Rao H, Moore KS, Wang J, Detre JA, Aguirre GK. Using perfusion fMRI to measure continuous changes in neural activity with learning. Brain Cogn. 2006;60:262–271. doi: 10.1016/j.bandc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Neuroergonomics: Research and practice. Theoretical Issues in Ergonomic Science. 2003;4:5–20. [Google Scholar]
- Parasuraman R, Wilson GF. Putting the brain to work: neuroergonomics past, present, and future. Hum Factors. 2008;50:468–474. doi: 10.1518/001872008X288349. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre R, Hofle T, Caramanos Z, Gotman J, Petrides M, Evans A. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007a;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007b;120:e1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, Tang K, Pan W, Detre JA. Imaging brain activity during natural vision using CASL perfusion fMRI. Hum Brain Mapp. 2007c;28:593–601. doi: 10.1002/hbm.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, Gais S, Schabus M, Desseilles M, Dang-Vu TT, Salmon E, Balteau E, Degueldre C, Luxen A, Maquet P, Cajochen C, Peigneux P. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Muller-Gartner HW, Willmes K. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Tinsley VF. Effects of memory demand and motivation on sustained attention in young and older adults. Am J Psychol. 1996;109:187–204. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude modulated continuous arterial spin labeling perfusion MRI with single coil at 3.0 Tesla. Radiology. 2004 doi: 10.1148/radiol.2351031663. in press. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS, Parasuraman R, Matthews G. Vigilance requires hard mental work and is stressful. Hum Factors. 2008;50:433–441. doi: 10.1518/001872008X312152. [DOI] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]


