Abstract
The ensheathment of neurons and their axons creates an ion-sensitive microenvironment that allows rapid conduction of nerve impulses. One of the fundamental questions about axonal ensheathment is how insulating glial cells wrap around axons. The mechanisms that underlie insulation of axons in invertebrates and vertebrates are not fully understood. In the present article we address cellular aspects of axonal ensheathment in Drosophila by taking advantage of glial mutants that illustrate a range of phenotypic defects including ensheathment of axons. From the findings of these mutant studies, we summarize that loss of glial cells, defects in glial membrane wrapping, failure of glial migration, and loss of specialized ladderlike septate junctions between ensheathing glial membranes result in axon-glial functional defects. These studies provide a broad perspective on glial ensheathment of axons in Drosophila and key insights into the anatomical and cellular aspects of axonal insulation. Given the powerful genetic approaches available in Drosophila, the axonal ensheathment process can be dissected in great detail to reveal the fundamental principles of ensheathment. These observations will be relevant to understanding the very similar processes in vertebrates, where defects in glial cell functions lead to devastating neurological diseases.
Keywords: peripheral glia, exit glia, glial migration, septate junctions, actin cytoskeleton
In 1928, neurobiologist P. D. Rio Hortega wrote,
Such minute corpuscles could not evoke any aesthetical emotions. Yet these cells refractory to every protoplasmic staining, because of their infinite numbers, their dissemination in encephalic terrains and their strategic location next to neurons and nerve fibers, deserved, at some point, to seize the attention of neurologists and to be placed on an equal rank with the classic neuroglia. This moment has arrived!
Glial cells are abundant in the nervous system, and the ratio of glia to neurons increases with increasing neural complexity. A thorough understanding of the diverse functions that glial cells perform and of the underlying molecular mechanisms that control axonal ensheathment is still lacking. Extensive studies have uncovered a variety of roles for glia in neuronal development and function. For example, glial cells provide growth factors that regulate neuronal proliferation and survival (Xiong and Montell, 1995; Booth et al., 2000; Lemke, 2001), help to compartmentalize to delimit regions of axonal outgrowth (Younossi-Hartenstein and Hartenstein, 1993; Oland and Tolbert, 2003), modulate axonal growth and fasciculation (Booth et al., 2000; Gilmour et al., 2002), and secrete factors essential for the maintenance and ensheathment of synapses (Barres and Raff, 1999; Fields and Stevens-Graham, 2002; Auld and Robitaille, 2003). In addition, glial cells act as the primary immune cells of the nervous system and perform engulfment functions to clear dying cells or invading pathogens in order to protect neural tissues (Freeman et al., 2003; Awasaki et al., 2006; MacDonald et al., 2006).
Among the various functions that glial cells perform in the nervous system, ensheathment or insulation of neurons and their axons is one of the functions that have high clinical relevance to human health and disease. Thus, the molecular mechanisms that underlie the ensheathment of axons remain an area of immense interest and are believed to be involved in the etiology of several debilitating myelin diseases such as Charcot-Marie-Tooth disease and multiple sclerosis that dramatically alter neuronal function. In vertebrates, myelin membranes are produced by Schwann cells in the peripheral nervous system (PNS) and by oligodendrocytes in the central nervous system (CNS). These cells generate a multilayered structure around axons that provides a high-resistance and low-capacitance barrier and organizes axons into distinct molecular domains essential for conduction of saltatory action potentials (Poliak and Peles, 2003; Bhat, 2003; Salzer, 2003). In contrast, glial ensheathment of peripheral axons in Drosophila is accomplished by ensheathing inner glial cells (Klambt and Goodman, 1991; Juang and Carlson, 1994; Leiserson et al., 2000; Banerjee et al., 2006a,b). Similar to that in vertebrate oligodendrocytes, ensheathing glial cell processes in Drosophila peripheral nerves wrap around individual axons or axon fascicles. A second outer glial cell wraps both the inner glial cell and the axons. In both vertebrates and invertebrates, the encasing of axons in insulating glial processes is a unique way to increase the conduction velocity of electrical impulses. This, in turn, increases the capacity of the nervous system to process information at a faster pace, decreasing the reaction time to stimuli. In addition, glial sheaths sort axons into appropriate fascicles in order to isolate them and enable them to conduct unique electrical impulses.
In the present article we address key aspects of axonal ensheathment by glial cells in Drosophila peripheral nerves and revisit some of the published studies on various glial mutants in order to highlight the critical steps involved in ensheathment of PNS axons.
GLIAL ENSHEATHMENT OF PERIPHERAL AXONS
Glial cells have been carefully classified and described using various enhancer trap markers that identify specific subsets of glial cells (Fredieu and Mahowald, 1989; Klambt and Goodman, 1991; Ito et al., 1995; Kania et al., 1995; Bellen and Schulze, 2003). The 6–8 PNS glia in each embryonic segment are derived from two lateral neuroglioblasts (Schmidt et al., 1997; Sepp et al., 2001; see Fig. 1). These glia become intermediate targets for motor neurites extending into the periphery. Initially exit glia migrate to the CNS/PNS border, where they first form a cone-shaped structure at the site where the growth cones of motor neurons exit the CNS (Sepp et al., 2001; Sepp and Auld, 2003a; Banerjee et al., 2006a). Nearly all glial cells are born in the CNS, but those that perform the ensheathment function of the peripheral nerves, namely, exit glia and peripheral glia, migrate out of the CNS along motor axon tracts during midembryonic development. Motor axons exit the periphery, and sensory axons grow toward the CNS and are all insulated by the same glial cells (schematic in Fig. 1O). Thus, the spatiotemporal distribution of neurons and glia in the embryonic nervous system suggests a highly coordinated mechanism of neuron–glia interactions and axonal insulation in which glial cell processes must undergo complex morphological changes to enable fewer glial cells to accommodate and insulate a large number of axons at the same time.
Fig. 1.
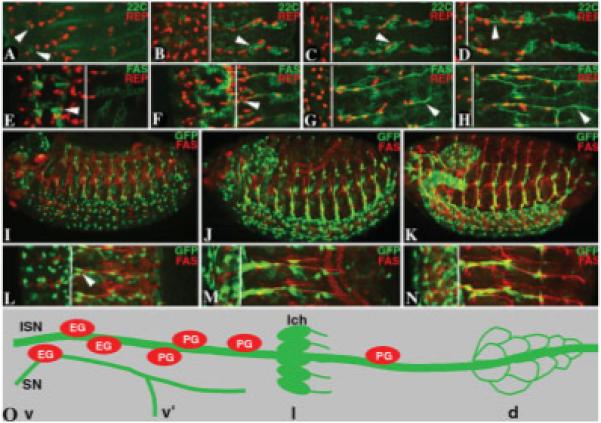
Distribution, migration and process extension of glial cells around axons in the Drosophila embryonic PNS. A–D: Embryonic segments from wild-type stage 12 (A), stage 14 (B), stage 15 (C), and stage 16 (D) embryos immunostained for sensory neuronal marker 22C10 (22C; green, white arrowheads) and glial nuclear marker Repo (REP; red) showing profile of sensory axon trajectories in the PNS. The sensory neurons were born in the periphery (A) and start sending their axons toward the CNS (B–D). The peripheral glial cells ensheath the sensory axon tracts as they grow toward the CNS. E–H: Portion of embryonic segments from wild-type stage 12 (E), stage 14 (F), stage 15 (G), and stage 16 (H) embryos immunostained for motor neuronal markers Fas II (FAS; green) and REP (red) shows motor axonal trajectories. As soon as the neurons and glia were specified in the CNS (E), they expressed these markers. The glial cells are in close proximity to the motor neurons in the neurectoderm, which produces both neuroblasts and glioblasts. F: Embryonic stage 14 shows migration of the glia toward periphery along the motor axons that exit the CNS (white arrowhead). G: Glial cells are lined along the axons as they are still in the process of migrating to their final destination. H: Stage 16 embryo marks the completion of glial migration to the periphery along with the motor axons. I–K: Whole-mount embryos of the genotype repo-GAL4::UAS-mCD8GFP at stage 12 (I), stage 14 (J), and stage 16 (K) show glial membrane ensheathment, highlighted by anti-GFP (green) around the peripheral motor axons stained for FAS (red). L–N: Higher magnification of embryos at stage 12 (L, arrowhead points to cone-shaped glia exiting CNS), stage 14 (M), and stage 16 (N) showing extension of glial processes around the motor axons as they migrated out of the CNS and made their way to the periphery. Not all of the axon tracts at stage 16 (N) were ensheathed as glial ensheathment is completed during the larval stages. White lines in B–H and L–N refer to the CNS/PNS transition zones. O: Schematic showing distribution of 7 peripheral glial cells in a single segment at stage 17 of embryonic development in the PNS. The glial cells nearer to the CNS region are exit glia (EG). One of the exit glia is closet to the segmental nerve (SN), and the rest of the glia line the intersegmental nerve (ISN). The neuronal clusters ventral (v), ventral’ (v’), lateral (l), and dorsal (d) are shown as a reference. The most prominent neuronal cluster is the lateral cluster with 5 chordotonal neurons (lch). Both the ISN and SN contain motor and sensory axons.
The distinction between the fate of glia and neurons is under the control of the glial cells missing (gcm/glide) gene (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). In gcm-mutant embryos, the presumed glial progenitors are directed toward a neuronal cell fate. Conversely, overexpression of gcm in the developing embryonic nervous system is able to transform most neurons into glia (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). Also, gcm can induce several glial differentiation markers in the mesoderm when expressed early in development, thus acting as a master regulator of glial cell development (Akiyama-Oda et al., 1998; Bernardoni et al., 1998). The transient expression of gcm has been shown to initiate glial differentiation and activation of its downstream targets, such as repo, which control both differentiation and maintenance of glial cell fate (Campbell et al., 1994; Halter et al., 1995). Repo is the most ubiquitous glial marker expressed in the nuclei of all glial subtypes and many PNS glial support cells. Repo is not expressed in midline glial cells (Campbell et al., 1994; Halter et al., 1995; Jones, 2001).
Visualization of axonal ensheathment has been impeded by not having a proper glial membrane marker. Although glial proteins, such as neurexin IV (Nrx IV), contactin (Cont), and neuroglian (Nrg), are localized to glial membranes, they are also highly expressed in ectodermally derived epithelia (Baumgartner et al., 1996; Faivre-Sarrailh et al., 2004; Banerjee et al., 2006a). We therefore took advantage of a glial-specific driver (repo-GAL4) to drive expression of a reporter transgene, UASmCD8-GFP, in glia during embryogenesis (Brand and Perrimon, 1993; Lee and Luo, 1999). UASmCD8-GFP encodes a transmembrane protein that is targeted to the cell membrane, with no apparent lethality or toxicity associated with overexpression (Lee and Luo, 1999). As shown in Figure 1, glial cells that ultimately become peripheral glial cells originate from the CNS/PNS border region (Fig. 1F). After these glial cells undergo proliferation (Fig. 1G), they begin their migration toward the periphery (Fig. 1H). This glial migration was followed in embryos of the genotype repo-GAL4::UAS-mCD8GFP double-immunostained using anti-GFP antibody in order to visualize the extent of glial processes and anti-Fas II as a marker for motor axons (Fig. 1I–N). The development of the peripheral nerve occurs in multiple stages beginning with specification of glial cells and growth of motor and sensory axons. By stage 16 (~16 hr after egg lay), a fully formed peripheral nerve contains approximately 7 peripheral glial cells in a single abdominal segment. Glial cells that remain nearer to the CNS region are exit glia (EG; see Fig. 1O). One exit glia stays associated with the segmental nerve (SN), and the rest of the glia line the long intersegmental nerve (ISN). Although no systematic ultrastructural analysis of developing peripheral nerve ensheathment has been carried out, ultrastructural characteristics show that by stage 17 (~17 hr after egg lay), glial ensheathment of the embryonic peripheral nerve appears to be complete, that is, glial processes surrounding axons and formation of glial–glial septate junctions (SJs; Banerjee et al., 2006a). Glial ensheathment appears continuous along the length of peripheral nerves, as it leaves no uninsulated areas that would resemble vertebrate nodes of Ranvier. In Drosophila thick glial membrane sheaths along peripheral nerves and any anatomically distinct domains in nerves are also absent. Thus, axonal insulation in Drosophila is accomplished by thin glial processes running continuously the length of peripheral axons (Banerjee et al., 2006a).
Invertebrates lack myelin, and most genes encoding myelin protein homologues are absent from the Drosophila genome. However, during ensheathment of adult Drosophila thoracic ganglia, glial wrapping somewhat resembles the vertebrate myelin as the glial membrane forms multiple layers around the neurons (Freeman and Doherty, 2006). Ultrastructural analyses have revealed myelin-like glial sheaths found in other invertebrates such as copepods to have similar structures and apparently the same functions (Weatherby et al., 2000). Genomewide analysis of the presence of myelin proteins across species is providing novel information about the evolution of myelin and myelin-related proteins (Gould et al., 2005; Schweigreiter et al., 2006). The Drosophila genome lacks most genes encoding major myelin proteins, for example, myelin basic protein, myelin P zero, and peripheral myelin protein. However, Drosophila has a homologue of myelin proteolipid protein (Gould et al., 2005). These finding suggest that multilayer membrane formation may require proteins present only in Drosophila (Freeman and Doherty, 2006). Thus, the ensheathment of peripheral nerves in flies more closely resembles that of unmyelinated peripheral axons, where nonmyelinating Schwann cells ensheath small-diameter axons, referred to as Remak bundles (Taveggia et al., 2005). Whether this structural resemblance translates into functional similarity in action potential conduction remains to be addressed.
In the embryonic nerve, the inner ensheathing glial cell wraps around individual axons or fascicles and plays an essential role in axonal insulation and axon–glial interactions (Fig. 2), and perineurial (outer) glial cell processes wrap around inner glial cells along with axons. In third instar larval peripheral nerves, perineurial cells undergo postembryonic proliferation to accommodate the growing larval nerve (Leiserson et al., 2000), whereas inner glial cells do not proliferate during postembryonic stages and accommodate nerve growth by extending longer processes (Sepp et al., 2000). It is interesting to note that in Drosophila terminal glial differentiation and the ensheathment of nerve tracts occur in larval stages. What starts out as CNS-derived peripheral glia during embryonic stages migrate peripherally positioning themselves along the ISN and SN completes the ensheathment of SN motor branches and the ISN only during larval development, with glia showing a preference for ensheathing sensory neuron tracts over motor axons (Sepp et al., 2000; Parker and Auld, 2004).
Fig. 2.
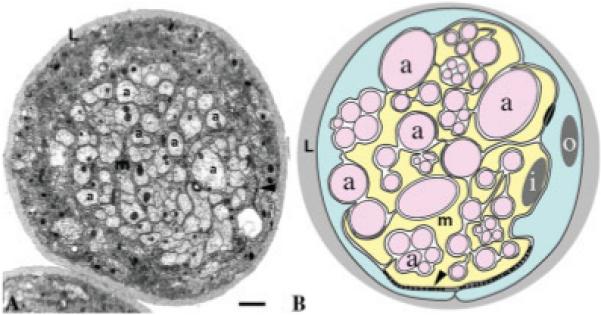
Peripheral nerve insulation in Drosophila. A: Transverse section through a Drosophila larval peripheral nerve showing individual axons (a) being wrapped by the membrane (m) of the inner glial cell. This in turn is wrapped by another glial cell, the perineurial glial cell. Extensive SJs were established between perineurial and inner glial membranes (arrowhead; Banerjee et al., 2006a). There is an outer-most layer of neural lamella (L) surrounding the nerve fibers. Scale bar = 1 μm. B: Schematic of a Drosophila peripheral nerve showing the axons and fascicles (a) surrounded by a sheath formed by the inner ensheathing glial cell (i), which in turn is surrounded by the outer perineurial glial cells (o). Arrowhead points to the areas where septate junctions formed between glial membranes.
EFFECTS OF LACK OF GLIAL CELLS ON PERIPHERAL NERVE ORGANIZATION
Because gcm is the master regulator gene for most glial cells in Drosophila, embryos mutant for gcm lack most glial cells in both the CNS and PNS. This provides an ideal situation for studying the effects of lack of glial cells on axon guidance, nerve fasciculation, and action potential conduction. Studies carried out on gcm mutants related to ensheathment, guidance, and fasciculation have revealed defects in nerve tracts in both the CNS and PNS (Sepp et al., 2001; Banerjee et al., 2006a). Embryos mutant for gcm display aberrant CNS longitudinal axon tracts and defasciculation of SN and ISN branches that exit the CNS (Sepp et al., 2001). Ultrastructural studies carried out on gcm mutant peripheral nerves demonstrated in greater detail the precise defects resulting from loss of glial cells and their ensheathing processes on the ensheathment of these nerves. In the absence of glial wrapping, the peripheral nerves of gcm mutants show total disorganization of axons in terms of their morphology and structural integrity and show signs of degeneration along with fluid buildup in the interaxonal spaces likely a result of the penetration of hemolymph inside nerve fibers (Banerjee et al., 2006a; Banerjee and Bhat, unpublished data).
A second gene, gcm2/glide2, was identified as both necessary and sufficient to promote glial differentiation (Kammerer and Giangrande, 2001; Alfonso and Jones, 2002). The gcm and gcm2 genes are about 27 kb apart, and the lack of both these proteins eliminates all Repopositive glial cells. Thus, these two genes encode all the glia-promoting activity in fly embryos. Similarly, a deficiency line, Df(2L)γ1 (Lane and Kalderon, 1993), removes both gcm and gcm2 genetic loci and displays a more severe phenotype of misrouted motor axons and complete lack of glial ensheathment compared with that in either mutant alone (Banerjee and Bhat, unpublished data). Because this deficiency removes many other genes in addition to both gcm genes, the severity of the mutant phenotype cannot be attributed solely to the complete loss of glial cells resulting from loss of gcm and gcm2. Despite these caveats, it is worth noting that even in the complete absence of glial cells, some motor axons do exit the CNS to travel toward the periphery. It raises an interesting possibility that apart from glial cells, other substrates or cell types might provide cues for axons to seek their targets. Although this area is still underinvestigated, there are reports showing that peripheral sensory axons are guided by multiple cues such as the trachea and musculature that ensure the high fidelity of axon trajectories evident in wild-type embryos (Younossi-Hartenstein and Hartenstein, 1993).
At about embryonic stage 13, the growth cones of motor axons extend their way through the array of peripheral glia at the CNS exit point and navigate a specific trajectory to pioneer the ISN tract (Sepp et al., 2000). Peripheral glia thus play an important role in the CNS–PNS transition zone through which motor axons grow out of the CNS and sensory axons grow toward the CNS. The Drosophila CNS–PNS transition zone is strongly reminiscent of the CNS–PNS interface in vertebrates, where boundary cap cells expressing Schwann cell markers are born in the neural crest and proceed to form compact arrays at the ventral transition zone and the dorsal root entry zone. Sensory and motor axons transit and interact with astrocytes and Schwann cells at this interface (Golding and Cohen, 1997). As in flies, vertebrate Schwann cells have also been shown to migrate along preestablished tracts determined by path-finder motor neurons, and glial processes never project beyond the leading edge of their neuronal migratory substrate (Carpenter and Hollyday, 1992).
Similar to that seen in gcm mutants, pathfinding errors as well as defasciculation are also seen in motor and sensory axons by targeted ablation of peripheral glia by expressing cell-death genes such as grim and ced-3 (Sepp et al., 2001). Similarly to that in vertebrates, virally mediated oligodendrocyte ablation causes defects in migration, dendritic arborization, and axon fasciculation, thereby profoundly affecting cerebellar neuronal development (Mathis et al., 2003).
Until recently, there were no available data to implicate the vertebrate homologues of Drosophila gcm in glial development and function. gcm1-Knockout mice die early during development because of failure of the placenta (Gunther et al., 2000; Schreiber et al., 2000), whereas gcm2-knockout mice are viable but lack a parathyroid gland (Gunther et al., 2000). Recent studies showed that Drosophila gcm genes are required in specific neuronal populations, in addition to glia in the larval visual system (Chotard et al., 2005; Yoshida et al., 2005). These rather puzzling observations were addressed in a recent study by Soustelle et al. (2007) in which the authors reevaluated the mode of gcm gene function in evolution. Using c-gcm, the chicken orthologue of Drosophila gcm, they demonstrated that a vertebrate gcm gene is expressed and required in the CNS. In Drosophila postembryonic development, gcm1 and gcm2 overexpression induced ectopic neuronal differentiation. This dual potential of gcm genes to perform both gliogenic and neurogenic roles in Drosophila but only a neurogenic role in vertebrates points to an interesting evolutionary aspect of the function of gcm genes (Soustelle et al., 2007). These findings also suggest that transcription factors may use cell-specific cofactors in a context-dependent manner to carry out their function in cellular specification and differentiation.
AXONAL ENSHEATHMENT FAILURE AND/OR IONIC IMBALANCE IN FRAY MUTANTS
The Drosophila fray locus encodes a 552-amino-acid protein with a serine–threonine kinase domain (Leiserson et al., 2000). The Fray kinase domain is most closely related to the PAK family of protein kinases, whose founding members are yeast STE20 and the mammalian PAKs (Ushiro et al., 1998; Manser and Lim, 1999). The closest members of the Fray kinase family include Fray, rat PASK, mouse SPAK, human OSR1, human Strerile-20-like, and C. elegans CAB6094. Thus, this family has been referred to as PF kinases, for PASK and Fray (Leiserson et al., 2000). Mutations in the fray gene result in defective axonal ensheathment in Drosophila larval peripheral nerves (Leiserson et al., 2000). Under light microscopy fray mutant third-instar larvae show unique nerve bulgings or swellings (Leiserson et al., 2000). Furthermore, the fray nerve phenotype seems to be manifested during larval development, suggesting it has a role in late glial development (as glial processes in fray mutants seem to elaborate and extend into the nerve to surround the axons but fail to form complete wraps). Immunostaining of fray mutant third-instar larval nerves reveals complete defasciculation and splitting of axons in the swollen regions (Banerjee and Bhat, unpublished data). Ultrastructural analyses of fray mutant nerves has demonstrated that the phenotype may be a result of the failure of the inner glial process to wrap the axons and not of a defect in the outer glial membrane (Leiserson et al., 2000). This is true for both the bulging and the nonbulging regions of the fray larval peripheral nerve. These initial ultrastructural observations have suggested that fray may modulate the glial cytoskeleton to bring about proper ensheathment of axons.
Recent biochemical and physiological studies suggest that the mammalian fray homologue PASK is required to maintain ionic homeostasis (Dowd and Forbush, 2003). It was reported that the sodium-potassium-chloride cotransporter (NKCC1) is the physiological substrate of PASK kinases and that phosphorylation of NKCC1 by PASK kinases results in enhanced transporter activity (Piechotta et al., 2002). Activation of NKCC1 was drastically reduced when a kinase-inactive, dominant-negative PASK mutant was overexpressed with the transporter. However, wild-type PASK overexpression caused only a modest increase in cotransporter activity (Dowd and Forbush, 2003). These mammalian studies have led to the idea that Drosophila Fray may also play an important role in ionic homeostasis in nerve fibers. Recent studies in Drosophila indicate that fray and a Drosophila cation chloride cotransporter display dominant genetic interactions (Leiserson and Keshishian, personal communication). These preliminary findings suggest that the fray nerve–bulging phenotype may result from an imbalance of ionic homeostasis, leading to fluid buildup and ultimately the fraying of the nerves in fray mutant larvae (Leiserson et al., 2000). These observations raise other questions about whether the phenotype displayed by fray mutant nerves is a secondary consequence of the physical damage to nerves as a result of fluid accumulation. The ultrastructural abnormalities displayed by fray mutant nerves at the level of the bulges indicate incomplete inner glial processes that fail to wrap the axons (Leiserson et al., 2000; Banerjee and Bhat, unpublished data). How does the fray phenotype manifest itself, and why do the bulges form at certain places and not throughout the nerve fibers? Are these hot spots for ionic exchange or places where cation cotransporters are localized? Future studies should be aimed at elucidating the mechanism by which Fray performs its function, and identification of Fray kinase substrates will provide insights into the signaling pathway that regulates ionic homeostasis and glial ensheathment.
GLIAL MIGRATION AND AXONAL ENSHEATHMENT
The ability of a cell to migrate over its substratum requires directed and asymmetrical organization of cellular activity. A migrating cell generates a protrusive force, usually associated with the formation and extension of lamellipodia in the direction of migration coupled with the development of new cell adhesions with the extracellular substrate. In addition, cell contractility is required to allow the cell body and the rear of the cell to follow the extending front. This protrusion and cell contraction are dependent on actin cytoskeleton and provide the driving force for cell migration. Myelinating glial cells like Schwann cells also migrate long distances over axonal surfaces prior to myelination. It has been shown that insulin-like growth factor–I increases Schwann cell process extension and motility by reorganizing the actin cytoskeleton via downstream activation of phosphatidylinositol 3-kinase, small GTPases, and focal adhesion kinase (Cheng et al., 2000). Similar to vertebrates, Drosophila peripheral glia are also known to migrate long distances from their site of origin in the CNS to ensheath peripheral axons. Using overexpression studies of dominant-negative and constitutively active RhoA and Rac1, it was demonstrated that interference with the glial actin cytoskeleton leads to defects in glial migration (Sepp and Auld, 2003a).
Many cell surface receptors signal through the Rho family of small GTPases to induce cytoskeletal changes to cause motility (Etienne-Manneville and Hall, 2002; Moon and Zheng, 2003; Starz-Gaiano and Montell, 2004). This family includes Rho, Rac, and Cdc42, which are capable of imparting a range of biological effects. For example, overexpression of dominant-negative Rac (N17) leads to failure of border cell migration (Murphy and Montell, 1996), whereas dominant-negative RhoA prevents germ-cell migration at an early stage (Kunwar et al., 2003). Alterations in the expression or function of Rho family members have been shown to contribute to inadequate regulation of cell motility, leading to invasive behavior of tumors (Schmitz et al., 2000).
The extent of the actin cytoskeleton in embryonic peripheral glia can be visualized by expressing actin-GFP in the glial cells under repo-GAL4 (Verkhusha et al., 1999; Sepp and Auld, 2003a). Actin-GFP highlights the glial actin cytoskeleton, and glial cell bodies and their processes can also be visualized in the early- and late-migrating phases. When RhoA and small GTPases are expressed in wild-type peripheral glial cells, subtle migration defects have been observed (Sepp and Auld, 2003a). Similarly, overexpression of constitutively active and dominant-negative RhoA and Rac1 shows severe glial migration defects, with peripheral glia stalling at the CNS–PNS transition zone, leading to ensheathment defects (Sepp and Auld, 2003a). In contrast, overexpression of RhoA as well as Rho dominant-negative and constitutively active forms using a pan-neuronal GAL4 driver (elav-GAL4) does not result in major glial migration phenotypes (Banerjee and Bhat, unpublished data) compared with using repo-GAL4. From these overexpression studies, it appears that the glial actin cytoskeleton plays an important role in glial migration and ensheathment of axons.
Rho kinase (ROCK) has been implicated in Schwann cell myelination in vertebrates (Melendez-Vasquez et al., 2004). ROCK, a major downstream effector of Rho, coordinates changes in glial cytoskeleton during spiral wrapping of glial processes via regulation of myosin phosphorylation and actomyosin assembly (Melendez-Vasquez et al., 2004). Given the striking similarity between Drosophila and vertebrate Rhos, elucidating regulation of Rho activity and the role of other Rho GTPases should provide important insights into the mechanisms of glial migration and ensheathment.
In addition to Rho GTPases, the Notch signaling pathway has been implicated in peripheral glial migration. Both loss- and gain-of-function mutations showed that Notch negatively regulates gcm and thereby gliogenesis in the Drosophila PNS (Van De Bor and Giangrande, 2001). Recent reports showed that Notch and Numb are required for normal migration of peripheral glia in Drosophila embryos, thereby implicating the Notch signaling pathway in peripheral glial migration (Edenfeld et al., 2007). Hypomorphic Notch alleles display a relatively normal-looking nervous system (Edenfeld et al., 2007). However, these mutants display abnormally positioned peripheral glial cells, suggesting that Notch may play a role during glial cell migration. Cell labeling experiments revealed that Notch mutant peripheral glia have an unusually enlarged growth cone, suggesting that the normal function of Notch may be to ensure proper neuron glial interaction by keeping the glial growth cone in close proximity to axonal membranes during glial migration (Edenfeld et al., 2007). In vertebrates, Notch regulates oligodendrocyte differentiation and the timing of axonal myelination (Wang et al., 1998; Hu et al., 2003). Interestingly, Delta-1 is also essential for proper migration and differentiation of neural crest cells, thereby implicating a function of Notch during cell migration in the vertebrates as well (De Bellard et al., 2002).
Recent studies indicate that glial cell differentiation is also controlled by the cytoplasmic assembly of a splicing factor, crooked neck (Crn). Crn is a conserved component of the splicing machinery that on translocation to the nucleus, promotes splicing of SJ-specific genes (Chung et al., 2002; Wang et al., 2003; Edenfeld et al., 2006). These crn mutants affect glial migration and differentiation, thus impairing proper wrapping of axons. For example, glial cells fail to form well-defined cellular processes around axonal fascicles and also seem to fail to establish SJs (Edenfeld et al., 2006). These findings further establish that glial migration coupled with differentiation is required for ensheathment of axons and link it to the assembly of cellular junctions formed by glial cells (Edenfeld et al., 2006). Interestingly, Crn does not have specific RNA-binding motifs to carry out alternative splicing. Crn accomplishes its splicing ability by interacting with held out wing (How; Lo and Frasch, 1997; Zaffran et al., 1997). The how locus in Drosophila encodes a number of RNA-binding proteins that display different subcellular expression patterns (Baehrecke, 1997). Indeed, biochemical experiments demonstrated that Crn binds to a short isoform of How (Edenfeld et al, 2006). The vertebrate homologue of How is Quaking, which is also required for glial differentiation, and Quaking mutants display tremors because of severe myelination defects (Sidman et al., 1964). Because Quaking and how mutants share defective axonal wrapping, it seems likely that the underlying molecular control of glial wrapping in invertebrates and vertebrates may have conserved mechanisms.
LOSS OF SJs RESULTS IN DEFECTIVE AXONAL ENSHEATHMENT AND COMPROMISED BLOOD–NERVE BARRIER
Another key aspect of peripheral axon ensheathment is maintenance of a proper blood–nerve barrier. This barrier is established by the close proximity of the inner and outer glial processes to form a tight seal that prevents the surrounding hemolymph from entering the nervous system and disrupting neuronal function. To this end, a number of glial mutants have been reported that show compromised blood–nerve barrier function because of partial or complete loss of functional SJs. The mutants identified in this category are gliotactin (gli; Auld et al., 1995) and nrx IV (Baumgartner et al., 1996). In gli mutants, the glia appear normal under light microscopy in terms of their birth, migration, and morphology. However, both ultrastructurally and physiologically they exhibit defects in glial SJs that lead to a compromised blood–nerve barrier (Auld et al., 1995; Baumgartner et al., 1996). The nrx IV–mutant peripheral nerves are in close proximity to glial membranes but completely lack glial SJs, resulting in a breach of the blood–nerve barrier (Banerjee et al., 2006a,b; Banerjee and Bhat, 2007). Since the discovery of Gli and Nrx IV, several SJ proteins have been identified in the nervous system. These include contactin, neuroglian, Lachesin, Moody, and Loco (Bainton et al., 2005; Schwabe et al., 2005; Banerjee et al., 2006a; Strigini et al., 2006). SJs and SJ-specific proteins such as Nrx IV in Drosophila and vertebrates show remarkable functional similarities in the formation and function of SJs (Bellen et al., 1998; Bhat et al., 2001; Banerjee et al., 2006a,b; Garcia-Fresco et al., 2006; Banerjee and Bhat, 2007).
The inner and outer glial processes establish distinct junctions that display unique ladder-like structures, the SJs. Because these junctions are formed between the outer and inner glial cell membranes, they could be considered heterotypic junctions. There is some evidence that SJs may also be formed between axons and inner glial membrane processes (Banerjee and Bhat, unpublished data). However, tissue fixation artifacts have not allowed us to conclusively establish the presence of axoglial SJs in fly nerves. SJs are also present in vertebrate myelinated axons in the paranodal region between myelin loops and the axolemma (Einheber et al., 1997; Bhat et al., 2001). These vertebrate axo-glial SJs are heterotypic junctions and are localized in the paranodal regions flanking a node of Ranvier between the myelin and the axonal membrane (Bhat, 2003). Functionally, SJs create an ionic barrier to protect the neural microenvironment (Baumgartner et al., 1996) and also serve as a fence that separates ion channels at nodes of Ranvier (Bhat et al., 2001; Rios et al., 2003). Each SJ may confer partial impermeability such that all the strands together form a barrier that protects neurons from a high potassium concentration in the hemolymph of insects (Hoyle, 1952; Juang and Carlson, 1992).
Not all SJ-specific proteins have been characterized for their role in nerve ensheathment or other aspects of the nervous system. One such family of proteins, found in both Drosophila and vertebrates, is the claudin family (Anderson and Van Itallie, 1995; Behr et al., 2003; Wu et al., 2004). Claudins are tight junction–specific proteins in vertebrates (Furuse and Tsukita, 2006). The SJs in invertebrates and the tight junctions of vertebrates were long thought to be distinct from both a morphological and a molecular standpoint. However, the identification of homologous epithelial junctional proteins of the claudin family in vertebrates and Drosophila has led to a change in this perspective (Behr et al., 2003; Wu et al., 2004).
A complex signaling pathway involving G-protein-coupled receptors, moody, loco, and the Gα genes Gi and Go are involved in the establishment of the intercellular SJs that generate the protective seal required for proper insulation of the CNS (Schwabe et al., 2005). Moody was identified in a screen for fly mutants with altered cocaine sensitivity and may thus provide interesting insights into the involvement of the blood-brain barrier in the physiological response to narcotics (Bainton et al., 2005). Phenotypic analysis of moody mutant embryos revealed that a junctional seal is created by the intercellular SJs formed by the surface glia that coalesce into single-layer epithelium and form contiguous SJ belts late in embryonic development (Schwabe et al., 2005). Using dye occlusion and onset of embryonic movement, the sealing of the nerve cord is complete by about 20 hr of development. The expression pattern of Moody, its G-protein α subunits Gi and Go and their regulator Loco in the surface glia, and the insulation defects and abnormal motor behavior phenotypes displayed by the respective mutants suggest that GPCR signaling plays a crucial role in the insulation of the nerve cord (Schwabe et al., 2005). In addition, both the moody and loco mutants were found to have insulating SJs of reduced length, irregular cell shape and size, and weaker and variable accumulation of cortical actin in the surface glia (Schwabe et al., 2005). These data suggest that the primary function of GPCR signaling may be linked to the organization of the cortical actin cytoskeleton, which in turn may be required to generate lengthy stretches of SJs. Future studies should unravel the genetic and molecular mechanisms of GPCR signaling in neuronal insulation and help to establish the role of Moody and its binding partners in organizing the glial cytoskeleton and forming the right complement of SJs.
One important aspect of glial ensheathment that remains to be addressed is the conduction of action potentials in glial mutants. To what extent do axons in glial mutants that have proper glial ensheathment but lack SJs conduct action potentials? For example, ultra-structural studies show that nrx IV and cont mutants have proper glial ensheathment but lack SJs (Banerjee et al., 2006a). Although synaptic transmission measurement has been carried out in glial mutants (Auld et al., 1995; Baumgartner et al., 1996), the rate at which mutant axons conduct action potentials remains to be established. It is estimated that the potassium (K+) ion concentration in Drosophila hemolymph is close to 40 mM, and at this K+ concentration, wild-type embryos with intact SJs display normal synaptic transmission, but synaptic transmission in nrx IV and gliotactin mutants is essentially abolished (Auld et al., 1995; Baumgartner et al, 1996; Banerjee et al., 2006a). That synaptic transmission can be restored to almost wild-type levels by decreasing the extracellular K+ concentration to 2 mM suggests that the key role of SJs is to maintain the ionic barrier (Auld et al., 1995; Baumgartner et al., 1996). To better understand the role played by glial ensheathment and SJs in action potential propagation, a thorough electrophysiological analysis of all glial mutants needs to be undertaken. Such studies, although technically challenging, will establish the role of individual loci in ensheathment, SJ formation, and ionic homeostasis and perhaps will help in separating various mutants into specific phenotypic categories.
CONCLUSIONS AND FUTURE DIRECTIONS
Although the study of Drosophila glia has come a long way since its inception, extensive studies are still needed to unravel the complex processes that underlie interactions between neuronal and glial cells. These coordinated mechanisms protect and insulate highly ion-sensitive neurons and their axons against hemolymph, as seen in Drosophila, and act as charge-selective barriers and molecular fences between ion-conducting channels, as seen in vertebrate myelinated nerve fibers. Most important, identification and characterization of novel components involved in neuronal–glial interactions will help in building a molecular framework to advance the understanding of the functional deficits that accompany demyelinating disorders and thus improve our ability to design strategies to prevent, diagnose, and treat human diseases like central and peripheral neuro-pathies that interrupt normal saltatory propagation of action potentials.
One of the lesser-known aspects of peripheral axon ensheathment is the possible involvement of various signaling pathways. Although recent studies have started to shed light on signaling aspects of the PNS in Drosophila, the existence of signaling pathways directly regulating glial ensheathment of axons remains largely unknown. It is an important area of research, as intercellular signaling among the various cell types in the peripheral nerves is likely to contribute to its proper growth and functioning. Axon contact is necessary for differentiation of peripheral glial cells. Certain aspects of axon ensheathment by peripheral glia have been shown to require EGFR signaling, which is known to express the necessary components of the DER/Ras/MAPK pathway (Sepp and Auld, 2003b). The inner glial cells that wrap around individual axons or fascicles also control the growth of perineurial glia (Yager et al., 2001). Recent studies using Drosophila peripheral nerves have identified molecules such as RasV12 that when specifically expressed in peripheral glia influence their thickness, which is mediated by phosphatidylinositol 3-kinase and Akt, suggesting that this pathway may promote nonautonomous growth of perineurial glia (Lavery et al., 2007).
Studies on the control of myelin thickness by the neuregulin/Erb B pathway have opened up new avenues for understanding the instructive roles of signaling mechanisms (Michailov et al., 2004). Do similar mechanisms exist in the fly axonal insulation? How is axonal ensheathment regulated in Drosophila nerves? Although our knowledge of the various signaling pathways involved in nerve ensheathment in the fly PNS is quite preliminary, it still provides a basic framework to unravel novel components that participate in signaling between neurons and glial cells.
ACKNOWLEDGMENTS
We thank Alan Fanning for comments on the manuscript and anonymous reviewers for their valuable suggestions.
Contract grant sponsor: National Institute of General Medical Sciences; Contract grant number: GM63074; Contract grant sponsor: National Institute of Neurological Disorders and Stroke of the National Institutes of Health; Contract grant number: NS050356; Contract grant sponsor: State of North Carolina.
REFERENCES
- Alfonso TB, Jones BW. gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–683. doi: 10.1006/dbio.2002.0740. [DOI] [PubMed] [Google Scholar]
- Akiyama-Oda Y, Hosoya T, Hotta Y. Alteration of cell fate by ectopic expression of Drosophila glial cells missing in non-neural cells. Dev Genes Evol. 1998;208:578–585. doi: 10.1007/s004270050217. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood–nerve barrier in Drosophila. Cell. 1995;81:757–767. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. who encodes a KH RNA binding protein that functions in muscle development. Development. 1997;124:1323–1332. doi: 10.1242/dev.124.7.1323. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006a;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006b;46:65–78. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood–brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin—novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Schulze KL. Invertebrate glia. In: Lazzarini RA, editor. Myelin biology and disorders. Elsevier Academic Press; Amsterdam, The Netherlands: 2003. pp. 199–222. [Google Scholar]
- Bernardoni R, Miller AA, Giangrande A. Glial differentiation does not require a neural ground state. Development. 1998;125:3189–3200. doi: 10.1242/dev.125.16.3189. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axo-glial junctions. Curr Opin Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127:237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Campbell G, Goring H, Lin T, Spana E, Andersson S, Doe CQ, Tomlinson A. RK2, a glial-specific homeodomain protein required for embryonic nerve cord condensation and viability in Drosophila. Development. 1994;120:2957–2966. doi: 10.1242/dev.120.10.2957. [DOI] [PubMed] [Google Scholar]
- Carpenter EM, Hollyday M. The distribution of neural crest-derived Schwann cells from subsets of brachial spinal segments into the peripheral nerves innervating the chick forelimb. Dev Biol. 1992;150:160–170. doi: 10.1016/0012-1606(92)90015-9. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Steinway ML, Russell JW, Feldman EL. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem. 2000;275:27197–27204. doi: 10.1074/jbc.M002534200. [DOI] [PubMed] [Google Scholar]
- Chotard C, Leung W, Salecker I. Glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Chung S, Zhou Z, Huddleston KA, Harrison DA, Reed R, Coleman TA, Rymond BC. Crooked neck is a component of the human spliceosome and implicated in the splicing process. Biochim Biophys Acta. 2002;1576:287–297. doi: 10.1016/s0167-4781(02)00368-8. [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Ching W, Gossler A, Bronner-Fraser M. Disruption of segmental neural crest migration and ephrin expression in delta-1 null mice. Dev Biol. 2002;249:121–130. doi: 10.1006/dbio.2002.0756. [DOI] [PubMed] [Google Scholar]
- Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- Edenfeld G, Altenhein B, Zierau A, Cleppien D, Krukkert K, Technau G, Klambt C. Notch and Numb are required for normal migration of peripheral glia in Drosophila. Dev Biol. 2007;301:27–37. doi: 10.1016/j.ydbio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Edenfeld G, Volohonsky G, Krukkert K, Naffin E, Lammel U, Grimm A, Engelen D, Reuveny A, Volk T, Klambt C. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila. Neuron. 2006;52:969–980. doi: 10.1016/j.neuron.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredieu JR, Mahowald AP. Glial interactions with neurons during Drosophila embryogenesis. Development. 1989;106:739–748. doi: 10.1242/dev.106.4.739. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Golding JP, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol Cell Neurosci. 1997;9:381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- Gould RM, Morrison HG, Gilland E, Campbell RK. Myelin tetraspan family proteins but no non-tetraspan family proteins are present in the ascidian (Ciona intestinalis) genome. Biol Bull. 2005;209:49–66. doi: 10.2307/3593141. [DOI] [PubMed] [Google Scholar]
- Gunther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000;406:199–203. doi: 10.1038/35018111. [DOI] [PubMed] [Google Scholar]
- Halter DA, Urban J, Rickert C, Ner SS, Ito K, Travers AA, Technau GM. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development. 1995;121:317–332. doi: 10.1242/dev.121.2.317. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Hoyle G. High blood potassium in insects in relation to nerve conduction. Nature. 1952;169:281–282. doi: 10.1038/169281a0. [DOI] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early learval ventral nerve cord. Rouxs Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Jones BW. Glial cell development in the Drosophila embryo. Bio-essays. 2001;23:877–887. doi: 10.1002/bies.1129. [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS. Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Juang JL, Carlson SD. Fine structure and blood–brain barrier properties of the central nervous system of a dipteran larva. J Comp Neurol. 1992;324:343–352. doi: 10.1002/cne.903240305. [DOI] [PubMed] [Google Scholar]
- Juang JL, Carlson SD. Analog of vertebrate anionic sites in blood-brain interface of larval Drosophila. Cell Tissue Res. 1994;277:87–95. doi: 10.1007/BF00303084. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Giangrande A. Glide2, a second glial promoting factor in Drosophila melanogaster. EMBO J. 2001;20:4664–4673. doi: 10.1093/emboj/20.17.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania A, Salzberg A, Bhat M, D’Evelyn D, He Y, Kiss I, Bellen HJ. P-element mutations affecting embryonic peripheral nervous system development in Drosophila melanogaster. Genetics. 1995;139:1663–1678. doi: 10.1093/genetics/139.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambt C, Goodman CS. The diversity and pattern of glia during axon pathway formation in the Drosophila embryo. Glia. 1991;4:205–213. doi: 10.1002/glia.440040212. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Starz-Gaiano M, Bainton RJ, Heberlein U, Lehmann R. Tre1, a G protein–coupled receptor, directs transepithelial migration of Drosophila germ cells. PLoS Biol. 2003;1:E80. doi: 10.1371/journal.pbio.0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane ME, Kalderon D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes Dev. 1993;7:1229–1243. doi: 10.1101/gad.7.7a.1229. [DOI] [PubMed] [Google Scholar]
- Lavery W, Hall V, Yager JC, Rottgers A, Wells MC, Stern M. Phosphatidylinositol 3-kinase and Akt nonautonomously promote perineurial glial growth in Drosophila peripheral nerves. J Neurosci. 2007;27:279–288. doi: 10.1523/JNEUROSCI.3370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Lemke G. Glial control of neuronal development. Annu Rev Neurosci. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. A novel KH-domain protein mediates cell adhesion processes in Drosophila. Dev Biol. 1997;190:241–256. doi: 10.1006/dbio.1997.8699. [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Manser E, Lim L. Roles of PAK family kinases. Prog Mol Subcell Biol. 1999;22:115–133. doi: 10.1007/978-3-642-58591-3_6. [DOI] [PubMed] [Google Scholar]
- Mathis C, Collin L, Borrelli E. Oligodendrocyte ablation impairs cerebellum development. Development. 2003;130:4709–4718. doi: 10.1242/dev.00675. [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates Schwann cell myelination and formation of associated axonal domains. J Neurosci. 2004;24:3953–3963. doi: 10.1523/JNEUROSCI.4920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Montell DJ. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J Cell Biol. 1996;133:617–630. doi: 10.1083/jcb.133.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Key interactions between neurons and glial cells during neural development in insects. Annu Rev Entomol. 2003;48:89–110. doi: 10.1146/annurev.ento.48.091801.112654. [DOI] [PubMed] [Google Scholar]
- Parker RJ, Auld VJ. Signaling in glial development: differentiation migration and axon guidance. Biochem Cell Biol. 2004;82:694–707. doi: 10.1139/o04-119. [DOI] [PubMed] [Google Scholar]
- Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Rio Hortega PD. Tercera aportacion al conocimiento morfologico e interpretacion functional de la oligodendroglia. Mem R Soc Esp Hist Nat. 1928;14:5–122. [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Schreiber J, Riethmacher-Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bosl MR, Wegner M. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol. 2000;20:2466–2474. doi: 10.1128/mcb.20.7.2466-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Schweigreiter R, Roots BI, Bandtlow CE, Gould RM. Understanding myelination through studying its evolution. Int Rev Neurobiol. 2006;73:219–273. doi: 10.1016/S0074-7742(06)73007-0. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003a;130:1825–1835. doi: 10.1242/dev.00413. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J Neurosci. 2003b;23:8221–8230. doi: 10.1523/JNEUROSCI.23-23-08221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia. 2000;30:122–133. doi: 10.1002/(sici)1098-1136(200004)30:2<122::aid-glia2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev Biol. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Dickie MM, Appel SH. Mutant mice (Quaking and Jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- Soustelle L, Trousse F, Jacques C, Ceron J, Cochard P, Soula C, Giangrande A. Neurogenic role of Gcm transcription factors is conserved in chicken spinal cord. Development. 2007;134:625–634. doi: 10.1242/dev.02750. [DOI] [PubMed] [Google Scholar]
- Starz-Gaiano M, Montell DJ. Genes that drive invasion and migration in Drosophila. Curr Opin Genet Dev. 2004;14:86–91. doi: 10.1016/j.gde.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol Cell Neurosci. 2006;32:91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro H, Tsutsumi T, Suzuki K, Kayahara T, Nakano K. Molecular cloning and characterization of a novel Ste20-related protein kinase enriched in neurons and transporting epithelia. Arch Biochem Biophys. 1998;355:233–240. doi: 10.1006/abbi.1998.0736. [DOI] [PubMed] [Google Scholar]
- Van De Bor V, Giangrande A. Notch signaling represses the glial fate in fly PNS. Development. 2001;128:1381–1390. doi: 10.1242/dev.128.8.1381. [DOI] [PubMed] [Google Scholar]
- Verkhusha VV, Tsukita S, Oda H. Actin dynamics in lamellipodia of migrating border cells in the Drosophila ovary revealed by a GFP-actin fusion protein. FEBS Lett. 1999;445:395–401. doi: 10.1016/s0014-5793(99)00124-6. [DOI] [PubMed] [Google Scholar]
- Vincent S, Vonesch JL, Giangrande A. Glide directs glial fate commitment and cell fate switch between neurones and glia. Development. 1996;122:131–139. doi: 10.1242/dev.122.1.131. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hobbs K, Lynn B, Rymond BC. The Clf1p splicing factor promotes spliceosome assembly through N-terminal tetratricopeptide repeat contacts. J Biol Chem. 2003;278:7875–7883. doi: 10.1074/jbc.M210839200. [DOI] [PubMed] [Google Scholar]
- Weatherby TM, Davis AD, Hartline DK, Lenz PH. The need for speed. II. Myelin in calanoid copepods. J Comp Physiol A. 2000;186:347–357. doi: 10.1007/s003590050435. [DOI] [PubMed] [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WC, Montell C. Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron. 1995;14:581–590. doi: 10.1016/0896-6273(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Yager J, Richards S, Hekmat-Scafe DS, Hurd DD, Sundaresan V, Caprette DR, Saxton WM, Carlson JR, Stern M. Control of Drosophila perineurial glial growth by interacting neurotransmitter-mediated signaling pathways. Proc Natl Acad Sci U S A. 2001;98:10445–10450. doi: 10.1073/pnas.191107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Soustelle L, Giangrande A, Umetsu D, Murakami S, Yasugi T, Awasaki T, Ito K, Sato M, Tabata T. DPP signaling controls development of the lamina glia required for retinal axon targeting in the visual system of Drosophila. Development. 2005;132:4587–4598. doi: 10.1242/dev.02040. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Hartenstein V. The role of the tracheae and musculature during pathfinding of Drosophila embryonic sensory axons. Dev Biol. 1993;158:430–447. doi: 10.1006/dbio.1993.1201. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Astier M, Gratecos D, Semeriva M. The held out wings (how) Drosophila gene encodes a putative RNA-binding protein involved in the control of muscular and cardiac activity. Development. 1997;124:2087–2098. doi: 10.1242/dev.124.10.2087. [DOI] [PubMed] [Google Scholar]


