Abstract
Breast cancer resistance protein (BCRP), a 72 kDa protein belongs to the subfamily G of the human ATP binding cassette transporter superfamily. Overexpression of BCRP was found to play a major role in the development of resistance against various chemotherapeutic agents. BCRP plays an important role in absorption, distribution and elimination of several therapeutic agents. BCRP expression and functional activity across human bronchial epithelium and its impact on pulmonary drug accumulation has not been established. The objective of this study is to identify and characterize the BCRP efflux transporter across human bronchial epithelium. Calu-3, a human bronchial epithelial cell line was employed as a model for this study. Reverse transcription-polymerase chain reaction (RT-PCR), western blot and immunocytochemical studies were performed to identify and characterize the expression of BCRP. RT-PCR studies detected ABCG2 mRNA levels in Calu-3 cells. A strong band for BCRP with a molecular weight of approximately 72 kDa was observed in Western blot analysis. Immunocytochemical studies confirmed the presence of BCRP on the apical membrane of human bronchial epithelium. Functional activity of BCRP was determined by performing uptake of radioactive substrate [3H]-mitoxantrone in the presence and absence of BCRP inhibitors. Uptake of [3H]-mitoxantrone was elevated significantly in the presence of GF120918 and fumitremorgin C. An increase in the accumulation of Hoechst 33342, a fluorescent dye was also detected in the presence of BCRP inhibitors when compared to control. In summary, this study provides evidence for the presence of an ATP dependent, membrane bound efflux transporter BCRP across human bronchial epithelial cell line, Calu-3.
Keywords: ABCG2, Breast cancer resistance protein, Mitoxantrone, Hoechst 33342, Calu-3 uptake, Western blot
1. Introduction
Breast cancer resistance protein (BCRP) was initially identified in a breast cancer derived cell line that showed drug resistance even in the presence of verapamil (a potent P-gp inhibitor) (Doyle et al., 1998). This protein was termed BCRP as it was first isolated from a human MCF-7 breast cancer cell line in an attempt to elucidate non-P-glycoprotein mechanisms of drug resistance. This efflux pump was also identified in a mitoxantrone-resistant human colon carcinoma cell line S1-M1−80 and hence gained the name MXR (Miyake et al., 1999).
BCRP/MXR is the second member of subfamily G of ATP-binding cassette (ABC) transporter superfamily. It is also referred to as ABCP (P stands for placenta), to indicate high levels of expression in placental tissue. BCRP, MXR and ABCP are homologous proteins differing only in one or two amino acid sequences. BCRP structurally diverges from the other prominent ABC transporters. BCRP is termed as half transporter having only six transmembrane helices and only one nucleotide binding domain. With the help of low resolution crystallography, it has been shown that this functional protein has a homodimeric structure (Henriksen et al., 2005; McDevitt et al., 2006).
BCRP is a high efficiency efflux pump with broad substrate specificity (Robey et al., 2009). BCRP expression is maximum in placenta and significantly high in the intestine and liver. Its expression in colon, brain, lungs, ovary and testis has also been reported (Mao and Unadkat, 2005). Various categories of drugs such as tyrosine kinase inhibitors, antivirals, HMG-CoA reductase inhibitors, carcinogens and flavonoids have been reported to be either substrates and/or inhibitors of this transporter system (Robey et al., 2009). Studies with ABCG2 knock out mice have revealed the physiological significance of this efflux transporter across barriers such as blood-brain, blood-testis and blood-fetal barriers (Vlaming et al., 2009).
Efflux transporters are believed to play an important role in preventing accumulation of potentially toxic xenobiotics in the lung (Scheffer et al., 2002). Efflux pumps along with mucociliary clearance, alveolar macrophages and bacteriocidal surfactants act as a protective barrier in the lungs. Recent studies have shown that majority of the BCRP substrates are basic lipophilic amines which readily accumulate in lung tissue. P-glycoprotein has also been shown to play a moderate role in the pulmonary accumulation of amine drugs (Roerig et al., 2004). Also, efflux transporters such as P-gp and MRPs have been attributed to drug resistance in lung cancers (Young et al., 2001; Young et al., 1999). BCRP is also involved in efflux of various chemotherapeutic agents employed in lung cancer which include mitoxantrone, doxorubicin, topotecan (Allen et al., 1999) and gefitinib (Yanase et al., 2004). Elevated mRNA levels have been correlated with resistance to these anticancer agents in lung cancer. BCRP expression in lungs might play a role in the disposition of drugs in cancer chemotherapy. Therefore, our objective is to identify and characterize the expression of BCRP in human bronchial epithelial cells. Various cell lines were utilized to investigate the expression and modulation of BCRP (Ceckova et al., 2006; Karla et al., 2009; Mao and Unadkat, 2005; Takada et al., 2005). Calu-3 cell line, derived from bronchial epithelium, has been employed as a model for the air way epithelium in a number of drug transport and metabolism studies (Florea et al., 2003; Sporty et al., 2008). In comparison to other models of the airway epithelium, several advantages of Calu-3 cell line such as tracheal epithelial sheets or primary tracheal cell cultures have been reported. Calu-3 cells form polarized monolayers with tight junctions producing high TEER values (Mathia et al., 2002). These cells express in vivo features of the airway epithelium (cilia, mucus production), several transport and metabolic systems relevant to drug absorption. These cells also produce several cell adhesion proteins (ZO-1 and E-cadherin) and generate mucosal secretions (Florea et al., 2003). In vitro-In vivo studies indicated good correlation (0.94) between permeability properties of Calu-3 cells with the rate of drug absorption from the rat lung (Mathia et al., 2002). Previous investigations established the functional activity of various efflux proteins belonging to ABC transporter super family such as MDR1 (Hamilton et al., 2001a) and MRP-1 (Hamilton et al., 2001b) in Calu-3 cells. Therefore, Calu-3 cell line has been selected as a model cell line to investigate the BCRP expression and to estimate its functional activity.
2. Materials and Methods
2.1. Materials
[3H]-Mitoxantrone (3 Ci/mmol) was purchased from Moravek biochemicals (Brea, CA, USA). Calu-3 cells were obtained from American Type Culture Collection (ATCC, USA). Cells between passages 20-40 were used for all the studies. DMEM-F12 (Dubelcco's minimum essential medium), fetal bovine serum, non essential aminoacids and trypsin-ethylenediaminetetraacetic acid were obtained from GIBCO (Invitrogen, Grand Island, NY, USA). Pencillin, streptomycin, sodium bicarbonate and HEPES were procured from Sigma Chemical (St. Louis, MO, USA). Culture flasks and twelve well uptake plates were obtained from Costar (Bedford, MA, USA). Hoechst 33342 was obtained from Invitrogen. NuPage Bis-Tris gels, sodium dodecyl sulfate running buffers, transfer buffer and sample loading buffers were supplied by Invitrogen. BXP-21 mouse monoclonal antibody was obtained from Santa Cruz biotechnology (Santa Cruz, CA, USA). All other reagents were obtained from Sigma and were of analytical grade.
2.2. Cell culture
Cells were cultured in DMEM-F12 medium supplemented with 10% heat inactivated fetal bovine serum, MEM non essential amino acids, HEPES, sodium bicarbonate, pencillin (100 μg/ml) and streptomycin (100 μg/ml). Cells were maintained at 37°C, in a humidified atmosphere of 5% CO2 and 90% relative humidity. Medium was replaced every alternate day until 5 days and subsequently every day until 11 days. Cells were subcultured by trypsinization with 0.25% trypsin containing 0.537 mM EDTA.
2.3. RT-PCR
Isolation of total RNA from cells was carried out with Trizol-LS® reagent (Invitrogen) according to manufacturer's instructions. RNA was mixed with 1.25 μl of oligo dT15 primer and denatured at 70°C for 10 minutes and 4°C for 5 minutes. Following denaturation, it was reverse transcribed to cDNA with 1 μl (10 units) of Moloney Murine Leukemia Virus Reverse Transcriptase (Promega, Madison, WI) per reaction mixture. After the first strand cDNA synthesis, 1 μl of cDNA was used for the PCR. GAPDH and ABCG2 primers were designed using OligoPerfect™ Designer (Invitrogen Corp. Carlsbad, CA). The forward and reverse primer sequences used for detection of GAPDH (NM_002046) were 5′-GGG AAG GTG AAG GTC GGA GT-3′ and 5′-GTC CAC CAC TGA CAC GTT GG-3′ respectively. Forward and reverse primer sequences used for the detection of ABCG2 (NM_004827) were 5′-GCT GCA AGG AAA GAT CCA AG-3′ and 5′-TTC CTG AGG CCA ATA AGG TG-3′ respectively. Briefly, the PCR mixture was adjusted to a final volume of 50 μl and contained template cDNA, 250 nM each of forward and reverse primers for the gene of interest, 1× Mg free PCR buffer (Promega), 3.75 mM MgCl2, 0.025U Taq Polymerase (Promega), and 200 μM dNTPs, The polymerase chain reaction conditions consisted of denaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 sec and 72°C for 1 min with a final extension at 72°C for 10 min. Ten microliters of PCR products obtained from PCR were separated by 2% agarose gel in tris-acetate-EDTA buffer along with 100 bp ladder.
2.4. Western blot analysis
Calu-3 cells grown in culture flasks were washed with phosphate-buffered saline (PBS) three times for ten minutes each. Cells were scrapped and homogenized in lysis buffer (PBS without calcium and magnesium, 1% Triton-x and protease inhibitor cocktail). The lysate was kept on ice for 30 minutes and then homogenized. Cell lysate was then centrifuged at 20,000 rpm for 10 minutes at 4°C. The supernatant was collected and stored at -80°C until further use. Protein content was measured with Bradford reagent. Three concentrations (25 μg, 50 μg and 75 μg) of protein were employed for gel electrophoresis. Protein samples were incubated at 100°C for 3 minutes and 15 μl samples was loaded onto a 4-12% NuPage Bis-Tris gel. Electrophoresis was carried out at 120V and subsequently transblotted at 15V for 90 minutes onto a polyvinylidene fluoride membrane. Blot was then blocked overnight with 3% non fat dry milk and 1.5% bovine serum albumin. The membrane was incubated with BXP-21 monoclonal antibody (1:200 dilution) for 2 hours at room temperature and then probed with secondary antibody tagged with horse radish peroxidase. Bands were visualized with ChemiImager 8900 digital imaging system (Alpha Innotech, San Leandro, CA).
2.5. Immunocytochemistry
Calu-3 cells grown on slides were used for these studies. Cells were washed with cold PBS for 10 minutes thrice and then fixed with 4% paraformaldehyde for 10 minutes. Fixed cells were washed and then permeabilized with 2% saponin and incubated for 2 min at room temperature. Non fat dry milk (3%) and bovine serum albumin (1.5%) were used for blocking for 2 hours. Membrane was incubated with mouse monoclonal antibody (BXP-21) for 2 hours. Following incubation with primary antibody, FITC tagged secondary antibody was added to cells and incubated for 1 hour at room temperature. For the negative control, slides without primary antibody were employed. Mounting medium was then added to chamber slides without any air bubbles and then stored at 4°C until further use. Slides were visualized using a fluorescence confocal microscope.
2.6. Uptake studies
At 11-13 days post seeding, cells were rinsed three times with DPBS (pH 7.4, 129 mM NaCl, 2.5mM KCl, 7.4mM Na2HP04, 1.3 mM KH2PO4, 1 mM CaCl2, 0.7 mM MgSO4 and 5.3 mM glucose) and equilibrated for 15 minutes with the buffer. [3H]-Mitoxantrone (166 nM) was used as a BCRP substrate to characterize functional activity. Time dependent uptake of [3H]-mitoxantrone was performed to determine the incubation time for all the studies. Uptake studies were performed by incubating the cells with a fixed amount of 0.5 μCi/ml of [3H]-mitoxantrone alone and in the presence of BCRP inhibitors at 37°C. GF120918 (0.1 to 200 μM), quercetin (50 μM) and saquinavir (50 μM) were added as BCRP inhibitors to determine the functional activity of BCRP. Following incubation, the reaction was stopped by addition of ice cold stop solution (210 mM KCl, 2 mM HEPES; pH 7.4). After three washings with stop solution, cells were lysed by keeping them overnight in 1 ml of 0.1% Triton-X solution in 0.3% NaOH. Following overnight lysis, 500 μl of the cell lysate from each well was transferred to scintillation vials containing 5 ml of scintillation cocktail (Fisher Scientific, Fair lawn, NJ). Samples were analyzed by liquid scintillation counter (Beckman instruments Inc., CA, USA) and uptake was normalized to the protein content in each well. Amount of protein in the cell lysate was measured by the Bio-Rad protein estimation kit with bovine serum albumin as standard.
2.7. Hoechst 33342 accumulation and cytotoxicity studies
Hoechst 33342, a fluorescent substrate was added to assess the efflux mediated by BCRP. Culture medium was first removed from the 96 well plates and washed with DPBS buffer at pH-7.4. Concentration dependent accumulation of Hoechst 33342 dye was measured to determine the experimental concentration for the remaining studies. Cells were incubated with varying concentrations of Hoechst 33342 dye ranging from 1 μM to 100 μM for 15 minutes. After 15 min incubation, reaction was arrested with stop solution (210 mM KCl and 2 mM HEPES; pH 7.4) and lysed with 1 ml of lysis buffer (0.1% Triton-X solution in 0.3% NaOH). Amount of intracellular dye was measured with a fluorescence spectrophotometer. Excitation and emission filters were set at 370 and 450nm and the intracellular mean fluorescence was normalized to protein content. For the remaining studies, cells were incubated with 5 μM Hoechst 33342 (100 μl) in DPBS buffer with or without BCRP inhibitors GF120918 (5 μM and 10 μM) and fumitremorgin C (1 μM and 5 μM). Similarly, ATP dependent accumulation study of Hoechst 33342 was performed after preincubating the Calu-3 cells for one hour in the presence of ouabain (1mM) and 2, 4, dinitrophenol (1 mM). Cytotoxicity studies were performed to study the toxicity of Hoechst 33342 dye, BCRP inhibitors and ATP modulators to Calu-3 cells for the incubation period mentioned previously. Hoechst dye was incubated alone and in the presence of BCRP inhibitors and ATP modulators at 37°C and the cell viability was evaluated by MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] assay obtained from Promega (G109). Calu-3 cells were grown in 96 well plates at a density of 100,000 cells per well and the tests were performed after 48 hours of seeding. 10 % DMSO was selected as a positive control for the cytotoxicity studies. Cytotoxicity tests were performed according to manufacturer's protocol.
2.8. Statistical Analysis
All the experiments were done in triplicate. All results were expressed as mean ± standard deviation. Statistical analysis between two groups of data was carried out with a student's t-test. A difference between mean values was considered significant at the P-value less than 0.05.
3. Results
3.1. RT-PCR
RT-PCR was carried out to determine the BCRP mRNA levels in Calu-3 cells. RNA was extracted from Calu-3 cells grown in DMEM-F12 medium with 10% FBS using trizol reagent. RT was performed using MMLV-RT enzyme and RNA was reverse transcribed to cDNA. Resulting cDNA was used as a template for PCR reaction. Primers specific to BCRP were designed using an Oligoperfect designer. GAPDH was used as an internal standard. Amplified PCR products obtained were separated by 2% agarose gel electrophoresis and bands were visualized. With BCRP specific primers, bands were detected at 508 bp following gel electrophoresis (Fig. 1). Band for GAPDH was detected at 723 bp.
FIGURE 1.
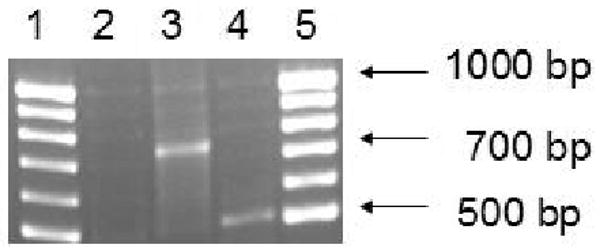
RT-PCR analysis of ABCG2 mRNA in Calu-3 cells. Agarose gel electrophoresis shows specific bands for human ABCG2 (∼ 508 bp). Lane-1 and lane-5 represents molecular ladder. Lane-2 represents the negative control. Lane-3 and lane-4 represents GAPDH and ABCG2 specific bands in Calu-3 cells. ABCG2 mRNA levels were detected in Calu-3 cells but not in negative control.
3.2. Western blot analysis
Western blot was performed to determine the expression of BCRP protein in Calu-3 cells. Protein samples were electrophoresed by SDS-PAGE and transferred to a PVDF membrane. BXP-21 monoclonal antibody was used to detect BCRP protein in Calu-3 cells. Lanes 2, 3 and 4 were loaded with 25, 50 and 75 μg protein extracted from Calu-3 cells. Fig. 2 illustrates the bands detected by chemiluminescence. Bands were observed at approximately 72 kDa corresponding to BCRP.
FIGURE 2.
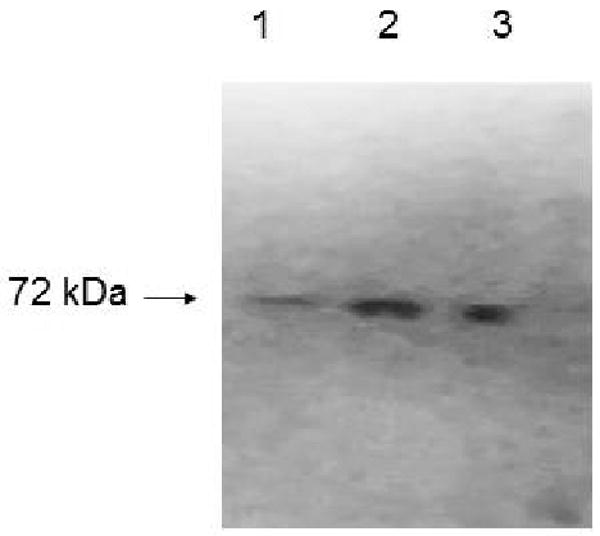
Immunodetection of breast cancer resistance protein using BXP-21 monoclonal antibody in Calu-3 cells. Total protein was isolated and protein samples (25 μg, 50 and 75 μg) were separated on a NuPAGE gel and electrotransferred to PVDF membrane. Blot shows specific bands of BCRP at ∼72 kDa. lane 1, 2 and 3 represent 25, 50 and 75 μg protein respectively.
3.3. Immunocytochemistry
BCRP expression was evaluated by immunocytochemical staining with a BXP-21 mouse monoclonal antibody. Calu-3 cells revealed strong plasma membrane localization (Fig. 3). In addition, some cytoplasmic staining was also observed with the use of BXP-21 antibody.
FIGURE 3.
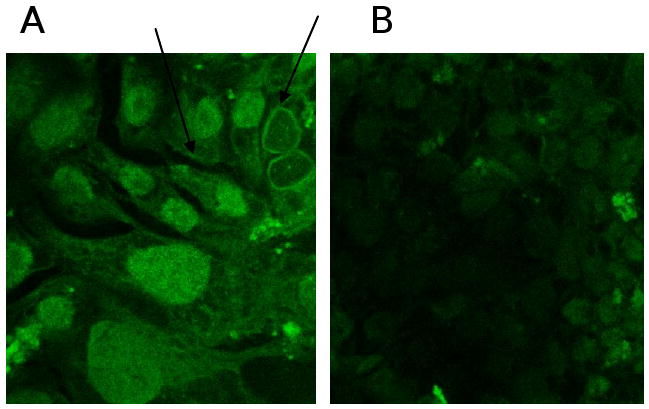
Immunocytochemical localization of ABCG2 in Calu-3 cells. BXP-21 mouse monoclonal antibody was used for detection of ABCG2. A and B represents the positive and negative control. Arrows indicate strong membrane localization.
3.4. Mitoxantrone uptake studies
Functional activity of BCRP was assessed by studying the uptake of [3H]-mitoxantrone in the presence of 0.1 to 200 μM GF120918. Time dependent uptake was performed to determine the uptake time for inhibition studies. Uptake of [3H]-mitoxantrone was found to be linear for 30 minutes as shown in Fig. 4A. So, a 15 minute time period was selected for all subsequent inhibition studies. Uptake of [3H]-mitoxantrone gradually increased significantly with increasing concentrations of GF120918. As shown in Fig. 4B, uptake of [3H]-mitoxantrone was found to be 112 ± 3.7%, 133 ± 4.2%, 145 ± 11.7% in the presence of 0.1, 1 and 10 μM GF120918 respectively as relative to control. Uptake of [3H]-mitoxantrone increased 2.67 times relative to control in presence of 200 μM GF120918. Dose dependent profile was found to be linear within the concentration range studied. Quercetin (50 μM) and Saquinavir (50 μM) enhanced the uptake of mitoxantrone to 141 ± 3.7%, 193 ± 2.6% respectively as compared to control (Fig. 4C).
FIGURE 4.
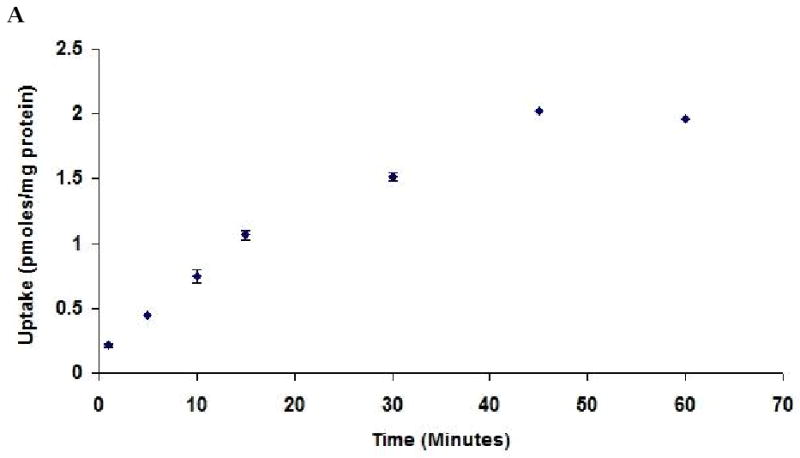
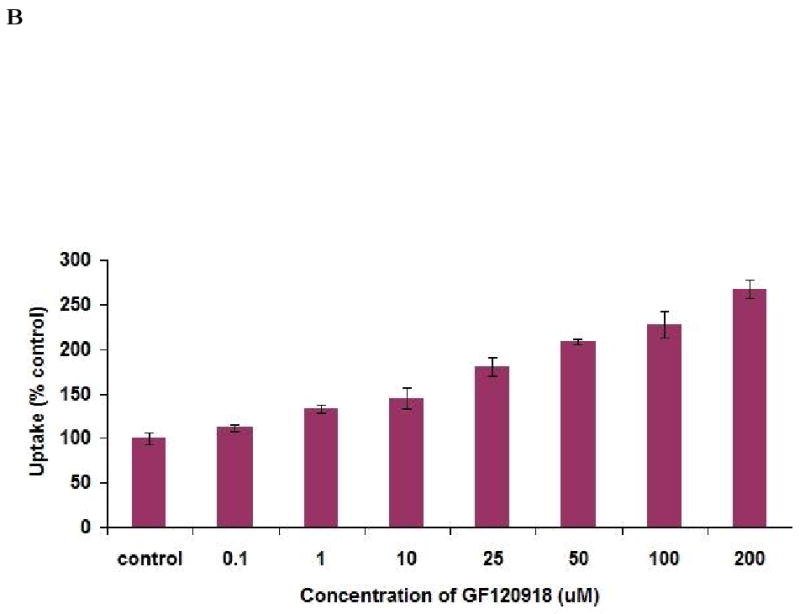
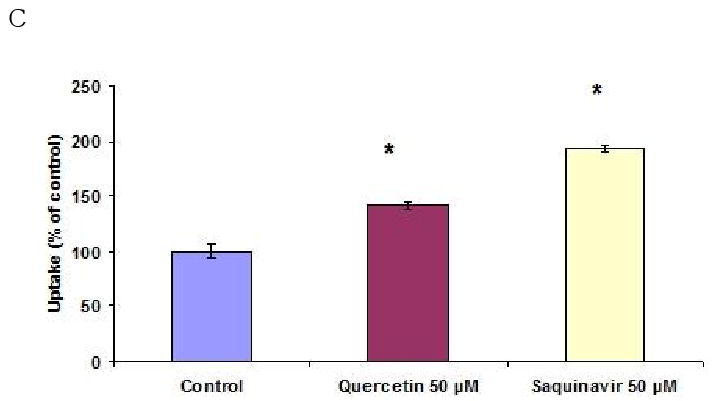
(A). Time dependent uptake of [3H]-mitoxantrone (0.5μCi/ml) by Calu-3 cells at 37°C, pH 7.4. (B). Uptake of [3H]-mitoxantrone in the presence of varying concentrations of GF120918. (C). Uptake of [3H]-mitoxantrone (0.5 μCi/ml) by Calu-3 cells in presence of 50 μM quercetin and saquinavir. Data are expressed as the mean±S.D. (n=3). Asterisk (*) represents significant difference from control (p <0.05).
3.5. Hoechst 33342 accumulation and cytotoxicity studies
BCRP functional activity was evaluated by measuring the accumulation of Hoechst 33342 in Calu-3 cells. Concentration dependent accumulation of Hoechst 33342 was also performed to select the 5 μM experimental concentration (Fig. 5A). ABCG2 specific inhibitors GF120918 and fumitremorgin C were selected for inhibition studies. As shown in Fig. 5B, Hoechst 33342 uptake was found to be 168.37 ± 11.7%, 159.37 ± 7%, 150.49 ± 6.5% and 164.34 ± 7.23% as compared to control in the presence of 5 μM GF120918, 10 μM GF120918, 1 μM fumitremorgin C and 5 μM fumitremorgin C. To study the energy dependency of Hoechst 33342 dye, uptake of the fluorescent dye was performed after preincubating the cells with 1 mM ouabain (Na+ /K+ -ATPase inhibitor) and 1 mM 2, 4-dinitrophenol (intracellular ATP reducer). Hoechst dye uptake increased significantly and the uptake was found to be 156.43 ± 12.76% and 175.68 ± 8.5% respectively as compared to control (Fig. 5C). In the course of our investigation, cytotoxicity studies were performed to determine whether the Hoechst 33342 dye, BCRP inhibitors and ATP modulators were cytotoxic to the cells. Hoechst 33342 and the inhibitors were incubated with the cells under the same conditions as the accumulation assay. Cell viability was expressed as percentage of control. Viability of Calu-3 cells was found to be unaffected in the presence of BCRP inhibitors and ATP modulators (Fig. 6).
FIGURE 5.
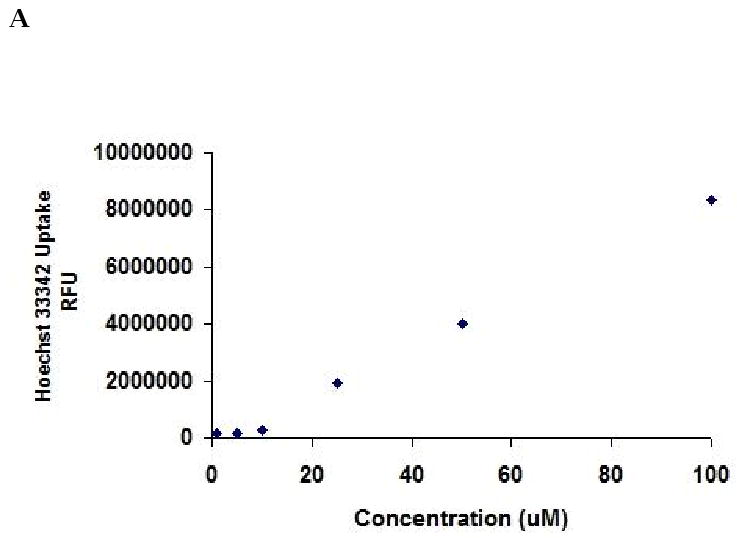
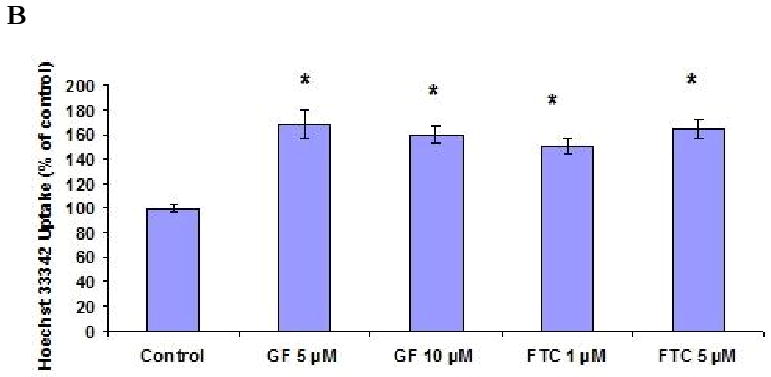
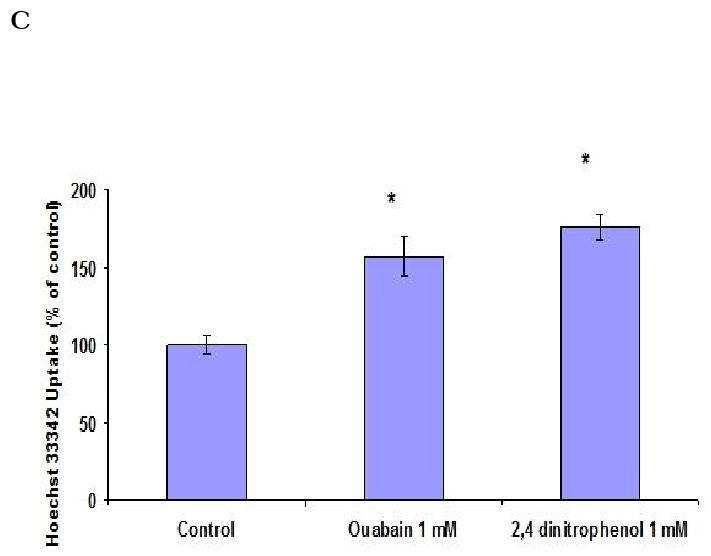
Hoechst 33342 dye accumulation assay by Calu-3 cells. (A). Concentration dependent accumulation of Hoechst 33342 dye. (B). Hoechst 33342 dye accumulation in presence of GF120918 and Fumitremorgin C. (C). Energy dependency of Hoechst 33342 dye. Accumulation was represented as percent control. Data are expressed as the mean±S.D. (n=6). Asterisk (*) represents significant difference from control (p <0.05).
FIGURE 6.
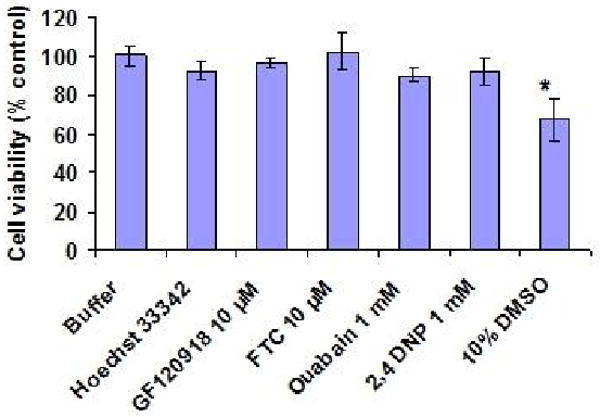
MTT assay to assess the cytotoxicity in Calu-3 cells. Cells were incubated with 5 μM Hoechst 33342 dye alone and in the presence of BCRP inhibitors and ATP modulators at 37°C. Cell viability was expressed as percent control. 10% DMSO was used as a positive control.
4. Discussion
Cellular drug resistance conferred by multidrug resistance (MDR) proteins is a major challenge in cancer chemotherapy. Tumor cells can acquire resistance to a single drug or to a class of cytotoxic drugs or to a broad spectrum of structurally and functionally diverse chemotherapeutic agents. The cellular mechanisms of MDR involve reduced drug uptake, activation of DNA repair and detoxification process, defective apoptotic signals (Lemos et al., 2008) and most commonly, active transport of drugs out of the cells mediated by efflux pumps (Borst and Elferink, 2002). P-glycoprotein (P-gp/ABCB1) has been widely investigated as a MDR transporter for many years. However, it is now established that various other ABC transporter proteins such as the multidrug resistance proteins (MRPs) (Deeley et al., 2006) and the breast cancer resistance protein (BCRP/ABCG2) (Sarkadi et al., 2006) are also major contributors to MDR.
BCRP plays an important role in absorption (intestine), distribution (placenta) and elimination (liver) of a variety of chemotherapeutic agents (Mao and Unadkat, 2005; Robey et al., 2009). BCRP is mainly localized in plasma membranes at the epithelial barriers such as blood- brain barrier, blood-testis barrier and blood-placental barrier. It plays a protective role across these barriers by active efflux of xenobiotics, pollutants, chemicals and toxins.
Pulmonary delivery has been utilized to administer several small molecule drugs, as well as therapeutic peptides and proteins. Small molecules delivered through inhalation route have higher bioavailability due to the lack of first pass metabolism and resistance to peptidases in the lungs. Lungs are constantly exposed to harmful xenobiotics and air borne toxins (Patton et al., 2004). Therefore, the organ displays protective efflux mechanisms to protect it from the external environment. Air-lung epithelial barrier is the most significant barrier to absorption for the inhaled drugs. Studies have shown that p-glycoprotein expressed in airway and bronchial epithelial cells is involved in the removal of environmental xenobiotics and cytotoxic drugs into the lumen and blood. Earlier studies from our lab have shown that p-glycoprotein limits the transport of anti-HIV protease inhibitors across Calu-3 cells (Patel et al., 2002). MRP1 has also been shown to be expressed on the basolateral side of the airway epithelium of the normal lung tissue. However, BCRP expression in bronchial epithelial cells and its impact on the kinetics of inhaled drugs has not been investigated.
The present study provides the first evidence for the expression of BCRP across air-lung epithelial barrier. Calu-3 cell line has been validated as a metabolic and transport model to study the mechanisms of drug delivery at respiratory epithelium. Therefore, we selected Calu-3 cell line as a model cell line to investigate the BCRP expression studies. Previous studies have shown that Calu-3 cells exhibited higher TEER values and stable morphology between 11-13 days. Calu-3 cells grown for 11-13 days were used for the functional activity studies. RT-PCR was carried out to determine the molecular evidence of ABCG2 in cultured Calu-3 cells. RT-PCR yielded a 508 bp product specific for ABCG2. Primers specific for ABCG2 and GAPDH were designed by OligoPerfect™ Designer. Specificity of the primers for their cDNA templates was evaluated by BLAST. Western blot was performed to study the expression of BCRP protein in Calu-3 cells. BXP-21, a mouse monoclonal antibody raised against amino acids 271-396 of ABCG2 was used for the western blot. BXP-21 does not cross react with other efflux transporters such as p-glycoprotein, MRP-2 and MRP-1. Bands for BCRP were detected at approximately 72 kDa with 25, 50 and 75 μg of protein used. Lee et al previously reported the expression of BCRP in human and rat brain cellular compartments using a BXP-21 as primary antibody and detected BCRP with a molecular weight of approximately 72 kDa (Lee et al., 2007). Immunocytochemical studies confirmed the apical membrane localization of BCRP.
Efflux transporters such as p-gp and BCRP have high capacity with wide substrate specificity. They reduce the intracellular accumulation of the cytotoxic drugs and modulate their absorption across biological barriers. To detect the functional activity of BCRP, active extrusion of BCRP substrates such as mitoxantrone, topotecan, rhodamine 123 and Hoechst 33342 have been studied extensively. In our studies, radioactive mitoxantrone was used as a substrate to determine the functional activity of BCRP. Mitoxantrone had been shown to be a high affinity substrate to BCRP and its accumulation correlated well with BCRP expression. Drug resistant cell lines that overexpress BCRP and cell lines transfected with BCRP cDNA were found to accumulate lower amounts of mitoxantrone (Litman et al., 2000; Yang et al., 2000). Radioactive mitoxantrone has been used previously as a radiolabeled substrate to determine the functional activity of BCRP. Radioactive mitoxantrone was incubated for 1.5 hours (Wang et al., 2006), 2 hours (Shi et al., 2009) and 3 hours (Pan and Elmquist, 2007) alone and in the presence of BCRP specific inhibitors. Time dependent uptake of mitoxantrone was performed to determine the optimal time of incubation. Based on the results, 15 minute incubation was selected for all the experiments. Results from the uptake studies reveal that uptake of mitoxantrone was increased in presence of BCRP inhibitors GF120918, quercetin and saquinavir. GF120918 is a potent BCRP inhibitor with an IC50 value of 50 nM (Giri et al., 2009; Mao and Unadkat, 2005; Zhang et al., 2005). Fumitremorgin c is a more specific and potent inhibitor for BCRP efflux pump than GF120918 (Shi et al., 2009). Previous studies by Allen et al 2002 have shown that higher concentrations (10-100 μM) of GF120918 and FTC were found to be cytotoxic to several cell lines (Allen et al., 2002). Miscellaneous potent inhibitors such as flavanoids (Zhang et al., 2004) and protease inhibitors (Gupta et al., 2004; Weiss et al., 2007) were also used to confirm the BCRP inhibition. Uptake increased significantly in the presence of BCRP inhibitors indicating the presence of functionally active BCRP. HIV protease inhibitors were found to be potent BCRP inhibitors and maximum concentration of saquinavir used for studies was 50 μM (Weiss et al 2004). Zhang et al have shown that flavanoids strongly inhibited the BCRP mediated accumulation of mitoxantrone. Mechanism of interaction between BCRP and its substrates and inhibitors is very complex due to the presence of multiple binding sites on BCRP. Also, ATP hydrolysis is one of the major mechanisms by which the inhibitors interact with BCRP. Flavanoids bind to the nucleotide binding domain of BCRP whereas mechanisms of interaction between BCRP and HIV protease inhibitors are yet to be understood completely.
Litman et al correlated BCRP expression with reduced accumulation of several fluorescent drugs such as mitoxantrone, daunorubicin, bisantrene, topotecan, lysotracker and rhodamine 123 in BCRP overexpressing cells (Litman et al., 2000). P-gp and Mrp1 substrates were not effluxed from BCRP overexpressing cells which indicated the specificity of BCRP mediated drug resistance. Kim et al studied the ability of BCRP to efflux the fluorescent dye, Hoechst 33342 in hematopoietic stem cells. In our studies, Hoechst 33342 dye accumulation assay was performed to determine the functional activity of BCRP. Hoechst 33342 was used at a concentration of 5 μM which was equivalent to approximately 3 μg/ml. Previous studies by Scharenberg et al and Kim et al used Hoechst 33342 dye at a concentration of 5 μg/ml and 4 μg/ml for BCRP mediated efflux studies (Kim et al., 2002; Scharenberg et al., 2002). Concentration dependent accumulation studies of Hoechst 33342 varying from 1 μM to 100 μM were done to select the experimental concentration within the linear range. A concentration of 5 μM Hoechst 33342 dye was selected for the fluorescent dye accumulation studies. Hoechst 33342 dye accumulation elevated significantly in presence of BCRP inhibitors such as GF120918 and fumitremorgin C. Fluorescent dye studies with ouabain and 2, 4-dinitrophenol indicated the energy dependent efflux of Hoechst dye in Calu-3 cells. These results suggest the involvement of energy dependent efflux process. MTT assay revealed the lack of toxicity of Hoechst 33342 alone and in the presence of BCRP inhibitors and ATP modulators to Calu-3 cells.
In conclusion, our present study demonstrates for the first time the presence of BCRP expression and functional activity across human bronchial epithelial cell line, Calu-3. Studies to modulate the expression of BCRP in Calu-3 are being carried out in our lab. Further studies are needed to delineate the role of BCRP on the pharmacokinetics of inhaled drugs.
Acknowledgments
This work was supported by National Institute of Health Grant GM64320-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59:4237–4241. [PubMed] [Google Scholar]
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Ceckova M, Libra A, Pavek P, Nachtigal P, Brabec M, Fuchs R, Staud F. Expression and functional activity of breast cancer resistance protein (BCRP, ABCG2) transporter in the human choriocarcinoma cell line BeWo. Clin Exp Pharmacol Physiol. 2006;33:58–65. doi: 10.1111/j.1440-1681.2006.04324.x. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea BI, Cassara ML, Junginger HE, Borchard G. Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. J Control Release. 2003;87:131–138. doi: 10.1016/s0168-3659(02)00356-5. [DOI] [PubMed] [Google Scholar]
- Giri N, Agarwal S, Shaik N, Pan G, Chen Y, Elmquist WF. Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in vitro assay design. Drug Metab Dispos. 2009;37:560–570. doi: 10.1124/dmd.108.022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- Hamilton KO, Backstrom G, Yazdanian MA, Audus KL. P-glycoprotein efflux pump expression and activity in Calu-3 cells. J Pharm Sci. 2001a;90:647–658. doi: 10.1002/1520-6017(200105)90:5<647::aid-jps1021>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther. 2001b;298:1199–1205. [PubMed] [Google Scholar]
- Henriksen U, Gether U, Litman T. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sci. 2005;118:1417–1426. doi: 10.1242/jcs.01729. [DOI] [PubMed] [Google Scholar]
- Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34:1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res. 2007;24:1262–1274. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- Lemos C, Jansen G, Peters GJ. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br J Cancer. 2008;98:857–862. doi: 10.1038/sj.bjc.6604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113(Pt 11):2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 2005;7:E118–133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathia NR, Timoszyk J, Stetsko PI, Megill JR, Smith RL, Wall DA. Permeability characteristics of calu-3 human bronchial epithelial cells: in vitro-in vivo correlation to predict lung absorption in rats. J Drug Target. 2002;10:31–40. doi: 10.1080/10611860290007504. [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- Pan G, Elmquist WF. Mitoxantrone permeability in MDCKII cells is influenced by active influx transport. Mol Pharm. 2007;4:475–483. doi: 10.1021/mp060083b. [DOI] [PubMed] [Google Scholar]
- Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig DL, Audi SH, Ahlf SB. Kinetic characterization of P-glycoprotein-mediated efflux of rhodamine 6G in the intact rabbit lung. Drug Metab Dispos. 2004;32:953–958. doi: 10.1124/dmd.104.000042. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332–339. doi: 10.1136/jcp.55.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, et al. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–793. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporty JL, Horalkova L, Ehrhardt C. In vitro cell culture models for the assessment of pulmonary drug disposition. Expert Opin Drug Metab Toxicol. 2008;4:333–345. doi: 10.1517/17425255.4.4.333. [DOI] [PubMed] [Google Scholar]
- Takada T, Suzuki H, Sugiyama Y. Characterization of polarized expression of point- or deletion-mutated human BCRP/ABCG2 in LLC-PK1 cells. Pharm Res. 2005;22:458–464. doi: 10.1007/s11095-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290:E798–807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, Sugimoto Y. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Mol Cancer Ther. 2004;3:1119–1125. [PubMed] [Google Scholar]
- Yang CH, Schneider E, Kuo ML, Volk EL, Rocchi E, Chen YC. BCRP/MXR/ABCP expression in topotecan-resistant human breast carcinoma cells. Biochem Pharmacol. 2000;60:831–837. doi: 10.1016/s0006-2952(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798–1804. [PubMed] [Google Scholar]
- Young LC, Campling BG, Voskoglou-Nomikos T, Cole SP, Deeley RG, Gerlach JH. Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res. 1999;5:673–680. [PubMed] [Google Scholar]
- Zhang S, Wang X, Sagawa K, Morris ME. Flavonoids chrysin and benzoflavone, potent breast cancer resistance protein inhibitors, have no significant effect on topotecan pharmacokinetics in rats or mdr1a/1b (-/-) mice. Drug Metab Dispos. 2005;33:341–348. doi: 10.1124/dmd.104.002501. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]


