Abstract
Dental pulp stem cells (DPSC) have drawn much interest for the regeneration of mineralized tissues, and several studies have compared DPSC to bone marrow-derived mesenchymal stem cells (BMMSC). However, conflicting results, possibly due to donor-associated variability, have been published and the regenerative potential of DPSC is currently unclear. In the present study we have sought to address this problem using a donor-matched experimental design to robustly compare the biological properties of DPSC and BMMSC. All experiments were performed using cells isolated from a single adult Sprague-Dawley rat. Our results show that DPSC and BMMSC had similar morphologies and flow cytometry profiles, were capable of forming colonies in vitro, and were capable of osteogenic, chondrogenic, and adipogenic differentiation. However, quantitative comparisons revealed that DPSC had a faster population doubling time and a higher percentage of stem/progenitor cells in the population as determined by clonogenic assays. Furthermore, while both cell populations formed mineral in vitro, DPSC had significantly higher alkaline phosphatase activity than BMMSC after three weeks in osteogenic medium. These data show several key differences between DPSC and BMMSC and support the possibility of using DPSC for mineralized tissue regeneration.
Keywords: dental pulp stem cells, bone marrow, mesenchymal stem cells, donor variation, proliferation, colony formation, clonogenicity, differentiation
1. Introduction
Dental pulp has been identified as a promising source of mesenchymal stem cells (MSC) for tissue engineering. The isolation of human dental pulp stem cells (DPSC) was first reported by Gronthos, Shi and coworkers, and they described DPSC as clonogenic, highly proliferative cells capable of self-renewal and multi-lineage differentiation (Gronthos et al. 2000; Gronthos et al. 2002). However, the primary attraction of dental pulp stem cells (DPSC) is their potential for cell banking. Several studies have demonstrated that DPSC retain their stem cell properties following cryopreservation (Papaccio et al. 2006), and we recently showed that DPSC cultures can be established from extracted human molars with a high efficiency, even after the whole tooth has been cryopreserved for up to one month (Perry et al. 2008). This finding represents a considerable advantage for tissue engineering and regenerative medicine, given that extracted human teeth are routinely discarded as medical waste.
Although bone marrow-derived mesenchymal stem cells (BMMSC) remain the most frequently used cells in bone tissue engineering, DPSC have attracted much interest for bone regeneration. Importantly, Laino, Papaccio, and colleagues have shown that human DPSC are capable of differentiating into osteoblasts and forming bone both in vitro and in vivo (Laino et al. 2005; Laino et al. 2006; d’Aquino et al. 2007; Graziano et al. 2008). In addition, several studies have compared DPSC to BMMSC in order to further evaluate the potential of DPSC for bone tissue engineering. However, conflicting results have been published. For example, Gronthos et al. transplanted human DPSC and BMMSC into immunocompromised mice using a granular calcium phosphate carrier, and they found that the DPSC formed a dentin-like tissue that surrounded a vascularized, pulp-like tissue, whereas BMMSC formed tissue resembling lamellar bone (Gronthos et al. 2000). While their results suggested that DPSC may be more useful for dentin regeneration, Otaki et al. performed virtually the same study and found that human DPSC form bone rather than dentin (Otaki et al. 2007). Similar results were published by Yu et al. using a rat model, and they also reported that DPSC formed mineral faster than BMMSC (Yu et al. 2007). Still yet, Zhang, Jansen and colleagues recently reported that human DPSC failed to form any mineralized tissue when seeded onto a macroporous calcium phosphate scaffold and implanted subcutaneously in immunocompromised mice for 10 weeks (Zhang et al. 2008).
One possible reason for the conflicting results published in the current literature is donor-associated variability. Differences in the biological properties of mesenchymal stem cell populations have previously been ascribed to donor-associated variability. For example, Phinney et al. studied donor-associated variability in human BMMSC and found that proliferation rate varied up to 12-fold, while alkaline phosphatase activity varied by up to 40-fold between donors (Phinney et al. 1999). Since such drastic differences are likely to exist between DPSC populations isolated from different donors too, a donor-matched comparison of DPSC and BMMSC is clearly desirable for evaluating the tissue engineering potential of DPSC. To this end, the objective of this study was to compare the biological characteristics of DPSC and BMMSC populations isolated from a single donor. Due to the difficulty of obtaining a matched pair of samples from a human donor, we chose to perform this study in a rat model. By using cells from the same donor, our experimental design facilitates a robust comparison between DPSC and BMMSC. To our knowledge, no studies of this nature have been reported.
2. Materials and methods
2.1. Isolation and culture of MSC
Under a protocol approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine, a single adult Sprague-Dawley rat (Harlan Sprague-Dawley, Indianapolis IN) was sacrificed to serve as a cell donor. To isolate DPSC, the rat’s incisors were extracted and cells were isolated from the dental pulp similar to that previously described (Perry et al. 2008). Special care was taken to prevent microbial contamination, as well as contamination by other dental cell populations (e.g. periodontal ligament cells (Gay et al. 2007) and apical bud cells (Ohshima et al. 2005)). Briefly, the rat’s mandible was removed and all soft tissue was blunt dissected away to reveal the incisor insertion. The incisors were then extracted from the mandible by cracking the mandible with forceps while pulling the tooth away from the socket. Any loose tissue on the root ends of the teeth was then trimmed off, and the external portions of the teeth were sterilized via immersion in 1% povidone-iodine for 2 minutes followed by immersion in 0.1% sodium thiosulfate for 1 minute and then a final rinse in sterile PBS. The teeth were then split length-wise to reveal the pulp chamber, and the pulp was removed from each tooth and placed in an enzymatic bath consisting of a combination of type I collagenase, type II collagenase, and thermolysin (a kind gift from Vitacyte, Indianapolis, IN). After a 40 minute incubation period at 37°C, the enzymes were neutralized with 10% serum in culture medium and the pulp digest was centrifuged at 500 × g for 5 minutes to yield a cell pellet. The cell pellet was then resuspended in fresh culture medium and plated at an initial concentration of 1 pulp digest per 25 cm2 flask.
BMMSC were isolated as we previously described for murine BMMSC (Li et al. 2009). Briefly, the rat’s tibiae and femora were isolated, the ends of the bones were clipped off, and a sterile 18 gauge needle with 5 ml syringe was used to flush the bone marrow cavity with culture medium. The resulting whole bone marrow suspension was washed with medium once, resuspended in fresh culture medium, and plated at an initial concentration of one bone per 25 cm2 flask.
Unless otherwise stated, all cells were cultured at 37°C and 5% CO2 in basal culture medium comprised of α-MEM (Invitrogen, Carlsbad, CA) with 20% FBS (Atlanta Biologicals, Lawrenceville, GA), 4 mM L-glutamine, 0.25 μg/ml amphotericin B, 100 IU/ml penicillin-G, and 100 μg/ml streptomycin (all from Invitrogen). Initially, medium was changed every other day to remove debris and non-adherent cells. Later, when cell growth was apparent, medium was changed twice weekly. The cells were passaged by detachment with trypsin when cultures became ≥70% confluent. The non-sorted or otherwise enriched DPSC and BMMSC populations were cultured in parallel so that both cell types were treated equally, and passage 3–5 cells were used for all experiments.
2.2. Comparison of cell surface markers
Antibodies to rat CD11b, CD29, CD45, CD59, CD90, and CD106 were obtained from BD Biosciences (San Jose, CA). DPSC and BMMSC were stained with the above antibodies and appropriate isotype controls as per the manufacturer’s instructions. Analysis was performed using a FACSCalibur instrument and CellQuest software (BD Biosciences).
2.3. Comparison of proliferation rates
Proliferation rates were compared on the basis of doubling time. DPSC and BMMSC were plated in parallel at 10,000 or 20,000 cells per well in 6 well plates. After 96 hours the cells were trypsinized, and cells from three wells were pooled and counted using a hemacytometer. The increase in cell number compared to the initial number of cells was used to calculate the doubling time by assuming an exponential growth model. The two plating densities were chosen in order to minimize any effects of density dependent cell growth (Javazon et al. 2001).
2.4. Comparison of colony-forming abilities
Clonogenic potential was assessed by two separate assays. First, DPSC and BMMSC were plated in parallel at 2,000 and 5,000 cells per dish in 15 cm dishes. Two plates at each density were set up. Two plating densities were used in order to minimize any effects of density dependent cell growth (Javazon et al. 2001). After 14–21 days of culture, the cells were stained with 2.5% Coomassie blue G-250 (Sigma, St. Louis, MO) and the number of colonies ≥1 mm in diameter per dish was counted on a white light transilluminator to determine the number of colonies per 1,000 cells plated.
In the second assay, a 10 cell/ml suspension was prepared of both DPSC and BMMSC, and 100 μL was deposited into wells of 96 well plates. After 21 days of culture, wells positive for clones were scored. Furthermore, 36 single DPSC and BMMSC clones were transferred from 96 to 24 well plates to determine if these clones had the proliferative potential after subcloning to achieve confluence in the 24 well plates. Confluent wells were scored after 14–21 days of growth.
2.5. Comparison of differentiation potentials
Chondrogenic, adipogenic, and osteogenic differentiation potentials of DPSC and BMMSC were evaluated using in vitro differentiation assays and the appropriate stains. Cells were plated at 20,000 cells per dish in 6 cm dishes and cultured in differentiation medium for up to 3 weeks with medium changes every 3–4 days. Chondrogenic differentiation medium consisted of basal medium supplemented with 10−8 M dexamethasone, 5 μg/ml ascorbic acid 2-phosphate, 10 mM β-glycerophosphate (all chemicals from Sigma) and 10 ng/ml transforming growth factor-β3 (PeproTech, Rocky Hill, NJ), adipogenic differentiation medium consisted of basal medium with 10 μg/ml insulin, 10−6 M dexamethasone, and 0.5 mM 3-isobutyl-1methyl xanthine, and osteogenic differentiation medium consisted of basal medium plus 10−8 M dexamethasone, 5 μg/ml ascorbic acid 2-phosphate, and 20 mM β-glycerophosphate.
To evaluate chondrogenic differentiation, the plates were fixed in 4% paraformaldehyde and proteoglycan deposition was detected by staining with 1% Alcian Blue (Sigma). For adipogenic differentiation, plates were stained with 0.5% Oil Red O (Sigma) in order to visualize lipid vacuole formation in the cells. Osteogenic differentiation was shown by alkaline phosphatase staining and staining for calcium deposition (see next paragraph). In addition to staining, differentiatiation was verified by gene expression using reverse transcription polymerase chain reaction (RT-PCR) for collagen type II (COL II), peroxisome proliferator activated receptor-γ (PPAR-γ), osteopontin (OPN), and runt-related transcription factor-2 (RUNX-2). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the control gene. RNA was isolated with the RNeasy mini kit (Qiagen, Valencia, CA), and gene expression was evaluated using a one-step RT-PCR kit (Qiagen) and the following thermocycling conditions: (1) 30 min at 50°C for reverse transcription, (2) 15 min at 95°C for PCR activation, (3) amplification cycles of 1 min at 94°C for denaturate, 30 sec at annealing temperature, and 30 sec at 72°C for extension, and (4) 10 min at 72°C for final extension. The annealing temperatures used were 64°C for COL II, 55°C for PPAR-γ, OPN, and GAPDH, and 57°C for RUNX-2. Primer sequences are presented in Table 1.
Table 1.
Primers for RT-PCR.
| Gene | Primer Sequence |
|
|---|---|---|
| Forward | Reverse | |
| COLII | GGAAGGCGTGAGGTCTTCTGT | CACCGCTAACGTCCAGATGAC |
| PPAR-γ | TGGAGCCTAAGTTTGAGTTGC | TGACAATCTGCCTGAGGTCTG |
| OPN | GATGAACCAAGCGTGGAAAC | TGAAACTCGTGGCTCTGATG |
| RUNX-2 | GCCAGGTTCAACGATCTGAG | GAGGCGGTCAGAGAACAAAC |
| GAPDH | AACTCCCATTCCTCCACCTT | GAGGGCCTCTCTCTTGCTCT |
Sequences for COLII and PPAR-γ were obtained from (Peng et al. 2008).
Sequences for OPN, RUNX-2, and GAPDH were obtained from (Kuroda et al. 2005).
To demonstrate mineralization potential, the cells were cultured in osteogenic medium for 3 weeks. The presence of mineralized nodules was then verified via staining with a 2% alizarin red S (Sigma) solution. Mineralization potential was quantitatively evaluated on the basis of alkaline phosphatase activity. DPSC and BMMSC were plated in triplicate at 20,000 cells per well in 6 well plates. After three weeks of culture in osteogenic medium, the cells were washed with PBS and then lysed by adding 1 ml of 0.05% Triton X-100 in PBS and performing two freeze-thaw cycles. Cell debris was pelleted by centrifugation, and the lysate was analyzed for alkaline phosphatase activity with a p-nitrophenyl phosphate-based assay (Sigma). The alkaline phosphatase activity was measured as the moles of p-nitrophenyl phosphate hydrolyzed per minute over a 30 minute period, and this value was normalized to the protein concentration in the lysate. Total protein concentration was determined using the BCA total protein assay (Pierce Biotechnology, Rockford, IL).
2.6. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Means were compared using the unpaired Student’s t test, or Welch’s unpaired t test if the SDs were significantly different. Fisher’s exact test was used to compare cloning and replating efficiency of DPSC and BMMSC. Statistical analyses were performed using InStat Version 3.05 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com).
3. RESULTS
3.1. Cell Morphologies and Flow Cytometry Profiles
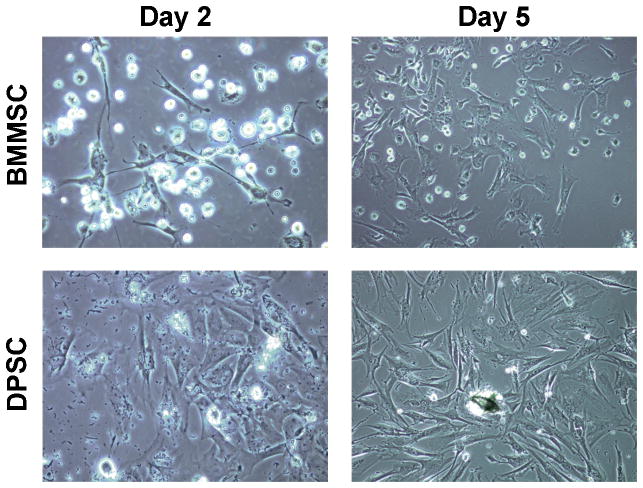
Two days after isolation debris from the pulp digest was apparent in the DPSC cultures, but it was removed when the culture medium was refreshed. By five days, both DPSC and BMMSC were proliferating, appeared healthy, and were exhibiting a fibroblastic morphology (Figure 1), which is characteristic of MSC (Kemp et al. 2005).
Figure 1. Appearance of early DPSC and BMMSC cultures.
Representative photographs (40× magnification) of DPSC and BMMSC cultures at days 2 and 5 show that both cell types appeared healthy, were proliferating, and exhibited a fibroblastic morphology typical of MSC.
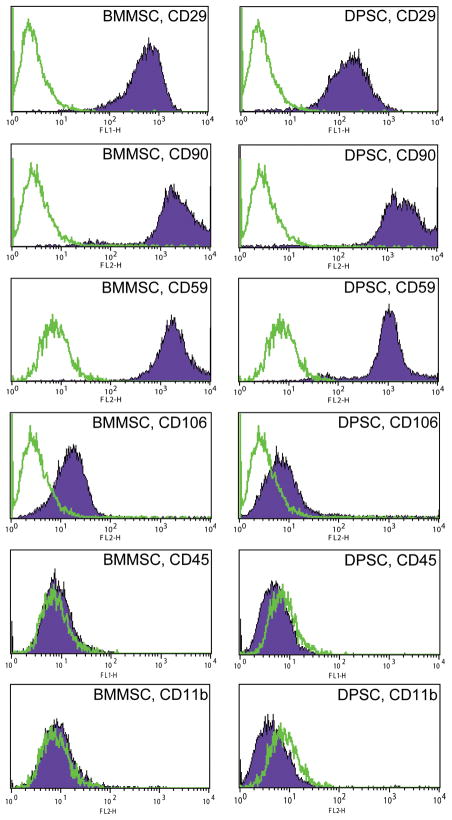
Cell surface markers for rat MSC are not as well established, nor are antibodies as widely available as for murine or human MSC. The cell surface markers chosen for this study (CD11b, CD29, CD45, CD59, CD90, and CD106) were based on the prior literature (Woodbury et al. 2000; Javazon et al. 2001; Schafer et al. 2007), as well as commercial availability. We found that passage 3–5 DPSC and BMMSC possessed a similar profile of cell surface markers by flow cytometry (Figure 2). Both cell populations were positive (defined as ≥ 95% positive cells) for a variety of adhesion molecules (CD29, CD59, CD90, and CD106), but were negative (≤ 5% positive cells) for the hematologic markers CD45 and CD11b.
Figure 2. DPSC and BMMSC express similar cell surface marker profiles.
Early passage DPSC and BMMSC were found to possess a similar profile of cell surface markers (positive for adhesion molecules CD29, CD59, CD90, and CD106; negative for hematologic markers CD45 and CD11b). The open histograms signify staining with isotype controls, and the filled histograms represent staining with the specified surface marker antibody.
3.2. Proliferation Rate
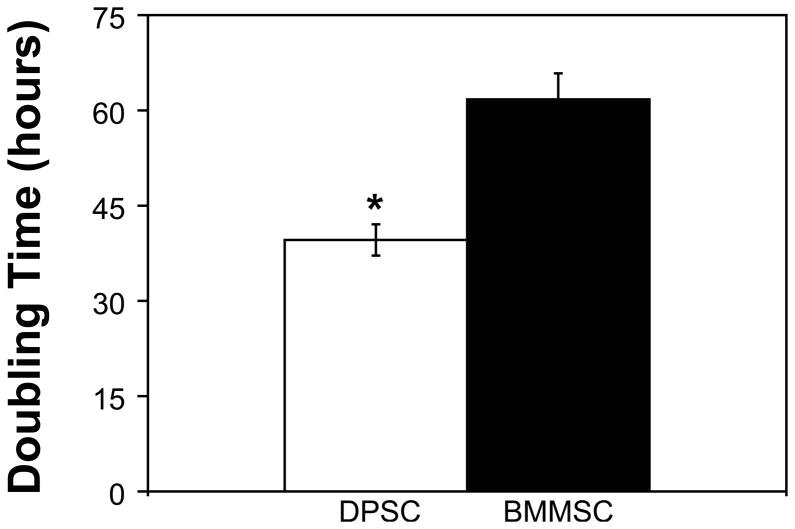
The proliferation rates of passage 3 DPSC and BMMSC cultures were measured by assuming exponential growth and then determining the amount of time needed for the cell population to double in number (Figure 3). Passage 3 DPSC were found to have a doubling time of 39.6 ± 2.5 hours, whereas passage 3 BMMSC had a significantly longer doubling time of 61.7 ± 4.1 hours (P = 0.0192).
Figure 3. DPSC have a significantly shorter population doubling time than BMMSC.
DPSC and BMMSC were plated at 10,000 and 20,000 cells per well in 6 well plates and cultured for 96 hours. DPSC were found to have a doubling time of 39.6 ± 2.5 hours, which was significantly shorter than the 61.7 ± 4.1 hours observed for BMMSC (N = 4; P = 0.0192).
3.3. Colony Formation and Clonogenic Potential
The ability of DPSC and BMMSC cultures to re-form colonies, a primitive measure of progenitor cell activity, was initially determined using a colony-forming assay (Figure 4A). Two concentrations of DPSC and BMMSC were plated to minimize any effects of plating density (Javazon et al. 2001). Colonies were scored after 14–21 days in culture, and the data were normalized to colonies per 1,000 cells plated. DPSC displayed significantly more colonies, with 151.5 ± 52.3 colonies per 1,000 cells plated compared to 63.8 ± 18.1 colonies per 1,000 cells plated for BMMSC (P = 0.023).
Figure 4. DPSC generate significantly more colonies and contain significantly more clonogenic potential than BMMSC.
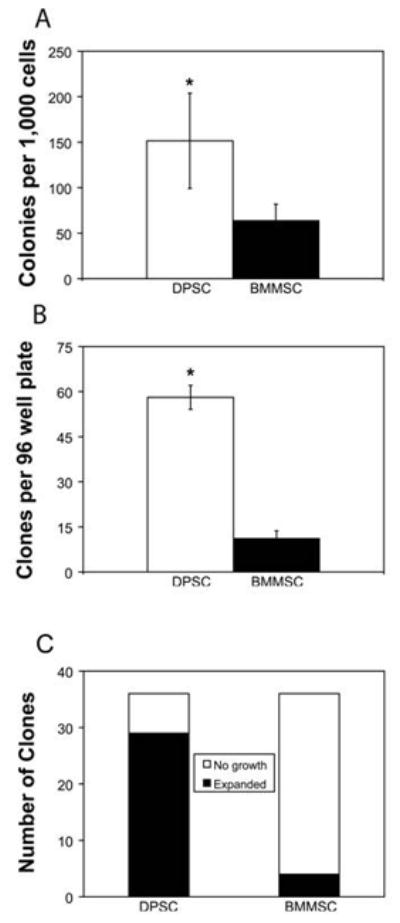
(A) DPSC and BMMSC were plated at 2,000 and 5,000 cells per dish in 15 cm dishes, and after 14–21 days in culture colonies ≥1 mm in diameter per dish were scored. DPSC had significantly more colonies, with 151.5 ± 52.3 colonies per 1,000 cells plated compared to 63.8 ± 18.1 colonies per 1,000 cells plated for BMMSC (N = 4; P = 0.023). (B) DPSC and BMMSC were plated at an average of one cell per well in 96 well plates. After 21 days DPSC had formed 58.1 ± 4 clones per plate, which was significantly greater than the 11.1 ± 2.6 clones per plate formed by BMMSC (P < 0.0001). (C) Thirty-six clones of each cell type were transferred to 24 well plates and evaluated on the basis expansion potential. While few BMMSC retained their ability to proliferate after replating (4/36), a significantly larger fraction of DPSC clones (29/36) proliferated (N = 8 plates from two independent experiments; P < 0.0001).
Based on these findings, the clonogenic and proliferative potential of DPSC and BMMSC were assessed with a more stringent assay. DPSC and BMMSC were plated at an average of one cell per well in 96 well plates, and the number of wells with clones present per 96 well plate was determined after 21 days (Figure 4B). DPSC formed significantly more clones per 96 well plate than BMMSC (58.1 ± 4 vs. 11.1 ± 2.6 clones per 96 well plate respectively; P < 0.0001). To determine what percentage of these clones retained the proliferative potential to expand upon replating, thirty-six clones of each cell type were transferred to 24 well plates and cultured for up to three weeks (Figure 4C). A much greater fraction of DPSC clones retained the ability to proliferate after replating than BMMSC clones (29/36 DPSC vs. 4/36 BMMSC clones; P < 0.0001).
3.4. Differentiation Potentials
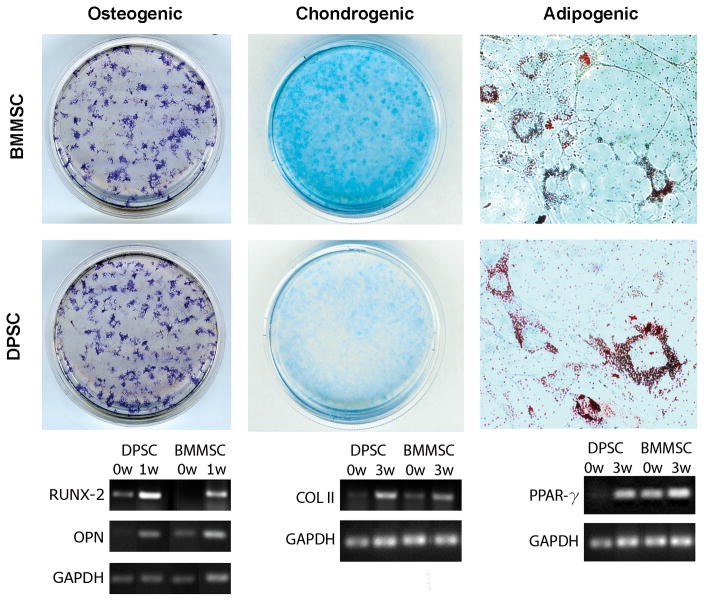
DPSC and BMMSC cultures both demonstrated tri-lineage differentiation potential after 3 weeks of culture in the appropriate differentiation medium (Figure 5). Proteoglycan deposition, which occurs during chondrogenic differentiation, was demonstrated for both DPSC and BMMSC by positive staining with Alcian Blue. Chondrogenic differentiation was also shown using RT-PCR for COL II. Both DPSC and BMMSC showed a clear upregulation of COL II after 3 weeks in chondrogenic medium. Similarly, after 3 weeks in adipogenic medium DPSC and BMMSC both underwent adipogenic differentiation, which was evident from the presence of abundant lipid vacuoles that stained positive with Oil Red O and upregulation of PPAR-γ. Finally, osteogenic differentiation was definitively demonstrated by positive staining for alkaline phosphatase and upregulation of osteopontin and RUNX-2 transcripts.
Figure 5. DPSC and BMMSC both undergo trilineage differentiation.
DPSC and BMMSC were cultured in osteogenic (left), chondrogenic (middle), or adipogenic (right) differentiation medium for three weeks and stained as described in the Materials and Methods. Representative macroscopic views show positive staining for osteogenic and chondrogenic differentiation. Adipogenic differentiation was indicated by the visualization of positively stained lipid vacuoles (40X magnification). RT-PCR results show that expression of COL II (40 amplification cycles), PPAR-γ (35 amplification cycles), osteopontin (20 amplification cycles), and RUNX-2 (35 amplification cycles) was upregulated in both DPSC and BMMSC, which confirmed differentiation.
Due to the interest in using DPSC for regenerating mineralized tissues, mineralization potential was evaluated in vitro. Both cell types formed mineralized nodules after 3 weeks of culture in osteogenic medium, as indicated by staining with alizarin red (Figure 6A, 6B). Quantitative comparison of alkaline phosphatase activity at 3 weeks (Figure 6C) showed that DPSC had a significantly higher alkaline phosphatase activity of 121.3 ± 31.3 nmole/mg*min, compared to 67.7 ± 5.4 nmole/mg*min for BMMSC (P = 0.0102).
Figure 6. DPSC exhibit significantly greater alkaline phosphatase activity than BMMSC.
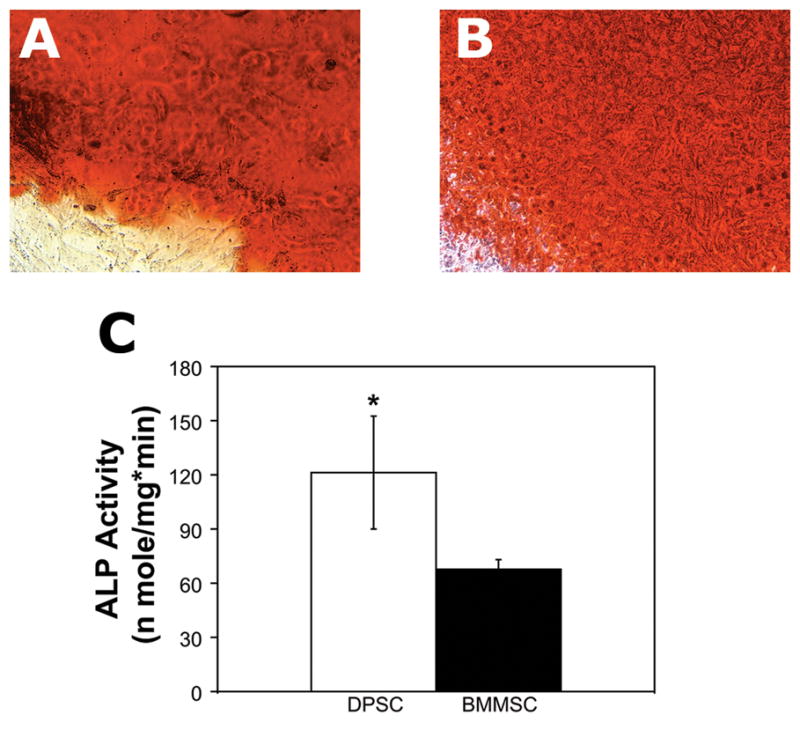
DPSC and BMMSC were cultured in osteogenic differentiation medium for three weeks. Positive staining with alizarin red showed that both DPSC (A) and BMMSC (B) formed mineralized nodules (20X magnification). (C) DPSC had a significantly higher alkaline phosphatase activity of 121.3 ± 31.3 nmole/mg*min, compared to 67.7 ± 5.4 nmole/mg*min for BMMSC (N = 3; P = 0.0102). Results shown are from one of two independent experiments performed in triplicate.
4. Discussion
Due to the interest in using DPSC for mineralized tissue regeneration, several studies comparing DPSC and BMMSC populations have previously been published. However, conflicting results have been reported by different research groups, which have led to ambiguity concerning the usefulness of DPSC. Donor-associated variability may be the source of these conflicting results, since this variable has been shown to account for drastic differences in the biological properties (e.g. proliferation rate, differentiation potential, etc.) of MSC populations (Phinney et al. 1999; Siddappa et al. 2007). Thus, in this study we have compared the properties of DPSC and BMMSC in vitro using a donor-matched experimental design. The present study is the first to compare DPSC and BMMSC isolated from the same donor animal.
Due to the ease of performing donor-matching in experimental animals, for this study we used cells isolated from an adult Sprague Dawley rat. The DPSC isolation protocol used in this study was identical to what we previously reported for human teeth (Perry et al. 2008), and precautions were taken to prevent contamination from other dental tissues. Whole populations of low passage cells were used for all experiments, rather than sorted subpopulations or clones, as these are likely the cells that would be used clinically. Our initial characterization of the cells showed several similarities between rat DPSC and BMMSC. Namely, we showed that both cell types exhibited a fibroblastic morphology in culture (Figure 1), and were positive for mesenchymal cell markers and negative for hematologic markers (Figure 2). We also showed that both cell types were capable of forming colonies (Figure 4) and undergoing osteogenic, adipogenic, and chondrogenic differentiation (Figure 5). These results were similar to what we previously observed for DPSC isolated from human teeth (Perry et al. 2008), and they are consistent with previously reported results demonstrating the MSC nature of DPSC (Gronthos et al. 2000; Shi et al. 2001; Gronthos et al. 2002; Shi et al. 2005; Laino et al. 2006; Wei et al. 2007). Of more interest, however, are our quantitative results comparing proliferation rate, colony formation, clonogenic potential, and mineralization potential. These characteristics of the populations are important because they are known to vary significantly between donors (Phinney et al. 1999; Siddappa et al. 2007), and they are key determinants of the regenerative potential of MSC.
Proliferation rate was evaluated on the basis of population doubling time (Figure 3), and we found that the doubling time for established DPSC cultures (39.6 ± 2.5 hours) was significantly faster than that of BMMSC (61.7 ± 4.1 hours). Gronthos et al. previously reported that human DPSC proliferate faster than BMMSC (Gronthos et al. 2000), whereas Jo et al. reported an opposite trend (Jo et al. 2007). However, neither of these studies compared donor-matched cells. Thus, our finding is important because it provides confirmation that DPSC proliferate faster than BMMSC. This characteristic of DPSC is important, given that the cell yield from teeth is considerably lower than from bone marrow. Interestingly, we noted that even though the total numbers of cells obtained from tooth digests (~ 0.5–1 × 106 cells/tooth) was drastically lower than for BMMSC isolation (~ 100 × 106 mononuclear cells/bone), cultures of one tooth digest or one femur equivalent of marrow cells per 25 cm2 flask initially plated reached confluence at roughly the same time. Differences in population doubling time could not have contributed to this occurrence. Thus, this observation indicated that dental pulp contains a higher percentage of more primitive, MSC-like cells.
To evaluate the number of stem/progenitor cells in the populations, we compared the DPSC and BMMSC cultures on the basis of colony formation. This assay is an adaptation of the colony-forming units-fibroblast assay, which is routinely used as a measure of the number of stem/progenitor cells in MSC populations (Castro-Malaspina et al. 1980; Derubeis and Cancedda 2004). Our results showed that DPSC had a significantly higher colonies per 1,000 cells plated than BMMSC (Figure 4A). This finding verified our prior observation, and it supports the data that Shi et al. previously reported for cells obtained from different donors (Shi et al. 2005). Moreover, because our assay used already-isolated MSC and not fresh tissue, the differences in DPSC and BMMSC colony-forming potential may be underestimated. Further investigation showed that in addition to having significantly greater clonogenic potential when single cell suspensions were plated (Figure 4B), DPSC clones also retained the ability to proliferate after subcloning, whereas BMMSC in general did not (Figure 4C). This finding is novel and has not previously been shown for DPSC, and it strongly suggests that DPSC populations possess more stem/progenitor cells with the proliferative potential necessary for tissue engineering applications than BMMSC.
In vivo, DPSC aid in reparative dentin formation by giving rise to new odontoblasts (Smith et al. 1995). As a result of this physiologic role, several studies have investigated the mineralization potential of DPSC (Gronthos et al. 2000; Shi et al. 2005; Otaki et al. 2007; Yu et al. 2007), and bone formation by human DPSC has been shown both in vitro and in vivo (Laino et al. 2005; Laino et al. 2006; d’Aquino et al. 2007; Graziano et al. 2008). In this study we demonstrated mineral formation and then quantified the mineralization potential of DPSC using an assay for alkaline phosphatase activity. Interestingly, we found that alkaline phosphatase activity was significantly higher in DPSC than in BMMSC after three weeks of induction in osteogenic medium (Figure 6). This result concurs with the findings of Yu et al. who showed that whereas rat DPSC formed mineralized, osteo-dentin like tissues after 14 days in a renal capsule model, minimal mineralization was observed for rat BMMSC (Yu et al. 2007). In contrast, Zhang, Jansen, and colleagues recently evaluated the ability of both rat and human DPSC and BMMSC to form mineralized tissue in vivo, and they found no evidence of mineral formation by rat or human DPSC after 10 weeks (Zhang et al. 2008). Although they concluded that DPSC have limited mineralization potential, their results could have been due to donor-associated variability. Thus, our finding adds support to the possibility of using DPSC to regenerate mineralized tissue.
In summary, our study was carefully conducted to eliminate differences in MSC function due to donor-to-donor variation. The data presented are similar to a case study in that cells were obtained from a single donor animal, but corroborating results have been obtained using additional donor rats (data not shown). Species-dependent biological differences have been noted for BMMSC (Kuznetsov and Gehron Robey 1996; Javazon et al. 2001), and it is likely that such differences also exist for DPSC. In contrast to human teeth, rodent incisors grow continuously (Ohshima et al. 2005), which may result in notable differences between rat and human DPSC. Nonetheless, our results appear to confirm several important differences between DPSC and BMMSC. In particular, DPSC have a faster proliferation rate, a higher number stem/progenitor cells in the population, and may have increased mineralization potential. These findings highlight the need for further investigation on the tissue engineering potential of DPSC using a donor-matched experimental design. Future studies will seek to determine if the differences noted in this study translate to differences in regenerative potential in vivo. We will also seek to extend our findings to human DPSC.
Acknowledgments
This work was supported by National Institutes of Health grants K08 HL75253 (WSG), 1R43RR023962 (EJW and WSG), the Riley Children’s Foundation, and General BioTechnology, LLC.
Footnotes
Conflict of interest statement
EJW is President and CEO of General BioTechnology, LLC. WSG is a medical director of General BioTechnology, LLC. Other authors report no potential conflicts of interest or financial interests.
References
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301. [PubMed] [Google Scholar]
- d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–71. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32(1):160–5. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10(3):149–60. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- Graziano A, d’Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, De Rosa A, Di Napoli D, Papaccio G. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008;41(1):1–11. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19(3):219–25. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13(4):767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46:1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Virdi AS, Dai Y, Shott S, Sumner DR. Patterns and localization of gene expression during intramembranous bone regeneration in the rat femoral marrow ablation model. Calcif Tissue Int. 2005;77(4):212–25. doi: 10.1007/s00223-004-0267-x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S, Gehron Robey P. Species differences in growth requirements for bone marrow stromal fibroblast colony formation In vitro. Calcif Tissue Int. 1996;59(4):265–70. doi: 10.1007/s002239900121. [DOI] [PubMed] [Google Scholar]
- Laino G, Carinci F, Graziano A, d’Aquino R, Lanza V, De Rosa A, Gombos F, Caruso F, Guida L, Rullo R, Menditti D, Papaccio G. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006;17(3):511–5. doi: 10.1097/00001665-200605000-00021. [DOI] [PubMed] [Google Scholar]
- Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20(8):1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- Laino G, Graziano A, d’Aquino R, Pirozzi G, Lanza V, Valiante S, Rosa AD, Naro F, Vivarelli E, Papaccio G. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206(3):693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen S, Yuan J, Yang Y, Li J, Ma J, Wu X, Freund M, Pollok K, Hanenberg H, Goebel WS, Yang FC. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg−/− mice in vivo. Blood. 2009;113(10):2342–51. doi: 10.1182/blood-2008-07-168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima H, Nakasone N, Hashimoto E, Sakai H, Nakakura-Ohshima K, Harada H. The eternal tooth germ is formed at the apical end of continuously growing teeth. Arch Oral Biol. 2005;50(2):153–7. doi: 10.1016/j.archoralbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, Matsuo O. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007;31(10):1191–7. doi: 10.1016/j.cellbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, Rosa AD, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208(2):319–325. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17(4):761–73. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75(3):424–36. [PubMed] [Google Scholar]
- Schafer R, Kehlbach R, Wiskirchen J, Bantleon R, Pintaske J, Brehm BR, Gerber A, Wolburg H, Claussen CD, Northoff H. Transferrin receptor upregulation: in vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology. 2007;244(2):514–23. doi: 10.1148/radiol.2442060599. [DOI] [PubMed] [Google Scholar]
- Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25(8):1029–41. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. 1995;39(1):273–80. [PubMed] [Google Scholar]
- Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99(8):465–74. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008;14(2):285–94. doi: 10.1089/tea.2007.0146. [DOI] [PubMed] [Google Scholar]