Abstract
The blood–brain barrier (BBB) is a major obstacle to gene therapy for genetic and infectious diseases in the central nervous system (CNS). At present, invasive techniques such as direct intracerebral injection of viral vector are necessary for clinical applications. Our laboratory is interested in developing a noninvasive cell-based gene delivery system for the CNS that exploits the natural ability of monocytes to enter the brain. With this in mind, fluorescently labeled monocytes were inoculated into the carotid artery of live mice, and their ability to enter the CNS was quantified by microscopic analysis of brain tissue sections obtained at necropsy. Labeled monocytes were detected in hippocampus, thalamus and cortex of experimental mice, and cell migration was confirmed by immunohistochemical analysis of tissue sections using a monocyte/macrophage-specific monoclonal antibody. Cell entry into the CNS could be enhanced by prior treatment of mice with intravenous bradykinin or hypertonic mannitol, with optimum results being obtained when bradykinin (5 mg/kg) or hypertonic mannitol was delivered 20 min prior to infusion of monocytes. These results suggest that it may be possible to transiently manipulate BBB permeability in such a way to achieve efficient migration of monocytes in the brain. This opens the way for the future use of vector-transduced monocytes as a novel delivery system to achieve effective gene transfer into the CNS.
Keywords: Brain blood barrier (BBB); Mouse brain, PHK26; Mannitol (MN); Bradykinin (BK); Monocyte trafficking; Intracarotid infusion
1. Introduction
The blood–brain barrier (BBB) strictly limits the transport of soluble materials, pathogens and circulating cells into the brain and thereby helps to maintain a protected neuronal microenvironment within the central nervous system (CNS) (Kroll and Neuwelt, 1998; Zhang and Pardridge, 2001). This protective barrier is essential for normal brain function but presents a considerable obstacle to the development of gene therapeutic interventions for inherited and acquired diseases of the CNS. Current CNS gene transfer studies therefore rely on the direct intracerebral injection of vectors or cells that harbor a therapeutic gene of interest (Bloch et al., 2004; Crystal et al., 2004; Janson et al., 2002). This poses obvious limitations, which include the risk for CNS damage as a consequence of drilling burr holes into the skull and introducing catheters to permit vector/cell infusion. Therefore, noninvasive and safer gene delivery methods are needed.
With this in mind, we have been interested in the ability of circulating monocytes to traverse the intact BBB and undergo differentiation within brain parenchyma, giving rise to long-lived brain-resident macrophages and microglia (Bart et al., 2000; Hickey, 1999). Monocyte trafficking into the CNS occurs in a highly regulated fashion and is dependent on cell–cell interactions that involve endothelial cells and astrocytes, as well as the local release of factors that promote BBB permeability. From a practical standpoint, the permeability of the BBB can be manipulated through the use of specific agents that transiently disrupt the barrier. Of particular interest in the clinical and laboratory setting are mannitol (Kroll and Neuwelt, 1998; Rapoport, 2000; Nilaver et al., 1995; Neuwelt et al., 1991) and bradykinin or its agonists (Elliott et al., 1996; Easton and Abbott, 2002; Borlongan and Emerich, 2003; Bartus et al., 2000). Intracarotid infusion of hypertonic mannitol has been used to osmotically disrupt the BBB to enhance entry of virus particles into the CNS (Nilaver et al., 1995). Bradykinin is one of several compounds including histamine and leukotrienes that can stimulate receptors present at the BBB and initiate second messenger systems, which induce opening of the tight junctions (Borlongan and Emerich, 2003). Cereport, a selective B2 bradykinin receptor agonist (also called RMP-7), has been shown to enhance CNS delivery of carboplatin, loperamide and cyclosporin-A, which are accompanied by enhanced chemotherapeutic, analgesic and neuroprotective effects, respectively (Borlongan and Emerich, 2003). We hypothesized that bradykinin and mannitol might be effective not only in increasing the permeability of the BBB to solutes or viral vectors, but also to viable monocytes. Experiments were therefore conducted to test this prediction, using fluorescently labeled monocytes. The results show that both bradykinin and hypertonic mannitol substantially enhance the trafficking of labeled monocytes into the CNS, following infusion via the intracarotid artery. These studies suggest that it may be feasible to develop monocytes as a novel noninvasive gene transfer vehicle for use in the CNS.
2. Results
2.1. BBB permeability to monocytes
Our initial experiments were directed at determining if PKH26-labeled mouse monocytes could cross the intact BBB and enter the brain. Quantitative measurements of monocyte trafficking into the brains of experimental mice was done by counting the number of PKH26-labeled monocytes in the CD and RM sections. As summarized in Table 1 and Fig. 1, CCA infusion of PKH26-labeled monocytes resulted in the detection of numerous fluorescent cells in brain sections from mice that received a CCA infusion of PKH26-labeled monocytes but not in control animals that did not receive any labeled cells.
Table 1.
Quantitative assessment of monocyte trafficking into the CD and RM regions of mouse brains
| Mice | Treatment | Brain region | |
|---|---|---|---|
| RM | CD | ||
| Group A | No monocytes | 0 | 0 |
| Group B | Monocytes + PBS | 7.8 ± 0.82 | 5.3 ± 0.89 |
| Group C | Monocytes + Bradykinin | 37.4 ± 3.56 ** | 27.9 ± 3.33 * |
The number of infiltrating monocytes (PKH26+) in the caudal diencephalon (CD) and rostral mesencephalon (RM) regions was determined by counting the number of PKH26-positive cells per high-powered microscope field (HPF) in the indicated groups of mice. Groups B and C received an internal carotid artery infusion of monocytes (1 × 106 cells in 50 µl) at 20 min following intravenous administration of 200 µl of bradykinin solution (1.0 g/L, Group C) or PBS (Group B).
P < 0.05.
P < 0.01 (when compared to group B).
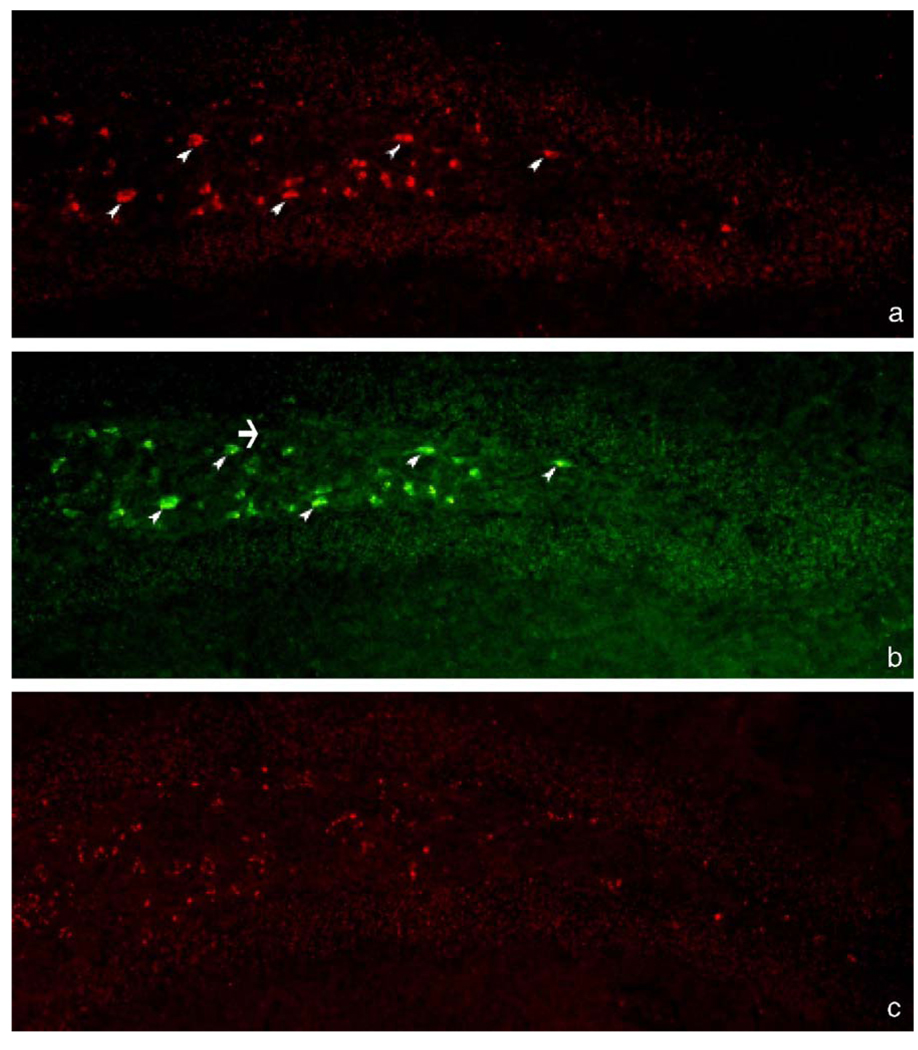
Fig. 1.
Detection of PKH26-labeled monocytes in the hippocampus of mice, following cell infusion and bradykinin treatment. (a) Fluorescence micrograph showing monocytes (➤) in the polymorph layer of the right hippocampal dentate gyrus of a mouse that received PKH26-labeled monocytes plus bradykinin (λex 546 nm). (b) Fluorescence micrograph from the same section as panel a showing MOMA-2-immunoreactive monocytes (FITC positive) (the few examples indicated by arrowheads; endogenous monocyte not stained in panel a is indicated by an arrow) (λex 489 nm). The section was treated with 0.1% Sudan black B after immunocytochemistry staining. (c) Fluorescence micrograph from the hippocampus of a control mouse that did not receive any monocyte infusion (λex 546 nm). Magnification: ×400, all panels.
When experimental animals were treated with bradykinin (i.v.) 20 min prior to monocyte infusion, many more PKH26-positive cells were detected within the CNS (an increase of 5.0 ± 0.2-fold, compared to animals that received PKH26-labeled cells plus PBS; Table 1). These data suggest that monocytes are able to traffic through the intact BBB and enter the brain, and that the efficiency of monocyte trafficking into the CNS can be significantly increased by intravenous administration of bradykinin (P value <0.05).
2.2. Effect of bradykinin concentration and timing of pretreatment on enhancement of trans-BBB migration of monocytes
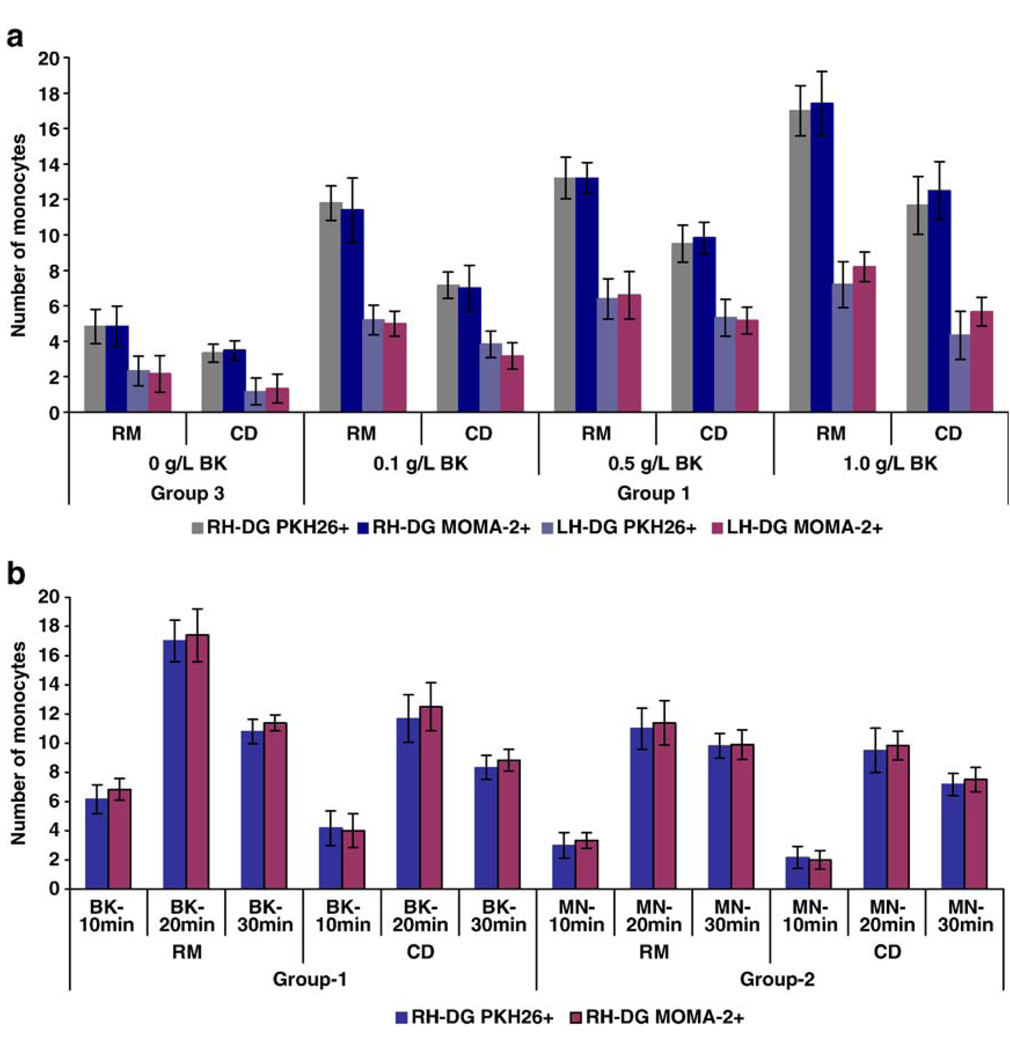
To establish optimized experimental conditions for enhancement of monocyte migration across an intact BBB, we examined the effects of bradykinin concentration and time of administration (in relation to monocyte infusion) of bradykinin and mannitol. As summarized in Fig. 2, intravenous administration of increasing concentrations of bradykinin led to increasing numbers of PKH26-labeled monocytes in the brain. An ANOVA analysis comparing differences between doses proved statistically significant (P < 0.01, Bonferroni adjusted) for all measurement methods (RM, CD for both RH-DG and LH-DG and both PKH26+ and MOMA2+). Compared to control mice (PBS-treated), the number of monocytes in the right hippocampus dentate gyrus (RH-DG) increased by 2.15-, 2.85- and 3.50-fold in the CD region, and by 2.44-, 2.73- and 3.50-fold in the RM region when bradykinin was administered 20 min prior to monocyte infusion, at increasing doses; the maximum increase was observed in animals that received 200 µl of a 1.0 g/L (w/v) stock of bradykinin via the intravenous route, 20 min prior to infusion of monocytes (equivalent to a dose of 5 mg/kg, in a 40-g mouse).
Fig. 2.
Effect of bradykinin (BK) and mannitol (MN) on BBB permeability to monocyte trafficking. (a) Significant increases in monocyte trafficking resulted from pretreatment with increasing amounts of bradykinin (P < 0.01). The number of monocytes present in the right and left hippocampal dentate gyrus (RH-DG and LH-DG) of the caudal diencephalon (CD) and rostral mesencephalon (RM) regions from mice that received an infusion of PKH26-labeled monocytes into the right intracarotid artery at 20 min after intravenous administration of the indicated concentrations of bradykinin was counted. Mean numbers of cells per field of view are shown when counted on the basis of either PKH26 positivity or MOMA2 immunoreactivity. (b) Timing-dependent increase of monocyte trafficking in response to pretreatment with bradykinin or mannitol. The number of monocytes in the RH-DG of mice that received an infusion of PKH26-labeled monocytes into the right intracarotid artery at 10, 20 or 30 min after intravenous administration of bradykinin (1.0 g/L) or mannitol (2.37 × 10−4 g/L) was counted. Mean numbers of cells per field of view are shown when counted on the basis of either PKH26 positivity or MOMA2 immunoreactivity.
To examine the relationship between the timing of bradykinin or mannitol delivery, and the subsequent entry of infused, PKH26-labeled monocytes into the brain, PKH26-labeled monocytes were infused through the right CCA at 10, 20 and 30 min, respectively, after intravenous drug administration. Fig. 2b shows the number of labeled monocytes that were detected in the RH-DG region of experimental mice following these treatments. PKH26-positive cells were most numerous within the CNS, when bradykinin or mannitol were administered 20 min prior to monocyte infusion; results were less impressive when the interval between drug delivery and cell infusion was only 10 min (Fig. 2b). In addition, the data in Fig. 2b show that bradykinin was more effective than mannitol, in terms of permitting monocyte entry into the brain—at least under the conditions examined here (maximum enhancement of monocyte uptake into the CNS was 3.5- fold for bradykinin and 2.8-fold for mannitol, as compared to the PBS control). In all cases, monocytes preferentially entered the right hippocampal dentate gyrus—presumably because the monocytes were infused via the right CCA (Fig. 2).
The stability of infused monocytes in the brain was evaluated by examining brain sections prepared from mannitol-treated animals at days 7, 14 and 21 postinfusion. The results indicate that, following an initial decline in monocyte counts at day 7 postinfusion, the number of PKH26-positive cells remained essentially unchanged until termination of the study (on day 21; data not shown). In addition, the experimental animals remained healthy, active and otherwise unremarkable in comparison to control mice.
Finally, a comparative analysis was conducted to determine whether monocyte infusion via the tail vein or intraperitoneal routes could also result in cell trafficking into the CNS. When compared to the intracarotid route of administration, entry of monocytes into the brain was reduced to 14% and less than 1%, respectively, when the cells were introduced via the tail vein or intraperitoneal route. Thus, the intracarotid delivery route was markedly superior—although the tail vein delivery method was also somewhat effective.
3. Discussion
Detection of PKH26-labeled mouse monocytes in the brain was conducted by using a fluorescent microscope to examine tissue sections. Results were confirmed by immunocytochemistry using a monoclonal antibody directed against MOMA-2, which is an intracellular marker expressed by monocytes and macrophages from all mouse strains (Kraal et al., 1987). As expected, there was excellent concordance between the detection of PKH26-positive cells and the detection of MOMA-2-positive cells because the antigen recognized by MOMA-2 is expressed only very weakly by dendritic cells (Kraal et al., 1987). However, we did also detect a few MOMA-2-stained cells in brain sections from control, noninfused mice (data not shown). This may reflect transit of endogenous blood-derived monocytes across the BBB (Bart et al., 2000), or it could be reflective of the rare population of phagocytic MOMA-2-positive microglia that has been previously identified (Ohmi et al., 2003).
In this study, we have evaluated the potential use of mannitol and bradykinin in transiently permeabilizing the mouse BBB so as to enhance monocyte trafficking into the brain. Hypertonic mannitol can cause the shrinkage of endothelial cells and subsequent opening of tight cellular junctions, whereas bradykinin, a vasodilatory molecule, acts by stimulating the B2 receptors on endothelial cells (Rapoport, 2000). Osmotic BBB disruption by mannitol is used clinically to alleviate brain swelling following injury and to increase delivery of chemotherapeutic agents; it has also been used in experimental animal model systems to enhance the delivery of viral gene transfer vectors into the brain (Nilaver et al., 1995; Neuwelt et al., 1999). Bradykinin has also been used in similar preclinical studies to modulate BBB permeability (Elliott et al., 1996; Easton and Abbott, 2002). Our data show that intravenous infusion of bradykinin is marginally more effective than mannitol in facilitating monocyte trafficking into the brain, at least under the experimental conditions described here. This is somewhat unexpected because bradykinin has been shown by others to have a very short half-life and narrow therapeutic index, requiring that it be infused into the carotid artery (Borlongan and Emerich, 2003). It would be interesting to know if RMP-7 will have a better enhancing effect on monocyte entry into the CNS than bradykinin because it has a longer half life, improved therapeutic index and better pharmacodynamic characteristics than bradykinin (Borlongan and Emerich, 2003).
We have carried out an initial optimization of the dose and timing of bradykinin delivery, with respect to enhancing monocyte entry into the brain. These experiments extend previous work on the use of bradykinin (Borlongan and Emerich, 2003) and mannitol (Nilaver et al., 1995; Neuwelt et al., 1991) for enhancing drug and viral vector uptake into the brain and show that these agents can also facilitate the transfer of intact, viable monocytes into the CNS. This could have a number of potential future applications, including the delivery of vector-transduced monocytes into the brain for gene therapy purposes. Studies directed at evaluating this possibility are underway.
Interestingly, most of the exogenous monocytes that we infused were detected within the hippocampus following intracarotid infusion. In comparison, previous studies of normal (noninfused) mice have detected rare H3 thymidine-labeled monocytes throughout the brain (Lawson et al., 1992), whereas the intravenous or intraperitoneal re-infusion of in vitro generated autologous macrophages into human subjects resulted in cell accumulation in the lungs and spleen, but only rarely in the brain (Andreesen et al., 1990). These results are consistent with our inability to deliver significant numbers of monocytes into the brain following intraperitoneal or tail vein infusion and suggest that additional studies are needed to explore the optimum route of monocyte administration for maximizing uptake into the brain. It will also be of interest to determine whether monocytes arising from different sources (bone marrow and blood), or purified by different methods (positive versus negative selection, CD14 versus adherence to plastic), exhibit different infiltration characteristics.
In conclusion, our results demonstrate that intravenously infused primary mouse monocytes can transmigrate across the intact BBB and into the brain. Furthermore, monocyte trafficking into the CNS can be significantly enhanced by transient BBB disruption using hypertonic mannitol or bradykinin. These data set the stage for future studies in which vector-transduced monocytes are employed as noninvasive cellular vehicles for the delivery of therapeutic genes or gene products into the CNS.
4. Experimental procedures
All experimental protocols followed National Institute of Health guidelines for animal research and were approved by the Institutional Animal Care and Use Committee of the University of Hawaii.
4.1. Monocyte isolation and PKH26-labeling
Mouse peripheral blood mononuclear cells (PBMCs) were obtained using the Lympholyte®-M reagent (Cedarlane, Hornby, Ontario, Canada). Mouse monocytes were then purified using miniMACS separation columns (Miltenyi Biotec, Germany). In brief, adult male mice (CD-1, weighing 35 ± 5 g) were euthanized with CO2 and bled by cardiac puncture. Cell suspensions were then layered over Lympholyte®-M and centrifuged for 20 min at 1000 × g at room temperature. The lymphocyte layer was removed, washed with RPMI 1640 and then resuspended in phosphate-buffered saline (PBS) supplemented with 2 mM ethylenediamine tetraacetic acid (EDTA) and 0.5% (w/v) bovine serum albumin (BSA). The lymphocytes were then incubated with MACS CD11b Microbeads for 15 min at 12 °C and monocytes were isolated by magnetic separation, following the manufacturer’s instructions. For tracking experiments, cells were labeled with the membrane dye PKH26 according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). The efficiency of cellular labeling (routinely 100%) was examined with a fluorescence microscope (Olympus, IX-70FLA).
4.2. In vivo BBB permeability
Experimental mice were anesthetized with 2% to 4% halothane in a mixture of 70% nitrous oxide and 30% oxygen and placed in a stereotactic frame. To examine monocyte trafficking across the BBB, adult CD-1 mice were equally divided into 3 groups: (1) Group A animals received no treatment or injection and (2) Groups B and C animals received an infusion of PKH26-labeled monocytes (1 × 106 cells/animal) through the right common carotid artery (CCA), 20 min after intravenous injection (tail vein) of 200 µl of PBS (Group B) or bradykinin solution (1.0 g/L) (Group C). Twenty to twenty-four hours following monocyte infusion, the animals were sacrificed and subjected to transcardial perfusion with 50 ml of 0.9% NaCl containing 5000 U of heparin at room temperature. Note that this perfusion step was used to remove labeled monocytes from tissue blood vessels, thereby ensuring that subsequent detection of cells within brain tissue would represent cells truly located within the brain parenchyma. Following perfusion, brain tissues were then removed and frozen at −80 °C until cryosection. After demonstrating that infused monocytes could traffic into the brain, and showing that this could be enhanced by using bradykinin, we next decided to optimize our experimental parameters—including the dose and timing of administration of both bradykinin and mannitol. In this test, experimental animals were divided into 4 groups, as follows: (1) Group A2 animals received 200 µl of bradykinin (0.1, 0.5 and 1 g/L in PBS); (2) Group B2 animals received 200 µl of 1.5 mol/L mannitol (Sigma) in PBS; (3) Group C2 animals received 200 µl of PBS; and (4) Group D2 animals received no treatment. At selected time points following the treatments (10, 20 and 30 min), the right CCA was exposed for all the mice in Groups A2, B2 and C2 following a standard anatomy protocol. An inoculum of 1 × 106 of PKH26-labeled monocytes was then infused slowly into the CCA using a Hamilton syringe (Beckton-Dickinson, Franklin Lakes, NJ), and cell entry into the CNS was examined by fluorescence microscopic inspection of brain sections, following sacrifice (see below). In some experiments, monocytes were also infused via intraperitoneal (i.p.) injection, following intravenous delivery of bradykinin (200 µl of a 1 g/L stock). In all experiments, a minimum of 3 mice were used in each experimental group.
4.3. Detection of PKH26-labeled monocytes
Serial coronal sections (20 µm thickness) from the caudal diencephalon (CD) and rostral mesencephalon (RM) were prepared using a cryostat microtome (Leica CM1900). Sections were transferred onto polylysine-coated microscopic slides and allowed to adhere in dust-free chambers for 3 h at room temperature. Each section was then examined for the presence of PKH26-labelled monocytes and photographed using a fluorescence microscope (λex 546 nm) (Olympus, IX-70FLA).
4.4. Monocyte immunohistochemistry
Identification of monocytes within the brain sections was confirmed by immunohistochemical staining with commercial antibodies against monocyte surface antigens. Briefly, brain sections were fixed with cold acetone for 15 min, blocked with 0.2% BSA/PBS for 30 min and washed with 0.5% Triton/PBS for 20 min before incubation with primary antibody. Sections were incubated for 1 h at room temperature with rat anti-mouse macrophage/monocyte monoclonal antibody (1:200) (MOMA-2, New England Biolabs, Ipswich, MA); note that MOMA-2 recognizes a highly specific intracellular marker in macrophages that is only very weakly expressed by dendritic cells and Langerhans cells (Kraal et al., 1987). Brain sections were then washed three times with PBS buffer containing 0.3% Triton X-100. The secondary antibody, rabbit anti-rat IgG (1:100) (Sigma-Aldrich, St. Louis, MO), was applied for 1 h at room temperature, followed by a detection antibody (goat anti-rabbit IgG conjugated with FITC, used at a dilution of 1:100) (Sigma-Aldrich), which was also incubated for 1 h at room temperature. To reduce tissue auto-fluorescence, we incubated sections with 0.1% Sudan Black B in 70% ethanol for 10 min after immunocytochemical staining. The slides were then washed with 70% ethanol and coverslipped with glycerol. Images were photographed using a fluorescence microscope (λex 546 nm) (Olympus, IX-70FLA).
4.5. Data analysis
Quantitative measurements of BBB permeability to monocytes were conducted by counting the number of PKH26-labeled cells from 10 serial CD and RM sections from each of three mice for each experimental group. In addition, the number of MOMA-2-positive cells was also determined by immunohistochemical staining of tissue sections from four brain regions (hippocampus, thalamus, olfactory and cortex). The results were expressed as mean number of cells per high-powered microscope field ± SD. The data were analyzed using ANOVA and paired Student’s t test with statistical significance defined as either P < 0.05 or P < 0.01.
Acknowledgments
The authors would like to thank Drs. Stephen Dewhurst and Martin Rayner for their critical comments on the manuscript and Dr. Peter Holck for his assistance in statistical analysis. This study was supported by grants from the National Institutes of Health (S11NS043499 and G12RR003061) and by the Hawaii Community Foundation.
REFERENCES
- Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl HG, Leser HG, Engler H, Lohr GW. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50:7450–7456. [PubMed] [Google Scholar]
- Bart J, Groen HJ, Hendrikse NH, vanderGraaf WT, Vaalburg W, deVries EG. The blood–brain barrier and oncology: new insights into function and modulation. Cancer Treat Rev. 2000;26:449–462. doi: 10.1053/ctrv.2000.0194. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Snodgrass P, Marsh J, Agostino M, Perkins A, Emerich DF. Intravenous cereport (RMP-7) modifies topographic uptake profile of carboplatin within rat glioma and brain surrounding tumour, elevates platinum levels, and enhances survival. J. Pharmacol. Exp. Ther. 2000;293:903–911. [PubMed] [Google Scholar]
- Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugieres P, Boisse MF, Baudic S, Cesaro P, Hantraye P, Aebischer P, Peschanski M. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum. Gene Ther. 2004;15:968–975. doi: 10.1089/hum.2004.15.968. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Emerich DF. Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, cereport. Brain Res. Bull. 2003;60:297–306. doi: 10.1016/s0361-9230(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P, Souweidane M, Hosain S, Heier L, Ballon D, Dinner M, Wisniewski K, Kaplitt M, Greenwald BM, Howell JD, Strybing K, Dyke J, Voss H. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum. Gene Ther. 2004;15:1131–1154. doi: 10.1089/hum.2004.15.1131. [DOI] [PubMed] [Google Scholar]
- Easton AS, Abbott NJ. Bradykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood–brain barrier. Brain Res. 2002;953:157–169. doi: 10.1016/s0006-8993(02)03281-x. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Hayward NJ, Huff MR, Nagle TL, Black KL, Bartus RT. Unlocking the blood–brain barrier: a role for RMP-7 in brain tumor therapy. Exp. Neurol. 1996;141:214–224. doi: 10.1006/exnr.1996.0156. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin. Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, Wang DJ, Shera D, Hurh P, Rupin J, Saslow E, Goldfarb O, Goldberg M, Larijani G, Sharrar W, Liouterman L, Camp A, Kolodny E, Samulski J, Leone P. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 2002;13:1391–1412. doi: 10.1089/104303402760128612. [DOI] [PubMed] [Google Scholar]
- Kraal G, Rep M, Janse M An immunohistochemical study. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. Scand. J. Immunol. 1987;26:653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Kroll RA, Neuwelt EA. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. (discussion 1099–1100). [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA, Dix RD. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood–brain barrier. J. Neurosurg. 1991;74:475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Abbott NJ, Drewes L, Smith QR, Couraud PO, Chiocca EA, Audus KL, Greig NH, Doolittle ND. Cerebrovascular biology and the various neural barriers: challenges and future directions. Neurosurgery. 1999;44:604–608. doi: 10.1097/00006123-199903000-00095. (discussion 608–609). [DOI] [PubMed] [Google Scholar]
- Nilaver G, Muldoon LL, Kroll RA, Pagel MA, Breakefield XO, Davidson BL, Neuwelt EA. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood–brain barrier disruption. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. Osmotic opening of the blood–brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000;20:217–230. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J. Neurochem. 2001;76:1597–1600. doi: 10.1046/j.1471-4159.2001.00222.x. [DOI] [PubMed] [Google Scholar]