Abstract
Forkhead L2 (FOXL2) is a member of the forkhead/hepatocyte nuclear factor 3 (FKH/HNF3) gene family of transcription factors and acts as a transcriptional repressor of the Steroidogenic Acute Regulatory (StAR) gene, a marker of granulosa cell differentiation. FOXL2 may play a role in ovarian follicle maturation and prevent premature follicle depletion leading to premature ovarian failure. In this study, we found that FOXL2 interacts with Ubc9, an E2-conjugating enzyme that mediates sumoylation, a key mechanism in transcriptional regulation. FOXL2 and Ubc9 are co-expressed in granulosa cells of small and medium ovarian follicles. FOXL2 is sumoylated by Ubc9, and this Ubc9-mediated sumoylation is essential to transcription activity of FOXL2 on the StAR promoter. As FOXL2 is endogenous to granulosa cells, we generated a stable cell line expressing FOXL2 and found that activity of the StAR promoter in this cell line is greatly decreased in the presence of Ubc9. The sumoylation site was identified at lysine 25 of FOXL2. Mutation of lysine 25 to arginine leads to loss of transcriptional repressor activity of FOXL2. Taken together, we propose that Ubc9-mediated sumoylation at lysine 25 of FOXL2 is required for transcriptional repression of the StAR gene and may be responsible for controlling the development of ovarian follicles.
Keywords: Sumoylation, transcriptional regulation, Forkhead, granulosa cell, premature ovarian failure, FOXL2, UBC9
1. Introduction
FOXL2 is a member of the forkhead/hepatocyte nuclear factor 3 (FKH/HNF3) gene family of transcription factors [1], which contains members that are essential in embryogenesis [2, 3], tumorigenesis [4-6], and cell differentiation [7-9]. Members of the FKH/HNF3 transcription factor family are characterized by the presence of a conserved winged helix domain that is essential for DNA binding, as well as more divergent transactivation or transrepression domains [10-12]. One forkhead transcription factor, FOXL2, was first identified to be mutated in patients with Blepharophimosis-Ptosis-Epicanthus Inversus (BPES) type I syndrome [13]. These patients have premature ovarian failure in association with a characteristic eyelid dysplasia, blepharophimosis, ptosis, and epicanthus inversus [13].
We previously identified FOXL2 as a transcriptional repressor of the Steroidogenic Acute Regulatory (StAR) gene [14]. StAR is a cholesterol transporter at the mitochondrial membrane, and controls the rate-limiting step in steroidogenesis [15-17]. StAR is present in the granulosa cell layer of large preovulatory and luteinized follicles, and is absent from immature follicles in several species [18-20]. StAR activity is present in the granulosa cell compartment during follicular differentiation, signaling early functional maturation of ovarian antral follicles [19, 20]. In contrast to StAR, FOXL2 shows a highly specific pattern of expression in the undifferentiated granulosa cells of small and medium follicles in the mouse ovary [14]. The expression of FOXL2 in undifferentiated granulosa cells and its repressor activity of the StAR gene, a marker of granulosa cell differentiation, suggest that FOXL2 may function as a determinant of cellular differentiation by inhibiting premature differentiation of granulosa cells. With the inhibition of granulosa cell differentiation, FOXL2 may control the number of primordial follicles that remain dormant and prevent the premature depletion of ovarian follicles and excessive follicle recruitment that is associated with premature ovarian failure.
Small ubiquitin-related modifer (SUMO) proteins are structurally related to ubiquitin and covalently bind to substrate proteins at lysine residues, in a three-step conjugation pathway similar to that involved in ubiquitination. Sumoylation starts when SUMO is activated by an E1-activiting enzyme and is transferred to the E2-conjugating enzyme. With the help of an E3-ligase, SUMO can then be transferred from the E2-conjugating enzyme to substrate proteins [21]. Independently, Ubc9 recognizes substrate proteins and catalyses the formation of an isopeptide bond between glycine 97 of SUMO and a lysine residue of the substrate protein [22]. Therefore, Ubc9 is a unique E2-conjugating enzyme and a key regulator of sumoylation.
Sumoylation is a dynamic process. Usually, less than 1% of the target protein will be sumoylated [23]. Also, sumoylation usually does not result in protein degradation, but enhances protein stability and plays a role in subcellular protein localization [23, 24]. Unlike ubiquitination, a consensus motif ψKXE/D has been identified for sumoylation. The ψ symbol represents a large hydrophobic residue which includes leucine (L), isoleucine (I), or valine (V). K, E, and D are lysine, glutamic acid, and aspartic acid, respectively. X represents any amino acid [23, 25-27]. However, around 23 to 26% of confirmed sumoylation sites do not contain this exact consensus motif [28, 29], and no specific sequence or secondary structure has been identified for those non-consensus sumoylation sites [30, 31].
The majority of currently-identified SUMO substrates are transcription factors or transcription cofactors. Whilst a few studies have described transcriptional activators whose activities are enhanced by sumoylation, e.g. tumor protein p53, Heat Shock Factor 1 (HSF1), cAMP response element-binding protein 1 (CREB) and T-cell transcription factor 4 (TCF4) [22, 32-35], the activities of most transcriptional factors are repressed by sumoylation, including the oncogene JUN, transcription factor Sp3, co-SMAD Smad4, sterol-regulatory element binding protein (SREBP), steroidogenic factor 1 (SF-1), transcriptional coactivator p300, testis receptor (TR2), and receptor-interacting protein 140 (RIP140) [36-44]. Further, studies of transcriptional repressors have shown that sumoylation enhances their repressive abilities, e.g. promyelocytic leukemia zinc finger (PLZF), the ETS transcription factor LIN-1, Survival of Motor Neuron interacting protein-1 (SIP1), SnoN, Pokemon 1 and erythroid Krüppel-like factor (EKLF) [45-50]. Thus, sumoylation plays a highly significant role in transcriptional regulation, usually resulting in transcriptional repression.
FOXL2 is a transcriptional repressor of the StAR gene, a marker of granulosa cell differentiation and follicular development, and loss of repression may lead to premature ovarian failure. Given that the process of premature follicle depletion is critical to reproduction, we hypothesized that there are more refined regulatory processes involved in the transcriptional regulation of FOXL2, a hypothesis supported by recent studies showing that FOXL2 is highly modified post-translationally [51]. As sumoylation plays such a significant role in transcriptional regulation, we tested whether Ubc9-mediated sumoylation may control FOXL2's activity as a transcriptional regulator, to better delineate the mechanisms underlying granulosa cell differentiation, ovarian follicle development and subsequent premature ovarian failure.
2. Materials and Methods
2.1. Plasmid construction and siRNA sequences
A 1.1 kb cDNA sequence encoding human FOXL2 [14] was released from a pcDNA3 backbone using the restriction enzymes Hind III and Xba I and subcloned into pFLAG-CMV-2 (Sigma, St. Louis, MO) and phCMV (Genlantis, San Diego, CA) to generate a FLAG-tagged FOXL2 cDNA and a HA-tagged FOXL2 cDNA. A 500 bp human Ubc9 cDNA sequence was PCR-amplified from human ovary cDNA (Ambion, Austin, TX) using the primers 5′-GCTAAAGCTTATGTCGGGGATCGCCCTCAGC-3′ and 5′-TACGTCTAGATTATGAGGGCGCAAACTTCTT-3′, which included Hind III and Xba I restriction sites respectively, and then also subcloned into a pcDNA3, pFLAG-CMV-2, and phCMV2 expression vectors. To generate the Ubc9 Cystine93Serine and FOXL2 Lysine25Arginine mutants, PCR-based mutagenesis (QuikChange site-directed mutagenesis kit, Stratagene, La Jolla, CA) was performed on the Ubc9/pcDNA3 and FOXL2/ pFLAG-CMV-2 constructs. The 93rd amino acid of Ubc9 was mutated from cysteine to serine using the primer 5′-CCTTCGGGGACAGTGAGCCTGTCCATCTTAGAGGAG-3′ and the 25th amino acid of FOXL2 was mutated from lysine to arginine using the primer 5′-GGTCGCACAGTCAGGGAGCCAGAAGGGCCG-3′. The results were confirmed by sequencing. An siRNA for Ubc9 (target sequence 5′-TCCGAGCTGGGTTGCAGAGGA-3′) and a non-silencing siRNA control were purchased from Qiagen (Qiagen, Valencia, CA).
2.2. Yeast two-hybrid screening
cDNAs encoding wild-type human FOXL2 was fused in-frame with the GAL4-binding domain into the pGBT9 yeast shuttle vector. Yeast cells were transformed with pGBT9 FOXL2 by screening 1.5 million transformants from a GAL4-activation domain-tagged ovarian fusion cDNA library prepared from rats primed with PMSG. Colonies were selected on plates deficient in tryptophan, leucine and histidine but containing 30 mM 3-amino-1,2,4-triazole (Sigma, St. Louis, MO). Plasmids were isolated from positive colonies and then transformed into KC18 Escherichia coli cells. Plasmids were then purified from KC18 cells and sequenced. Subsequently, the specific interaction between wild-type FOXL2 and Ubc9 was confirmed based on activation of the GAL1-HIS3 reporter gene.
2.3. Immunohistochemistry
Ovaries were dissected from immature Swiss Webster outbred female mice at 21 days of age and fixed in formalin overnight. The fixed ovaries were then embedded in paraffin, cut at 7 μm thickness and placed on poly-L-Lysine coated slides. The slides were de-waxed by incubating in xylene two times for 5 minutes each and re-hydrated by placing them in 100%, 95%, 70% ethanol for 5 minutes each. The slides were washed with distilled water and PBS for 5 minutes each. Endogenous peroxidases were inactivated by incubating the slides in 0.3% hydrogen peroxide in methanol for 10 minutes. The slides were then pretreated with antigen retrieval buffer (DAKO, Carpinteria, CA) in a steamer for 20 minutes and cooled for 20 minutes. After washing with PBS, the slides were blocked with 10% normal goat serum (Vector, Burlingame, CA) for 1 hour. The slides were hybridized with a custom-made FOXL2 antibody (Zymed Laboratories, San Francisco, CA) or with a Ubc9 antibody (BD, Franklin Lakes, NJ) for 1 hour, followed by incubation with a biotinylated anti-rabbit secondary antibody (Vector, Burlingame, CA) for another 30 minutes. The slides were then incubated with ABC solution (Vector, Burlingame, CA) for 30 minutes and stained by adding fresh DAB solution for 2 minutes. The slides were then counterstained with haematoxylin (Sigma, St. Louis, MO) and mounted in Vectamount AQ (Vector, Burlingame, CA).
2.4. Cell culture, DNA transfection and siRNA transfection
Chinese hamster ovary (CHO) cells were cultured in DMEM/F12 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM glutamine in a humidified atmosphere with 5% CO2 at 37°C.
For DNA transfections, the cells were plated and then transfected with the desired plasmids one day later using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The cells were incubated with the plasmids in Opti-MEM medium (Invitrogen, Carlsbad, CA) for 4 hours followed by incubation in fresh culture media for 24 hours. The cells were then lysed using either RIPA buffer, reporter lysis buffer (Promega, Madison, WI) or immunoprecipitation buffer for subsequent experiments. For siRNA transfection, cells were plated shortly before transfection and incubated at 37°C with 5% CO2. HiPerFect transfection reagent (Qiagen, Valencia, CA) was mixed with the desired siRNA, incubated for 10 minutes and then added to cells for a final siRNA concentration of 5nM, in accordance with the manufacturer's instructions. Cells were cultured with siRNA for 48 hours. Twenty-four hours after siRNA transfection, cells were transfected with FOXL2 plasmids with or without StAR luciferase contructs and β-gal expression plasmids. The cells were subsequently lysed using either RIPA buffer, reporter lysis buffer or immunoprecipitation buffer for subsequent experiments.
2.5. Promoter activity assays
Cells were co-transfected with 0.5 μg of the −95 bp human StAR promoter luciferase construct [52] either with an expression construct containing wild type human FOXL2 cDNA cloned into a pFLAG-CMV-2, or the pFLAG-CMV-2 vector backbone. The indicator plasmid pCMV-β-galactosidase was used to estimate transfection efficiency. The total DNA concentration was maintained at 1μg per well in 24 well plate through the inclusion of empty pFLAG-CMV-2 vector. Twenty-four hours after transfection, the cells were lysed using reporter lysis buffer and repeated freeze/thaw cycles. The luciferase activities in the cell lysates were determined using the luciferase assay system (Promega, Madison, WI) and FLUOstar OPTIMA microplate reader (BMG Labtech Inc., Durham, NC). Results are reported as relative luminescence units and normalized with β-galactosidase activity.
2.6. Immunoprecipitation
CHO cells were transfected with FLAG-tagged FOXL2 or FLAG-tagged Ubc9 for 24 hours and then lysed in an immunoprecipitation buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA and 0.2 mM PMSF. The cell lysates were incubated with anti-FLAG M2 affinity gel (Sigma, St. Louis, MO) at 4°C for 4 hours. The gel was then washed with TBS (50 mM Tris-HCl, pH 7.4 and 150 mM NaCl) to eliminate non-specific binding and incubated with 100 μg/mL FLAG peptide (Sigma, St. Louis, MO) in TBS at 4°C for 30 minutes to elute the bound proteins. The eluted samples or cell lysates were added to 4× SDS sample buffer and heated at 95°C for 5 minutes to denature the proteins. The proteins of interest in the eluted samples or cell lysates were then analyzed by Western blotting.
2.7. Western blotting
Cells lysates in RIPA buffer as well as the immunoprecipitation products described in the previous section were added to 4× SDS sample buffer and heated at 95°C for 5 minutes to denature the proteins. The cell lysates were then separated on 7.5% or 12% SDS-PAGE gels and transferred to PVDF membranes. The membranes were incubated with primary antibodies, our custom-made FOXL2 antibody, M2 FLAG, HA (Sigma, St. Louis, MO), Ubc9, SUMO-1 (Cell Signaling, Danvers, MA), or SUMO-2/3 (Cell Signaling, Danvers, MA) antibodies followed by HRP-conjugated secondary antibodies. Chemiluminescent detection was performed using ECL western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). To analyze proteins in whole-cell lysates, membranes were first probed with the FOXL2 or Ubc9 antibodies, and then stripped with Restore Western Blot Stripping Buffer (Pierce, Rockford, IL) for 1 hour. The stripped membranes were then re-probed using a tubulin antibody (NeoMarker, Fremont, CA) as a loading control. Quantification of the intensities of the protein bands detected by western blotting was performed using Science Lab 2003 Multi Gauge Software Version 2.2 (Fujifilm, Japan).
2.8. In vitro sumoylation assay
The FOXL2/pcDNA3 construct was used as a template for in vitro synthesis of the FOXL2 protein, which was performed using TNT Quick Coupled Transcription/Translation Systems (Promega, Madison, WI). The synthesized FOXL2 protein was then used as a substrate for an in vitro sumoylation assay, performed using a SUMOylation kit (Enzo Life Sciences International, Inc., Plymouth Meeting, PA) according to the manufacturer's instructions. The assay includes SUMO-activating enzyme (Aos1/Uba2), Ubc9, and SUMO1, to which the FOXL2 protein was added. The assay mixture was then incubated at 30°C for 4 hours. The products of the sumoylation assay were then denatured by adding SDS sample buffer and heating them at 60°C for 20 minutes, after which they were analyzed by western blotting.
2.9. Generation of a stable cell line expressing FOXL2
To generate a CHO cell line stably expressing FOXL2, a plasmid encoding wild type FOXL2 in a pcDNA3 vector backbone [14] was transfected into CHO cells. The transfectants were grown in the presence of 1000 μg/mL G418 for 10 days, and the surviving colonies were picked and cultured. Cell lysates from these cultures were screened by Western blotting with our custom antibody to FOXL2. Cell lines with stable expression of FOXL2 were maintained in CHO cell culture medium with 1000 μg/mL G418 at 37°C and 5% CO2.
2.10. Immunostaining
CHO cells stably expressing wild type FOXL2 were grown on poly-L-Lysine-coated coverslips for 24 hours. Cells were fixed with 4% paraformaldehyde in PBS for 20 minutes and then permeabilized with PBS containing 0.5% Triton X-100 and 1% bovine serum albumin for 20 minutes. The cells were then incubated with primary antibodies to FOXL2 and Ubc9 (1:100 dilutions in PBS) for 2 hours at room temperature. After incubation with secondary antibodies (Molecular Probe, Eugene, OR), the cells were washed and the cell nuclei were identified by staining with DAPI (Molecular Probe, Eugene, OR). The cells were then washed with ice-cold PBS and mounted using a ProLong Antifade mounting kit (Molecular Probes, Eugene, OR).
2.12. Statistical analysis
To compare the amount of target proteins between samples, intensities of protein bands detected by Western blotting were quantified by Science Lab 2003 Multi Gauge Version 2.2. (Fujifilm). The results were similar for at least three separate experiments. For promoter assay studies, experiments were performed in quadruplicate and the standard error (±SE) reported. Statistical analysis was performed using paired t-test, significance was at p<0.001.
3. Results
3.1. FOXL2 interacts with Ubc9
In order to identify FOXL2-interacting proteins, we performed a yeast two-hybrid screen using wild type human FOXL2. This construct was fused in frame with the GAL4-DNA binding domain of the pGBT9 yeast shuttle vector. The vector was used to identify FOXL2-interacting proteins by screening 1.5 million transformants from a GAL4 activation domain-tagged ovarian fusion cDNA library prepared from rats [53]. The results of this screening indicated that the E2-conjugating enzyme Ubc9 interacts strongly with wild type FOXL2. In order to confirm the interaction between these two proteins, we subcloned cDNAs of FOXL2 and Ubc9 into pFLAG-CMV-2 and phCMV2 vectors to express the FLAG-tagged and HA-tagged proteins. FLAG-tagged FOXL2 expressing plasmid or pFLAG-CMV-2 backbone vector was co-transfected with HA-tagged Ubc9 in CHO cells for 24 hours, and the FLAG-tagged FOXL2 was immunoprecipitated. As shown in Fig. 1A, FLAG-tagged FOXL2 (FLAG-FOXL2) was expressed in CHO cells and was immunoprecipitated (Lysate and IP). HA-tagged Ubc9 (HA-Ubc9) was also present in the cell lysate (Lysate), and was co-immunoprecipitated with FLAG-tagged FOXL2 (IP). When HA-tagged FOXL2 (HA-FOXL2) was present in the cell lysate (Lysate), it co-immunoprecipitated with FLAG-tagged Ubc9 (IP) (Fig. 1B). From the results of yeast two-hybrid and co-immunoprecipitation in mammalian cells, we can conclude that FOXL2 and Ubc9 interact with each other in vivo.
Fig. 1.
FOXL2 interacts with Ubc9 in CHO cells. CHO cells were transfected with different combinations of FLAG or HA tagged FOXL2 and Ubc9. The cell lysates were immunoprecipitated with an anti-FLAG antibody, and proteins in the cell lysates and immunoprecipitates (IP) were resolved by SDS-PAGE and immunoblotted with antibodies to FLAG and HA tags. A. HA-tagged Ubc9 (HA-Ubc9) was co-transfected with or without FLAG-tagged FOXL2 (FLAG-FOXL2) into CHO cells. When cells were transfected with HA-Ubc9 and FLAG-FOXL2, a 60 kDa FLAG-FOXL2 band was present in the cell lysates and immunoprecipitates. When cells transfected with HA-tagged Ubc9 alone or combined with FLAG-FOXL2, a 20 kDa HA-Ubc9 was expressed in the cell lysates. Moreover, HA-Ubc9 was observed co-immunoprecipitating with FLAG-tagged FOXL2 (IP, HA-Ubc9), when cells co-expressed FLAG-FOXL2 and HA-Ubc9. B. HA-tagged FOXL2 (HA-FOXL2) was co-transfected with or without FLAG-tagged Ubc9 in CHO cells. When cells were transfected with FLAG-Ubc9, a 20 kDa FLAG-Ubc9 band was present in the cell lysates and immunoprecipitates. When cells were transfected with HA-FOXL2 alone or combined with FLAG-Ubc9, cells expressed a 60 kDa HA-FOXL2 in the cell lysates. Similar to the previous result (A), HA-FOXL2 co-immunoprecipitated with FLAG-Ubc9 (IP, HA-FOXL2) when cells co-expressed FLAG-Ubc9 and HA-FOXL2. The experiment was repeated three times with similar results.
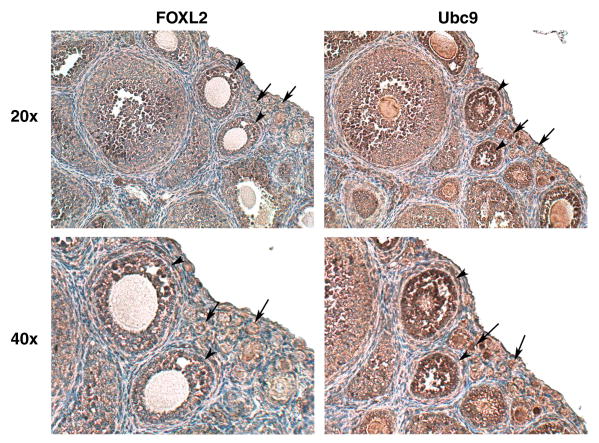
3.2. FOXL2 and Ubc9 are co-expressed in granulosa cells in the mouse ovary
Both FOXL2 and Ubc9 have been identified in the human ovary [1, 54], and the FOXL2 transcript has been shown to be specifically expressed in the granulosa cells of small and medium follicles in the mouse ovary [14]. To further investigate the respective locations and expression levels of FOXL2 and Ubc9, immunohistochemical analysis was performed using mouse ovarian tissue sections. As the FOXL2 transcript is known to be expressed in the less differentiated granulosa cells of small and medium follicles, serial sections of ovaries from immature (21-day old) mice were used for these studies. As shown in Fig. 2, FOXL2 and Ubc9 were found to have overlapping patterns of expression in the granulosa cells of small (arrows) and medium (arrowheads) follicles in the immature mouse ovary. The identification of Ubc9 as a FOXL2-interacting protein, together with their co-expression in granulosa cells, suggests that Ubc9 may be involved in mediating FOXL2's activity as a transcriptional regulator via sumoylation during follicular development.
Fig. 2.
FOXL2 and Ubc9 are co-expressed in mouse granulosa cells. Immunohistochemical staining was performed on serial sections of ovaries from 21 day-old immature mice using antibodies to FOXL2 or Ubc9, followed by counterstaining with hematoxylin. Both FOXL2 (left) and Ubc9 (right) are expressed in granulosa cells of immature ovarian follicles, small (arrow) and medium (arrowhead) follicles. The experiment was performed three times with identical results.
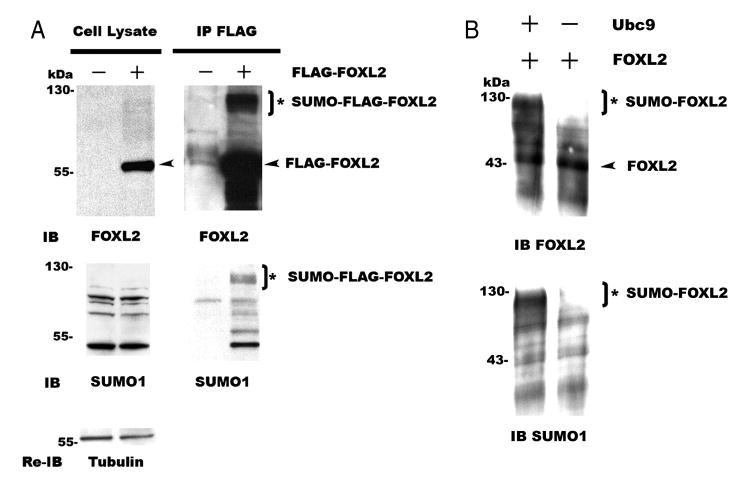
3.3. FOXL2 is sumoylated in vivo and in vitro
To test whether FOXL2 is sumoylated in mammalian cells, FLAG-tagged FOXL2 was transfected into CHO cells and then immunoprecipitated to analyze modifications to the FOXL2 protein. As shown in Fig. 3A, FOXL2 was not detected in CHO cells when cells were transfected with the FLAG-CMV2 backbone vector (cell lysate). This demonstrates that CHO cells do not express endogenous FOXL2. However, when cells were transfected with FLAG-tagged FOXL2, a 60 kDa FLAG-tagged FOXL2 band (arrowhead) was detected by FOXL2 antibody (cell lysate and IP). In addition, a 120 kDa band, presumed to be sumoylated FLAG-FOXL2 (asterisk) was present in the IP product. When these IP products were probed with an antibody to SUMO1, the 120 kDa band was again identified, suggesting that this product is sumoylated FLAG-tagged FOXL2 (asterisk, SUMO-FLAG-FOXL2). When the blot was re-probed with an antibody to SUMO2/3, this band was no longer present (data not shown). These results suggest that FOXL2 is sumoylated by SUMO1, but not SUMO2/3, in vivo. To further verify that FOXL2 is sumoylated by SUMO1, in vitro sumoylation assays were also performed. As shown in Fig. 3B, FOXL2 (arrowhead, 45 kDa) was available as a substrate, and was sumoylated (asterisk, SUMO-FOXL2, 110 kDa) when Ubc9 was present in the assay mixture. In contrast, FOXL2 was not sumoylated when Ubc9 was absent from the assay mixture (Fig. 3B). These results indicate that FOXL2 is sumoylated by SUMO1 in vivo and in vitro.
Fig. 3.
FOXL2 is sumoylated in vivo and in vitro. A. CHO cells were transfected with FLAG-tagged FOXL2. The cell lysates were immunoprecipitated with an anti-FLAG antibody, and proteins in the cell lysates and immunoprecipitates (IP) were resolved by SDS-PAGE and immunoblotted with antibodies to FOXL2 and SUMO1. Endogenous FOXL2 was not detected in CHO cells, but cells transfected with FLAG-FOXL2 expressed a 60 kDa FLAG-FOXL2 band (arrowhead, cell lysates). This 60 kDa FLAG-FOXL2 band and a 120 kDa band (asterisk) were seen in the IP products when immunoblotted with an antibody to FOXL2. The 120 kDa band was then confirmed to be SUMO1 modified FLAG-tagged FOXL2 (asterisk, SUMO-FLAG-FOXL2) by immunoblotting with a SUMO1 antibody. The blots of the cell lysates were then re-probed with a tubulin antibody to control for equal loading. The experiment is representative of three similar experiments. B. A 45 kDa FOXL2 protein (arrowhead) was synthesized from the FOXL2/pcDNA3 construct using TNT Quick Coupled Transcription/Translation Systems, and was then used as a substrate for in vitro SUMO1-sumoylation reactions. When Ubc9 was present in the reaction, a 110 kDa band (asterisk) was observed when the assay products were immunoblotted with an antibody to FOXL2. When Ubc9 was absent from the reaction, this 110 kDa band was not observed. This 110 kDa band was then confirmed to be SUMO1-modified FOXL2 (asterisk, SUMO-FOXL2) by immunoblotting the assay products with a SUMO1 antibody. The experiment is representative of three similar experiments.
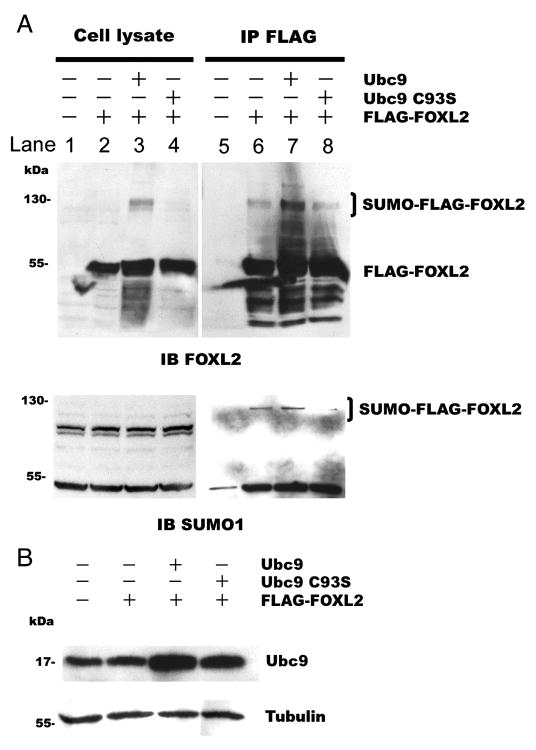
3.4. Ubc9 is required for sumoylation of FOXL2
To test whether Ubc9 is required for sumoylation of FOXL2, FLAG-tagged FOXL2 was expressed alone, with Ubc9 or with a dominant negative mutant Ubc9 [Cysteine93Serine (C93S)] in CHO cells. Mutation of the cysteine residue to serine at the active site of Ubc9 prevents the formation of a thioester bond, rendering the mutant protein incapable of SUMO conjugation [55]. The cells were then lysed, and the lysates were immunoprecipitated using FLAG-tagged FOXL2. Sixty kDa FLAG-tagged FOXL2 (Fig. 4A, lysate) and 18 kDa wild type or mutant Ubc9 (Fig. 4B) were detected in the cell lysate by immunoblotting with antibodies to FOXL2 and Ubc9. Additionally, when FLAG-tagged FOXL2 was co-expressed with wild type Ubc9, the 120 kDa SUMO-FLAG-FOXL2 band was present (Fig. 4A, lane 3). Immunoprecipitates were also analyzed by immunoblotting with antibodies to FOXL2. As shown in Fig. 4A, when FLAG-tagged FOXL2 was expressed alone, both the 60 kDa FLAG-tagged FOXL2 band and the 120 kDa sumoylated FLAG-FOXL2 band (SUMO-FLAG-FOXL2) were again detected using the FOXL2 antibody. When FLAG-tagged FOXL2 was co-expressed with wild type Ubc9, the intensity of the SUMO-FLAG-FOXL2 band increased by 2.5 times compared to basal (Fig. 4A, lane 7) as quantified by ScienceLab 2003 MultiGuage software. In contrast, when FLAG-tagged FOXL2 was co-expressed with the dominant negative mutant Ubc9 (UBC9 C93S), the intensity of the SUMO-FLAG-FOXL2 band decreased by 32% from baseline (Fig. 4A, lane 8). When these IP products were probed with an antibody to SUMO1, the 120 kDa sumoylated FLAG-FOXL2 bands (SUMO-FLAG-FOXL2) were again identified with similar results. These results suggest that Ubc9 is involved in the transfer of SUMO to FOXL2, resulting in a 120 kDa SUMO-FLAG-FOXL2 product.
Fig. 4.
Overexpression of Ubc9 enhances the sumoylation of FOXL2. CHO cells were transfected with 0.4μg FLAG-FOXL2 plasmid alone, 0.4μg FLAG-FOXL2 and 2μg Ubc9 plasmids, or 0.4μg FLAG-FOXL2 and 2μg dominant negative mutant Ubc9 C93S plasmids. The cell lysates were immunoprecipitated. Proteins in the cell lysates and immunoprecipitates were resolved by SDS-PAGE and immunoblotted with an antibody to FOXL2 or SUMO1 (A) or Ubc9 (B). A. Cells transfected with FOXL2 along or with wild type or mutant Ubc9 exhibit a 60 kDa FLAG-FOXL2 product (cell lysate, lane 2, 3 & 4). After immunoprecipitation of FLAG-tagged proteins, cells transfected with FLAG-FOXL2 alone exhibit both a 60 kDa FLAG-FOXL2 product and a 120 kDa sumoylated FLAG-FOXL2 product (SUMO-FLAG-FOXL2) (lane 6). Co-expression of FLAG-FOXL2 with Ubc9 increased the amount of the 120 kDa product (lane 7), but co-expression with dominant negative mutant Ubc9 C93S did not (lane 8). B. Transfection with wild type Ubc9 or dominant negative mutant Ubc9 C93S increased the amount of Ubc9 in these cells. The blots of the cell lysates were then re-probed with a tubulin antibody to control for equal loading. The experiment was repeated three times with similar results.
To further determine whether Ubc9 is required for FOXL2 sumoylation, Ubc9 siRNA was used to deplete endogenous Ubc9 in CHO cells. Hence, CHO cells were transfected with non-silencing siRNA or Ubc9 siRNA, and then transfected with FLAG-tagged FOXL2. Cells were then lysed and analyzed by immunoblotting with an antibody to Ubc9. After transfection with Ubc9 siRNA, 77% of the Ubc9 protein was knocked down, as quantified by ScienceLab 2003 MultiGuage software (Fig. 5A). To examine the effect of depleting Ubc9 on FOXL2 sumoylation, the cell lysates were immunoprecipitated with the anti-FLAG antibody and immunoblotted with the FOXL2 antibody. As shown in Fig. 5B, when CHO cells were transfected with non-silencing siRNA, both the 60 kDa FLAG-tagged FOXL2 band and the 120 kDa SUMO-FLAG-FOXL2 band were observed. When CHO cells were transfected with Ubc9 siRNA, the 120 kDa SUMO-FLAG-FOXL2 band was no longer present (Fig. 5B). The IP products were also probed with a SUMO1 antibody. Again, the 120 kDa sumoylated FLAG-FOXL2 bands (SUMO-FLAG-FOXL2) were greatly decreased when cells were transfected with Ubc9 siRNA. Taken together, these results demonstrate that Ubc9 is involved in and necessary for sumoylation of FOXL2.
Fig. 5.
Ubc9 is required for the sumoylation of FOXL2. A. CHO cells were transfected with 5nM non-silencing siRNA or 5nM Ubc9 siRNA. After 24h, the cells were transfected with FLAG-FOXL2, cultured for another 24 h, lysed and immunoblotted with an antibody to Ubc9. Transfection with Ubc9 siRNA depleted the amounts of Ubc9 (18 kDa) in CHO cells. B. The cell lysates were immunoprecipitated, and probed with an antibody to FOXL2 or SUMO1. The amount of sumoylated FLAG-FOXL2 (SUMO-FLAG-FOXL2, indicated by the bracket) decreased following transfection with Ubc9 siRNA (lane 4), but not after transfection with non-silencing siRNA (lane 2). The experiment was repeated three times with similar results.
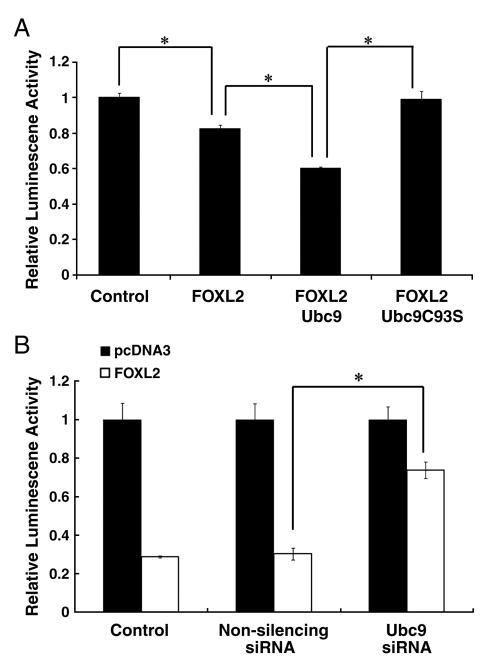
3.5. Sumoylation of FOXL2 enhances its repressive effect on the StAR gene promoter
FOXL2 acts as a key repressive transcriptional factor during follicle development, and is known to interact directly with the promoter of the StAR gene. In previous studies, we showed that FOXL2 can repress the activity of a -95-bp human StAR promoter-luciferase construct in CHO cells [14]. Therefore, we used this construct to examine whether Ubc9-mediated sumoylation is involved in or required for repression of the StAR promoter by FOXL2. CHO cells were co-transfected with the -95-bp human StAR promoter-luciferase reporter plasmid and FLAG-tagged FOXL2 alone, FLAG-tagged FOXL2 and Ubc9, or FLAG-tagged FOXL2 and dominant negative mutant Ubc9 C93S. Twenty-four hours after transfection, the activity of the StAR promoter was assessed by measuring luciferase activity in the cell lysates. As shown in Fig. 6A, FLAG-tagged FOXL2 was found to repress basal StAR promoter activity, as reflected by a decrease in luciferase activity. When Ubc9 was over-expressed in these cells with FOXL2, luciferase activity was further reduced. In contrast, when dominant negative mutant Ubc9 was expressed with FLAG-tagged FOXL2, luciferase activity was restored to basal levels. We then examined the repressive activity of FOXL2 in Ubc9-depleted cells. CHO cells were first transfected with non-silencing siRNA or Ubc9 siRNA, followed by transfection with FLAG-tagged FOXL2 and the reporter plasmid. StAR promoter activity was determined by measuring luciferase activity in the cell lysates. Using the empty pcDNA3 vector as a control, transfection of FOXL2 resulted in transcriptional repression of the StAR promoter as demonstrated by the decrease in luciferase activity. When Ubc9 was depleted using Ubc9 siRNA, but not non-silencing siRNA, the ability of FOXL2 to suppress StAR promoter activity was greatly reduced (Fig. 6B). These results suggest that SUMO modification of FOXL2 plays a role in its transcription repressor activity of the StAR gene.
Fig. 6.
Ubc9 is essential for the repression of the StAR gene promoter by FOXL2. A. CHO cells were co-transfected with −95-bp StAR promoter-luciferase and either 20ng FLAG-FOXL2 alone, 20ng FLAG-FOXL2 and 200ng Ubc9, or 20ng FLAG-FOXL2 and 200ng Ubc9 C93S, and the resulting luciferase activities were determined. Luciferase activity values were normalized against activity levels in cells transfected with the StAR promoter plasmid and pcDNA3 backbone vector (Control, value = 1). StAR promoter activity is repressed following transfection with FOXL2, and is further repressed following transfection with FOXL2 and Ubc9. However, in the presence of the mutant Ubc9 C93S, FOXL2 repressor activity is lost. B. CHO cells were transfected with 5nM non-silencing siRNA or 5nM Ubc9 siRNA. After 24 h, the cells were transfected with the −95-bp StAR promoter-luciferase alone or with 50ng FLAG-FOXL2, incubated for 24 h and then lysed for determination of luciferase activity. Luciferase activity values were normalized as described above. FOXL2 maintains its ability to repress StAR promoter activity when co-transfected with non-silencing siRNA, but loses this repressor activity when co-transfected with Ubc9 siRNA. The results were similar for at least three separate experiments. The standard error (±SE) and statistically significant differences are shown (*, p<0.001).
3.6. FOXL2 and Ubc9 are expressed in the nucleus
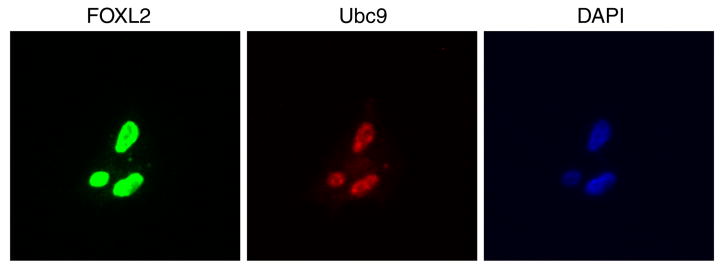
As FOXL2 is absent in CHO cells but endogenous to granulosa cells, we generated a stable CHO cell line expressing wild type FOXL2. We first determined the location of FOXL2 and Ubc9 within these stable cells by immunostaining. As expected, FOXL2 and Ubc9 were both located in the nucleus, which was identified by DAPI staining (Fig. 7). Co-localization of FOXL2 and Ubc9 in the nucleus suggests that sumoylation takes place in the nucleus for rapid regulation of FOXL2's transcriptional activity.
Fig. 7.
FOXL2 and Ubc9 are localized to the nucleus. CHO cells stably expressing FOXL2 were grown on poly-L-lysine coverslips for 24 h. Cells were then immunostained with FOXL2 and Ubc9 antibodies. The cell nucleus was identified by DAPI staining (blue). Both FOXL2 (green) and Ubc9 (red) are localized in the cell nucleus. The experiment is representative of three separate experiments.
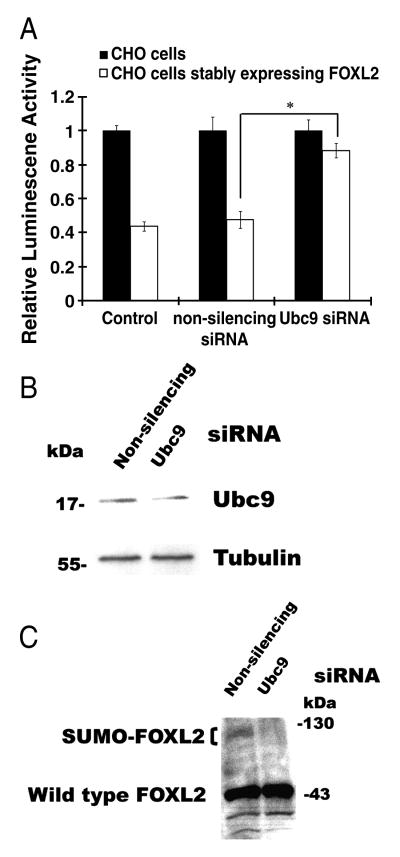
3.7. Sumoylation by Ubc9 is required for FOXL2's repressive activity in a stable cell line
Within these stable cell lines, FOXL2 is functional, as demonstrated by repression of StAR promoter activity (Fig. 8A). The basal level of StAR promoter activity in these stable cells was 50% of that in wild type CHO cells, suggesting that the expressed FOXL2 was functional and repressed StAR promoter activity. Even in the presence of non-silencing siRNA, StAR promoter activity was repressed. However, when Ubc9 was knocked down in this stable cell line using Ubc9 siRNA, the level of StAR promoter activity was restored to 90% of that seen in wild type CHO cells, indicating that the repressive activity of FOXL2 was severely affected (Fig. 8A). Further, the levels of Ubc9 in cells treated with Ubc9 siRNA were decreased by 42% compared to cells treated with non-silencing siRNA, as quantified by ScienceLab 2003 MultiGuage software (Fig. 8B). These results are consistent with those seen in transiently transfected CHO cells (Fig. 5A). Sumoylation of FOXL2 was also examined in this stable cell line. When cells stably expressing FOXL2 were transfected with non-silencing siRNA, a 45 kDa wild type FOXL2 band and a 100kDa band corresponding to sumoylated FOXL2 (SUMO-FOXL2) was observed (Fig. 8C). However, when Ubc9 was depleted in this stable cell line, the 100kDa putative SUMO-FOXL2 band disappeared (Fig. 8C). These results indicate that co-expression of FOXL2 and Ubc9 is required for the repressive effect of FOXL2 on the StAR gene promoter.
Fig. 8.
Co-expression of FOXL2 and Ubc9 is required for repression of the StAR gene promoter. A. Native CHO cells and CHO cells stably expressing FOXL2 were transfected with 5nM non-silencing siRNA or 5nM Ubc9 siRNA. After 24 h, the cells were transfected with −95-bp StAR promoter-luciferase, incubated for 24 h and then lysed for determination of luciferase activity. Luciferase activity values were normalized against activity levels in cells transfected with StAR promoter plasmid and pcDNA3 backbone vector (Control, value = 1). StAR promoter activity is repressed in cells stably expressing FOXL2, compared to that in native CHO cells which lack FOXL2 expression. This repression is unaffected by non-silencing siRNA, but is reduced following transfection with Ubc9 siRNA. B. and C. CHO cells stably expressing FOXL2 were transfected with 5nM non-silencing siRNA or 5nM Ubc9 siRNA. Forty-eight hours after siRNA transfection, cells were lysed and Ubc9 (B) and FOXL2 protein expression (C) in cell lysates were detected by immunoblotting. Transfection with Ubc9 siRNA reduces the amounts of Ubc9 in these cells (B) and also reduces the amount of SUMO-modified FOXL2 (SUMO-FOXL2, indicated by the bracket) (C). The results were similar for at least three separate experiments. The standard error (±SE) and statistically significant differences are shown (*, p<0.001).
3.8. Sumoylation site on FOXL2
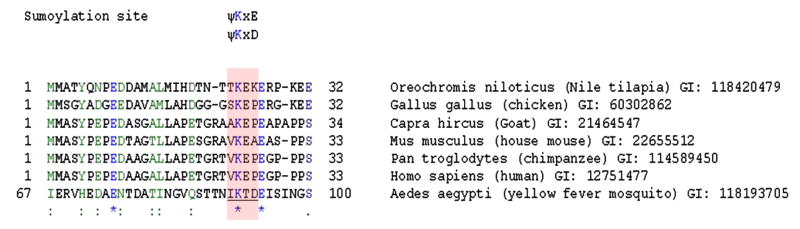
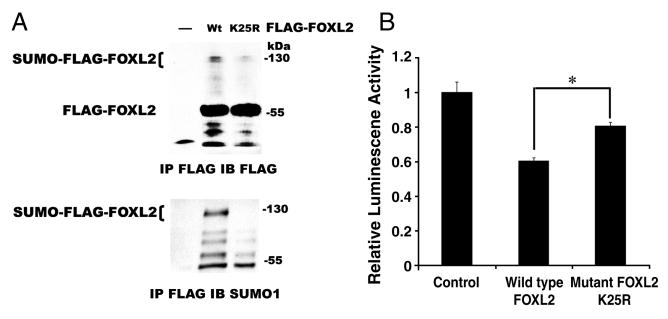
FOXL2 does not have the consensus sumoylation site, ψKXE/D. Therefore, to identify the most likely sumoylation site on FOXL2, we aligned the protein sequence of human FOXL2 to FOXL2 sequences of other species, nile tilapia, chicken, goat, mouse, and chimpanzee, and FOXL of yellow fever mosquito, which is similar to FOXL2 in mammals (Fig. 9). The lysine 25 of 24VKEP27 in human FOXL2 is aligned to the lysine within the consensus sumoylation site of yellow fever mosquito FOXL, 91IKTD94 (Fig. 9). Additionally, this area of human FOXL2 is highly homologous to FOXL2 of other species. The region 24VKEP27 of FOXL2 was also confirmed as a high probability sumoylation site with a score of 0.82 by SUMOplot™ prediction software (Abgent, San Diego, CA). We thus mutated lysine 25 of human FOXL2 to arginine and generate a mutant, FLAG-tagged FOXL2 K25R. This mutant FOXL2 was transfected into CHO cells and immunoprecipitated by anti-FLAG antibody to examine the expression and its sumoylation. When cells were transfected with wild type or mutant FLAG-tagged FOXL2, a 60 kDa FLAG-tagged FOXL2 band was detected in the immunoprecipitates (Fig. 10A). As we expected, when cells were transfected with wild type FOXL2, the 120 kDa SUMO-FLAG-FOXL2 band was observed. However, when cells were transfected with mutant FOXL2 K25R, the 120 kDa SUMO-FLAG-FOXL2 band was markedly diminished. The IP products were then reprobed with SUMO1 antibody. The 120 kDa sumoylated FLAG-FOXL2 band (SUMO-FLAG-FOXL2) was only present in cells which expressed wild type FLAG-tagged FOXL2, and was absent from cells which expressed mutant FLAG-tagged FOXL2 K25R. These results suggested that lysine 25 of FOXL2 is sumoylated. We then used our StAR promoter activity assay to test the repressor activity of mutant FOXL2 K25R. When cells were transfected with StAR promoter-luciferase reporter and wild type FOXL2, the luciferase activity dropped by 40% compared to cells that were transfected with StAR promoter-luciferase reporter and pFLAG-CMV2 backbone vector (control). However, when cells were transfected with StAR promoter-luciferase reporter and mutant FOXL2 K25R, the transcriptional repressor activity was lost compared to wild type FOXL2. This data confirmed that sumoylation of FOXL2 is involved in its transcriptional regulation. Taken together, results of our studies suggest that Ubc9 is essential for the sumoylation of FOXL2 and this enhances the ability of FOXL2 to repress StAR gene promoter activity.
Fig. 9.
Sumoylation consensus site aligned in sequences of FOXL2 from different species. The sumoylation consensus site is ψKXE/D with a hydrophobic residue, such as Valine (V), Isoleucine (I), or Leucine (L). Sequences of FOXL2 of nile tilapia, chicken, goat, mouse, chimpanzee, and human, and FOXL of yellow fever mosquito were aligned. A consensus sumoylation site is localized in FOXL of yellow fever mosquito, 91IKTD94 (underlined), and is highly homologous to sequences of other FOXL2 protein sequences (red box).
Fig. 10.
Sumoylation mutant of FOXL2 loses transcriptional repression activity. A. CHO cells were transfected with wild type (Wt) or sumoylation mutant (K25R) FLAG-tagged FOXL2. The cell lysates were immunoprecipitated with an anti-FLAG antibody, and proteins in immunoprecipitates were resolved by SDS-PAGE and immunoblotted with M2 FLAG or SUMO1 antibodies. Cells transfected with wild type FOXL2 (Wt) exhibit both a 60 kDa FLAG-FOXL2 product and a 120 kDa sumoylated FLAG-FOXL2 product (SUMO-FLAG-FOXL2). A 60 kDa FLAG-FOXL2 product with similar intensity was also present in cells transfected with mutant FOXL2 K25R. However, the 120 kDa sumoylated FLAG-FOXL2 product (SUMO-FLAG-FOXL2) was markedly diminished. B. CHO cells were co-transfected with −95-bp StAR promoter-luciferase and either 10ng wild type FOXL2 or 10ng sumoylation mutant FOXL2 (K25R), and the resulting luciferase activities were determined. Luciferase activity values were normalized against activity levels in cells transfected with StAR promoter plasmid and FLAG-CMV2 backbone vector (Control, value = 1). StAR promoter activity is repressed following transfection with wild type FOXL2, but repression is diminished following transfection with the sumoylation mutant FOXL2 K25R. The results were similar for at least three separate experiments. The standard error (±SE) and statistical significances were shown (*, p<0.001).
4. Discussion
In this study, we investigated the mechanisms by which the activity of FOXL2 as a transcriptional regulator is modulated. We found that wild type FOXL2 interacts with the E2-conjugating enzyme Ubc9, and immunohistochemical analyses reveals that FOXL2 and Ubc9 are co-expressed in the granulosa cells of small and medium follicles in ovaries of immature mice. Further, we have demonstrated that FOXL2 is sumoylated via Ubc9-mediated sumoylation and, using promoter-luciferase assay, have shown that the activity of FOXL2 as a repressor of the StAR gene is dependent on Ubc9-mediated sumoylation. We also showed that stable co-expression of FOXL2 and Ubc9 in CHO cells, similar to the environment in undifferentiated granulosa cells, represses StAR promoter activity, and that this repression is lost when Ubc9 is depleted in these cells using siRNA. Finally, we identified the sumoylation site on FOXL2, lysine 25, and mutation of this lysine diminishes the transcriptional repressor activity of FOXL2. Based on these results, we propose that Ubc9-mediated sumoylation of FOXL2 is necessary for the transcriptional repression of the StAR gene, a marker of granulosa differentiation. Regulation of FOXL2 through sumoylation may inhibit granulosa cell differentiation, maintaining the number of follicles that remain dormant and prevent the premature depletion of ovarian follicles
A recent study by Benayoun et al. (2008) indicated that FOXL2 is highly modified post-translationally, validating our findings [51]. We have found that one of these post-translational modifications is sumoylation. Sumoylation typically occurs at a ψKXE/D consensus motif. However, this exact sumoylation consensus site could not be found in the protein sequence of FOXL2. Based on the conservation of the site among species, lysine 25 of FOXL2 was defined to a potential SUMO site. By mutation studies, we confirmed that SUMO-1 is conjugated at lysine 25 of FOXL2, suggesting that the unconventional sumoylation site 24VKEP27 of FOXL2 may be the site for sumoylation. Similar to the sumoylation site of FOXL2, some other unconventional sumoylation sites are also evolutionarily conserved, such as the sumoylation site of PCNA (proliferating-cell nuclear antigen) [31].
In our study, we believe that the major FOXL2 sumoylation product is conjugated with a tri-SUMO chain, the molecular weight of this tri-SUMO-FOXL2 is 125kDa, and smaller products which correspond in size to mono- or bi-SUMO conjugated FOXL2 at 80 and 100 kDa respectively (Fig. 3A). In addition to studies describing poly-SUMO2/3 chain modification of proteins, such as HDAC4 [56] and HID-1 [57], there are also studies demonstrating other proteins are poly-SUMO1 modified. For example, human topoisomerase I was identified as being modified by a poly-SUMO1 chain in vitro [58]. Human topoisomerase I has only one major sumoylation site at Lys117 [58], but was found to be multi-SUMO1 modified in HeLa cells after camptothecin induction [59]. These studies indicate that human topoisomerase I is poly-SUMO1 chain conjugated. Furthermore, NK-kB2/p100 is also SUMO1 conjugated, and while the molecular weight of NK-kB2/p100 is 100 kDa, the sumoylated NK-kB2/p100 band is approximately 250 kDa in size. Although NK-kB2/p100 has 4 sumoylation sites, a mutation at 3 of 4 lysines (S3 mutant in the article) only decreased the intensity of the 250 kDa band, and did not reduce the molecular weight [60]. These results suggest that NK-kB2/p100 is conjugated with a poly-SUMO1 chain at one lysine, rather than mono-sumoylated at 4 different lysines. Similar to topoisomerase I and NK-kB2/p100, FOXL2 is likely,poly-SUMO1 modified, with the major FOXL2 sumoylation product conjugated with a tri-SUMO chain.
Sumoylation is well established as a mechanism for transcriptional modulation, and usually results in repression of transcription. Recent studies have revealed a mechanism by which sumoylation downregulates gene transcription, showing that sumoylation promotes the interaction of transcription factors with co-repressors. For example, histone deacetylases (HDACs) and death-associated protein 6 (Daxx) have been shown to act as co-repressors, and contribute to SUMO-mediated repression of transcription. Sumoylated Elk-1 and p300 bind to HDAC2 and HDAC6 respectively, resulting in repression of transcription [42, 61]. Daxx interacts with sumoylated transcription factors including androgen receptor (AR) and Smad4 [62, 63], and can itself be sumoylated [64], all of which result in repression of transcription. Specifically DP103, a member of the DEAD-box family of RNA helicases, interacts with sumoylated SF-1 and enhances SF-1 sumoylation to repress SF-1 responsive transcription [65]. Furthermore, we have previously demonstrated that DP103 also interacts with FOXL2 [66]. Thus, like SF-1, sumoylation of FOXL2 may lead to interaction with DP103 and further recruitment of co-repressors, leading to transcriptional repression activity of genes such as StAR. We have previously shown that FOXL2 is a transcriptional repressor of the StAR gene [14], and have now demonstrated that sumoylation of FOXL2 is important for its activity as a repressor of the StAR promoter. The results of future studies may determine if sumoylation promotes interactions between FOXL2 and other co-repressors, such as DP103, leading to differences in transcriptional activity of StAR and/or other gene promoters.
StAR is important in cholesterol transport and steroidogenesis, and is a marker of granulosa cell differentiation. The StAR gene is activated by several transcription factors, including SF-1 and SREBP [67-69], and is suppressed by FOXL2 and chicken ovalbumin upstream promoter transcription factor (COUP-TF) [14, 70]. Recent studies have shown that COUP-TF and SF-1 can competitively bind to the same steroidogenic enzyme promoters, such as human StAR promoter, steroid 17-alpha-hydroxylase (CYP17) and steroid 11/18-beta-hydroxylase (CYP11B2) [70-73]. Similarly, FOXL2 shares a binding region in the StAR promoter with SF-1 and SREBP [14, 67-69], suggesting that competition for binding sites may occur between the transcriptional repressor FOXL2 and the transcriptional activators SF-1 and SREBP, further modulating the transcription of the StAR gene.
BPES type I is an autosomal dominant disorder. In patients with BPES type I, mutations of FOXL2 exhibit loss of repressor activity, which likely leads to acceleration of granulosa cell differentiation and subsequent follicle recruitment. An acceleration in the initial recruitment of follicles could prematurely deplete the limited number of follicles in the ovary, leading to premature ovarian failure. Our data demonstrate that Ubc9-mediated sumoylation is involved in FOXL2's activity as a repressor of StAR gene transcription. Further, inhibition of FOXL2 sumoylation results in a loss of its repressor activity. We therefore suggest that sumoylation of FOXL2 by UBC9 is important for its transcriptional repressor activity of genes controlling granulosa cell differentiation, such as StAR, and subsequent follicle development, leading to maintenance of the ovarian follicle pool and prevention of premature depletion that is associated with premature ovarian failure.
Acknowledgments
This work was supported by R01HD047603 from the National Institute of Child Health and Human Development (NICHD) and the Office of Research on Women's Health (ORWH) (MP) and by a grant from the Helping Hands of Los Angeles, Inc. (MP).
Footnotes
Disclosure Statement: F-TK, I B-B, JB and GB have nothing to disclose. MP is supported by grants from the NICHD/ORWH (R01HD047603) and the Helping Hands of Los Angeles, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. Nat Genet. 2001 Feb;27(2):159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 2.Weigel D, Jurgens G, Kuttner F, Seifert E. Jackle H Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki K, Minami T, Tojo M, Honda Y, Uchimura Y, Saitoh H, Yasuda H, Nagahiro S, Saya H, Nakao M. Biochem Biophys Res Commun. 2003 Jun 20;306(1):32–38. doi: 10.1016/s0006-291x(03)00910-0. [DOI] [PubMed] [Google Scholar]
- 4.Parry P, Wei Y, Evans G. Genes Chromosomes Cancer. 1994;11(2):79–84. doi: 10.1002/gcc.2870110203. [DOI] [PubMed] [Google Scholar]
- 5.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. Cancer Res. 2002;62(16):4773–4780. [PubMed] [Google Scholar]
- 6.Radisavljevic Z. Cancer. 2003;97(5):1358–1363. doi: 10.1002/cncr.10081. [DOI] [PubMed] [Google Scholar]
- 7.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. Dev Cell. 2003;4(1):119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 8.Brissette JL, Li J, Kamimura J, Lee D, Dotto GP. Genes Dev. 1996;10(17):2212–2221. doi: 10.1101/gad.10.17.2212. [DOI] [PubMed] [Google Scholar]
- 9.Dottori M, Gross MK, Labosky P, Goulding M. Development. 2001;128(21):4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann E, Knochel W. Mech Dev. 1996;57(1):3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson P, Mahlapuu M. Dev Biol. 2002;250(1):1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. Trends Genet. 2003;19(6):339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 13.Zlotogora J, Sagi M, Cohen T. Am J Hum Genet. 1983;35(5):1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 14.Pisarska MD, Bae J, Klein C, Hsueh AJ. Endocrinology. 2004 Jul;145(7):3424–3433. doi: 10.1210/en.2003-1141. [DOI] [PubMed] [Google Scholar]
- 15.Clark BJ, Wells J, King SR, Stocco DM. J Biol Chem. 1994;269(45):28314–28322. [PubMed] [Google Scholar]
- 16.Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Science. 1995;267(5205):1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 17.Stocco DM. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 18.Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., 3rd J Clin Endocrinol Metab. 1997;82(12):4243–4251. doi: 10.1210/jcem.82.12.4445. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WE, Powell J, Thomas KH, Whittaker JA. J Histochem Cytochem. 1999;47(6):769–776. doi: 10.1177/002215549904700606. [DOI] [PubMed] [Google Scholar]
- 20.Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Endocrinology. 1998;139(1):303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- 21.Kerscher O, Felberbaum R, Hochstrasser M. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP, Hay RT. EMBO J. 1999 Nov 15;18(22):6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson ES. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Liang M, Liang YY, Brunicardi FC, Feng XH. J Biol Chem. 2003 Aug 15;278(33):31043–31048. doi: 10.1074/jbc.C300112200. [DOI] [PubMed] [Google Scholar]
- 25.Hay RT. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Melchior F, Schergaut M, Pichler A. Trends Biochem Sci. 2003;28(11):612–618. doi: 10.1016/j.tibs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Denison C, Rudner AD, Gerber SA, Bakalarski CE, Moazed D, Gygi SP. Mol Cell Proteomics. 2005;4(3):246–254. doi: 10.1074/mcp.M400154-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, He Y, Qiang B, Yuan J, Peng X, Pan XM. BMC Bioinformatics. 2008;9:8. doi: 10.1186/1471-2105-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Y, Zhou F, Fu C, Xu Y, Yao X. Nucleic Acids Res. 2006;34(Web Server issue):W254–257. doi: 10.1093/nar/gkl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pichler A, Knipscheer P, Oberhofer E, van Dijk WJ, Korner R, Olsen JV, Jentsch S, Melchior F, Sixma TK. Nat Struct Mol Biol. 2005;12(3):264–269. doi: 10.1038/nsmb903. [DOI] [PubMed] [Google Scholar]
- 31.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. Nature. 2002;419(6903):135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 32.Gostissa M, Hengstermann A, Fogal V, Sandy P, Schwarz SE, Scheffner M, Del SG. EMBO J. 1999 Nov 15;18(22):6462–6471. doi: 10.1093/emboj/18.22.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong Y, Rogers R, Matunis MJ, Mayhew CN, Goodson ML, Park-Sarge OK, Sarge KD. J Biol Chem. 2001 Oct 26;276(43):40263–40267. doi: 10.1074/jbc.M104714200. [DOI] [PubMed] [Google Scholar]
- 34.Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, Taylor CT. Proc Natl Acad Sci USA. 2003 Feb 4;100(3):986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto H, Ihara M, Matsuura Y, Kikuchi A. EMBO J. 2003 May 1;22(9):2047–2059. doi: 10.1093/emboj/cdg204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller S, Berger M, Lehembre F, Seeler JS, Haupt Y, Dejean A. J Biol Chem. 2000 May 5;275(18):13321–13329. doi: 10.1074/jbc.275.18.13321. [DOI] [PubMed] [Google Scholar]
- 37.Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G. EMBO J. 2002 Oct 1;21(19):5206–5215. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long J, Wang G, He D, Liu F. Biochem J. 2004 Apr 1;379(Pt 1):23–29. doi: 10.1042/BJ20031867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano Y, Murata S, Tanaka K, Shimizu M, Sato R. J Biol Chem. 2003 May 9;278(19):16809–16819. doi: 10.1074/jbc.M212448200. [DOI] [PubMed] [Google Scholar]
- 40.Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. J Biol Chem. 2004 Sep 10;279(37):38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K. Mol Endocrinol. 2004 Oct;18(10):2451–2462. doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- 42.Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, Garcia-Wilson E, Perkins ND, Hay RT. Mol Cell. 2003 Apr;11(4):1043–1054. doi: 10.1016/s1097-2765(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 43.Gupta P, Ho PC, Huq MM, Ha SG, Park SW, Khan AA, Tsai NP, Wei LN. Proc Natl Acad Sci U S A. 2008;105(32):11424–11429. doi: 10.1073/pnas.0710561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rytinki MM, Palvimo JJ. J Biol Chem. 2008;283(17):11586–11595. doi: 10.1074/jbc.M709359200. [DOI] [PubMed] [Google Scholar]
- 45.Kang SI, Chang WJ, Cho SG, Kim IY. J Biol Chem. 2003 Dec 19;278(51):51479–51483. doi: 10.1074/jbc.M309237200. [DOI] [PubMed] [Google Scholar]
- 46.Leight ER, Glossip D, Kornfeld K. Development. 2005 Mar;132(5):1047–1056. doi: 10.1242/dev.01664. [DOI] [PubMed] [Google Scholar]
- 47.Long J, Zuo D, Park M. J Biol Chem. 2005 Oct 21;280(42):35477–35489. doi: 10.1074/jbc.M504477200. [DOI] [PubMed] [Google Scholar]
- 48.Hsu YH, Sarker KP, Pot I, Chan A, Netherton SJ, Bonni S. J Biol Chem. 2006 Nov 3;281(44):33008–33018. doi: 10.1074/jbc.M604380200. [DOI] [PubMed] [Google Scholar]
- 49.Roh HE, Lee MN, Jeon BN, Choi WI, Kim YJ, Yu MY, Hur MW. Cell Physiol Biochem. 2007;20(14):167–180. doi: 10.1159/000104164. [DOI] [PubMed] [Google Scholar]
- 50.Siatecka M, Xue L, Bieker JJ. Mol Cell Biol. 2007 Dec;27(24):8547–8560. doi: 10.1128/MCB.00589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benayoun BA, Auer J, Caburet S, Veitia RA. Proteomics. 2008;8(15):3118–3123. doi: 10.1002/pmic.200800084. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara T, Holt JA, Kiriakidou M, Strauss JF., III Biochemistry. 1996 Jul 16;35(28):9052–9059. doi: 10.1021/bi960057r. [DOI] [PubMed] [Google Scholar]
- 53.Kaipia A, Hsu SY, Hsueh AJ. Endocrinology. 1997;138(12):5497–5504. doi: 10.1210/endo.138.12.5588. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe TK, Fujiwara T, Kawai A, Shimizu F, Takami S, Hirano H, Okuno S, Ozaki K, Takeda S, Shimada Y, Nagata M, Takaichi A, Takahashi E, Nakamura Y, Shin S. Cytogenet Cell Genet. 1996;72(1):86–89. doi: 10.1159/000134169. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarti SR, Sood R, Ganguly S, Bohlander S, Shen Z, Nucifora G. Proc Natl Acad Sci USA. 1999 Jun 22;96(13):7467–7472. doi: 10.1073/pnas.96.13.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. J Biol Chem. 2001;276(38):35368–35374. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- 57.Bylebyl GR, Belichenko I, Johnson ES. J Biol Chem. 2003;278(45):44113–44120. doi: 10.1074/jbc.M308357200. [DOI] [PubMed] [Google Scholar]
- 58.Yang M, Hsu CT, Ting CY, Liu LF, Hwang J. J Biol Chem. 2006 Mar 24;281(12):8264–8274. doi: 10.1074/jbc.M510364200. [DOI] [PubMed] [Google Scholar]
- 59.Mao Y, Sun M, Desai SD, Liu LF. Proc Natl Acad Sci U S A. 2000;97(8):4046–4051. doi: 10.1073/pnas.080536597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vatsyayan J, Qing G, Xiao G, Hu J. EMBO Rep. 2008;9(9):885–890. doi: 10.1038/embor.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang SH, Sharrocks AD. Mol Cell. 2004;13(4):611–617. doi: 10.1016/s1097-2765(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 62.Lin DY, Fang HI, Ma AH, Huang YS, Pu YS, Jenster G, Kung HJ, Shih HM. Mol Cell Biol. 2004;24(24):10529–10541. doi: 10.1128/MCB.24.24.10529-10541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang CC, Lin DY, Fang HI, Chen RH, Shih HM. J Biol Chem. 2005;280(11):10164–10173. doi: 10.1074/jbc.M409161200. [DOI] [PubMed] [Google Scholar]
- 64.Jang MS, Ryu SW, Kim E. Biochem Biophys Res Commun. 2002;295(2):495–500. doi: 10.1016/s0006-291x(02)00699-x. [DOI] [PubMed] [Google Scholar]
- 65.Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. Mol Cell Biol. 2005;25(5):1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee K, Pisarska MD, Ko JJ, Kang Y, Yoon S, Ryou SM, Cha KY, Bae J. Biochem Biophys Res Commun. 2005 Oct 28;336(3):876–881. doi: 10.1016/j.bbrc.2005.08.184. [DOI] [PubMed] [Google Scholar]
- 67.Parker KL, Schimmer BP. Endocr Rev. 1997 Jun;18(3):361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- 68.Christenson LK, Osborne TF, McAllister JM, Strauss JF., III Endocrinology. 2001 Jan;142(1):28–36. doi: 10.1210/endo.142.1.7867. [DOI] [PubMed] [Google Scholar]
- 69.Shea-Eaton WK, Trinidad MJ, Lopez D, Nackley A, McLean MP. Endocrinology. 2001 Apr;142(4):1525–1533. doi: 10.1210/endo.142.4.8075. [DOI] [PubMed] [Google Scholar]
- 70.Buholzer CF, Arrighi JF, Abraham S, Piguet V, Capponi AM, Casal AJ. Mol Endocrinol. 2005 Jan;19(1):65–75. doi: 10.1210/me.2004-0061. [DOI] [PubMed] [Google Scholar]
- 71.Shibata H, Kobayashi S, Kurihara I, Suda N, Yokota K, Murai A, Ikeda Y, Saito I, Rainey WE, Saruta T. Endocr Res. 2004 Nov;30(4):795–801. doi: 10.1081/erc-200044042. [DOI] [PubMed] [Google Scholar]
- 72.Shibata H, Kurihara I, Kobayashi S, Yokota K, Suda N, Saito I, Saruta T. J Steroid Biochem Mol Biol. 2003 Jun;85(25):449–456. doi: 10.1016/s0960-0760(03)00217-6. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi S, Shibata H, Kurihara I, Yokota K, Suda N, Saito I, Saruta T. J Mol Endocrinol. 2004 Feb;32(1):69–86. doi: 10.1677/jme.0.0320069. [DOI] [PubMed] [Google Scholar]