Abstract
N-acylethanolamines (NAEs) are endogenous lipids that are synthesized in response to tissue injury, including ischemia and stroke, suggesting they may exhibit neuroprotective properties. We hypothesized that NAE 16:0 (palmitoylethanolemine) is neuroprotective against ischemia-reperfusion injury in rats, a widely employed model of stroke, and that neuroprotection is mediated through an intracellular mechanism independent of known NAE receptors. Administration of NAE 16:0 from 30 min. before to 2 hrs after stroke significantly reduced cortical and subcortical infarct volume, and correlated with an improvement of the neurological phenotype, as assessed by the neurological deficit score. We here show that NAE 16:0-mediated neuroprotection was independent of cannabinoid (CB1) and vanilloid (VR1) receptor activation, known NAE receptors on the plasma membrane, as determined by inclusion of specific inhibitors. The inclusion of an NAE uptake inhibitor (AM404), however, completely reversed NAE 16:0-mediated neuroprotection, suggesting that NAE 16:0’s effects are through an intracellular mechanism. NAE 16:0 produced a significant reduction in the number of cells undergoing apoptosis and reversed ischemia-induced up-regulation of several proteins, including inducible nitric oxide synthase and transcription factor NFκB. Our findings suggest that NAE 16:0-mediated neuroprotection is due to the reduction of neuronal apoptosis and inflammation in the brain.
Keywords: neurodegeneration, neuroprotection, receptors, ischemia
Stroke is the third leading cause of death in the United States with approximately 5.8 million current cases and approximately 780,000 new cases every year, making it a significant health problem (Lloyd-Jones et al., 2009). Potential neuroprotective treatment and intervention strategies have been tested over the last several years, but with limited success (White et al., 2000; Lo et al., 2003). Studies utilizing animal surgical models of stroke, such as ischemia-reperfusion injury induced by middle cerebral artery occlusion (MCAO), have been utilized to identify potential neuroprotective agents (Wen et al., 2004a, b; Hu et al., 2004; Green and Ashwood, 2005). Assessment of the efficacy of neuroprotective compounds against ischemia-reperfusion injury is an important in vivo experimental strategy relevant to ischemic stroke. Pharmacological agents used in these studies include anti-epileptic drugs (Calabresi et al., 2003), COX-2 inhibitors (Iadecola and Gorelick, 2005), estrogens (Wen et al., 2004a, b, 2007; Gibson et al., 2006), free radical scavengers (Green and Ashwood, 2005)and tissue plasminogen activator (Sheehan and Tsirka, 2005).
N-acylethanolamines (NAEs) are lipids present in the central nervous system and involved in cellular signaling and a variety of physiological funtions (for review, see Fride, 2002). One particular NAE, NAE 20:4 (arachidonylethanolamine; AEA), is an endogenous ligand for cannabinoid (CB1) receptors (Fride, 2002) and activation of CB1 receptors protects cultured cortical neurons from excitotoxicity and oxidative stress (Marsicano et al., 2003; Kim et al., 2005, 2006; Shouman et al., 2006). AEA has since been demonstrated to have significant neuroprotective effects in experimental models for stroke (Sinor et al., 2000). Other targets of NAE action include vanilloid receptors, protein kinases, nitric oxide synthase and possibly ion channels (Di Marzo et al., 2002; Fride, 2002).
Of particular relevance, some NAE species and NAE precursor molecules, N-acylphosphatidylethanolamines (NAPEs), are increased in response to multiple chemical and traumatic insults (Epps et al., 1979; Epps et al., 1980; Natarajan et al., 1986; Moesgaard et al., 1999; Schabitz et al., 2002; Berger et al., 2004), suggesting a role in cytoprotection. In addition, NAEs and NAPEs occur at higher levels in aged rat cortical neuron cultures and aged rats lose the ability to accumulate NAPE in the brain in response to ischemia (Moesgaard et al., 2000).
We previously hypothesized based on the structural and biochemical similarities to AEA that other NAEs that do not activate cannabinoid receptors are neuroprotective as well (Koulen and Chapman, 2006). Recently, the lesser characterized non-cannabinoid NAE 16:0 has been shown to reduce infarct volume in rats following ischemia/reperfusion injury (Schomacher et al., 2008). However, the therapeutic window for NAE 16:0 and the mechanisms underlying this NAE 16:0-mediated protection remain unclear.
We here determined the therapeutic window of NAE 16:0-mediated neuroprotection against ischemia/reperfusion injury in the middle cerebral artery occlusion (MCAO) model of ischemic stroke, and show that neuroprotection occurs by an intracellular mechanism independent of CB1 and vanilloid receptor 1 (VR1) activation, by inducing a reduction in activity of apoptotic and neuroinflammatory pathways.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague Dawley rats weighing 300–325 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). The animals were acclimatized for one week in the animal care facility prior to use in present studies. Animals were maintained in a temperature-controlled room (22–25 °C) with 12-hour light/dark cycles. Rats had free access to food and water. All animal experiments had been reviewed and approved by the Institutional Animal Care and Use Committee.
Middle cerebral artery occlusion (MCAO) to induce focal cerebral ischemia
For MCA occlusion and reperfusion, an intraluminal filament model was used as described by us previously (Wen et al., 2004a; Wen et al., 2004b; Wen et al., 2007). Briefly, animals were anesthetized with Ketamine (60 mg/kg) and Xylazine (10 mg/kg). For MCAO the left common carotid artery, left internal carotid artery (ICA) and the left external carotid artery were exposed, and a 3-0 monofilament nylon suture (Ethilon; Ethicon Inc., Sommerville, N.J., USA) was introduced into the ICA lumen through a puncture and was gently advanced until proper resistance was felt. After 90 min., the suture was gently withdrawn from the ICA and 24 hrs of reperfusion followed the MCAO. Rectal temperature of the animals was maintained at 37.0 ± 0.5° C throughout the surgery procedure using heating lamps. After recovery from anesthesia, animals were returned to their cages with free access to food and water.
Experimental groups
Animals were randomly divided into eight experimental groups for the present study: (1) control group of sham-operated rats with vehicle (ethyl alcohol) treatment, (2) control ischemic-reperfusion group (I/R, 90 min. of MCAO followed by 24 hrs of reperfusion) with vehicle treatment, (3) I/R with NAE 16:0 (10 mg/kg, i.p.) pretreatment, 6 hrs and 30 min. before MCAO, (4) I/R + NAE 16:0 concomitant with MCAO, (5) I/R + NAE 16:0 at 2 hrs after MCAO, (6) I/R + NAE 16:0 at 3 hrs after MCAO, (7) AM251 (10 mg/kg, i.p., 15 min. before MCAO) + Capsazepine (CPZ) (10 mg/kg, i.p., 10 min. before MCAO) + I/R + NAE 16:0 at 0 hrs after MCAO, (8) AM404 (10 mg/kg, i.p., 15 min. before MCAO) + I/R + NAE 16:0 at 0 hrs after MCAO. All parameters were measured at 24 hrs after 90 min. of MCAO. All compounds used in this study were administered intraperitoneally (i.p.) at indicated times and dosed with ethyl alcohol as the vehicle control.
Measurement of cerebral infarct volume
For evaluation of infarct volume and other parameters an overdose of pentobarbital was given to rats prior to decapitation. Brains were quickly removed and placed in ice-cold saline for 5 min. Seven coronal slices of 2 mm thickness were cut from each brain and incubated in 2% 2,3,5- triphenyltetrazolium chloride (TTC) for 15 min at 37 °C. In the TTC stained sections, pale colored region indicated infarct area and colored region indicated viable areas. Stained brain sections were stored in 10% formalin and refrigerated at 4 °C for further processing and storage. Analysis for infarct volume in each brain slice was done using Simple PCI version 5.3.1 High Performance Imaging Software (Compix, Inc., Cranberry Township, PA).
Infarction volume was calculated with a previously described method to compensate for brain swelling in the ischemic hemisphere (Swanson et al., 1990). Briefly, the infarction area in each section was calculated by subtracting the non-infarct area of the ipsilateral side from the area of the contralateral side. Infarction areas on each section were summed and multiplied by section thickness to give the total infarction volume, which is expressed as a percentage of total volume.
Neurological evaluation
Neurological evaluation was performed before euthanasia at 24 hrs of reperfusion after MCAO for neurological deficits and scored as described elsewhere as follows: 0, no observable neurological deficit (normal); 1, failure to extend left forepaw on lifting the whole body by tail (mild); 2, circling to the contralateral side (moderate); 3, leaning to the contralateral side at rest or no spontaneous motor activity (severe) (Huang et al., 1994).
Hematoxylin and Eosin stain
Hematoxylin and eosin (HE) staining was conducted on animals sacrificed 24 hrs after sham surgery or I/R with and without NAE 16:0 treatment at the time of occlusion. Brains were flash frozen in liquid nitrogen, cryosectioned at 16 m onto StarFrost adhesive slides (Mercedes Medical, Sarasota, FL) and stored at −80 °C until staining. Sections were fixed in 4% (w/v) paraformaldehyde in phosphate puffer (pH 7.4) for one hour at room temperature and washed two times for 7 min. each with PBS containing 0.5% (w/v) glycine. HE staining was performed on these sections (Hu et al., 2004). Five microscopic fields in each section (from areas marked in Fig. 3C) were analyzed. Images were acquired using a Zeiss Axiovert 200M inverted microscope equipped with an axiocam MRC5 camera (Carl Zeiss, Thornwood, NY, USA).
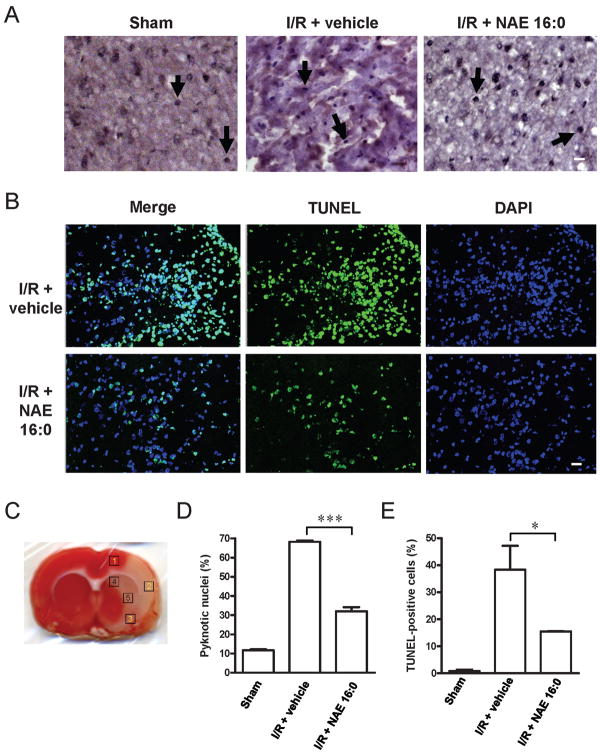
Figure 3. NAE 16:0 protects from I/R-induced apoptosis.
(A) Representative photomicrographs of hematoxilin/eosin (HE) staining within the ischemic area of the cortex in sham-operated and ischemic-reperfusion rats with or without NAE 16:0 treatment. Arrows indicate pyknotic nuclei, indicative of cells undergoing apoptosis. Fewer pyknotic nuclei were observed following I/R in the presence of NAE 16:0. Scale bar: 100 μm. (B) TUNEL labeling revealed a substantially reduced number of TUNEL-positive cells in the presence of NAE 16:0. In sham-operated animals, almost no TUNEL-positive cells were identified (data not shown). Scale bar: 50 μm. (C) For quantification of pyknotic nuclei and TUNEL-positive cells, five areas in each section were analyzed. Areas are indicated here on a TTC-stained section of a vehicle-treated rat following I/R. Areas 1–3 are cortical and lie in the ischemic core and penumbral zone, whereas areas 4–5 represent subcortical areas mainly falling in the ischemic penumbral zone. (D) Treatment with NAE 16:0 reduced the number of pyknotic nuclei following MCAO/reperfusion significantly by 53%, compared with vehicle-treated control. (E) Similarly, the number of TUNEL-positive cells was 60% lower in NAE 16:0 treated brain sections, compared with vehicle control, indicating that NAE 16:0 treatment protects from induction of apoptosis and cell-death pathways. Data are presented as mean ± s.e.m. for each group. * p<0.05, *** p<0.001, using one-way ANOVA with Newman Keuls multiple comparison post-hoc test.
TUNEL stain
DNA fragmentation was detected using terminal deoxynucleotidyltransferase, recombinant enzyme mediated dUTP Nick-End Labeling (TUNEL) method with the DeadEnd Fluorometric TUNEL assay according to the manufacturer’s protocol (Promega, Madison, WI). Animals were sacrificed at 24 hrs after post MCAO reperfusion, brains were removed, flash frozen on liquid nitrogen, cryosectioned at 16 μm onto adhesive slides and stored at −70 °C until further processing. Sections were fixed in 4% (w/v) paraformaldehyde in PB (pH 7.4) for 30 min. at room temperature and washed two times for 7 min. each with PBS containing 0.5 % (w/v) glycine. Sections were then permeabilized for 10 min. in 0.2% (v/v) Triton X-100 and then further processed for staining with DeadEnd Fluorometric TUNEL. As a negative control, sections of ischemic brain were used after the standard procedures, but recombinant terminal deoxynucleotidyltransferase (rTdT) was omitted. To counterstain with DAPI for detecting the total number of cells the sections were mounted in Prolong Gold containing DAPI (Invitrogen, Carlsbad, CA). Total of five sections from each rat brain were taken and five microscopic fields (see Fig. 3C) in each section were acquired and analyzed. Optically sectioned images were acquired by using a Zeiss LSM 510 Duo META confocal laser-scanning microscope (Carl Zeiss, Thornwood, NY, USA). For counting cells stereologically in each field, the maximum projection of optically sectioned images was used. From each image the total number of cells (nuclei stained with DAPI) and the number of TUNEL positive cells is counted and percentage of cells (TUNEL positive) in each image was calculated using Simple PCI version 5.3.1 software (Compix, Inc., Cranberry Township, PA).
Immunohistochemistry
Immunohistochemistry was performed on tissue from animals sacrificed 24 hrs after reperfusion following MCAO. Brains were flash frozen in liquid nitrogen, cryosectioned at 16 m onto adhesive slides and stored at −70°C until staining. Sections were fixed in 4% (w/v) paraformaldehyde in PB (pH 7.4) for one hour at room temperature and washed two times for 7 min. each with PBS containing 0.5 % (w/v) glycine. Sections were then permeabilized with 0.5% (v/v) Triton X-100 (25 min.) and blocking buffer was added containing 0.5% (w/v) gelatin and 0.5% (w/v) BSA in PBS for one hour. Sections were incubated overnight with the following antibodies: rabbit anti- iNOS (Cell Signaling Technology), rabbit anti- nNOS (Chemicon Internatinal), rabbit anti- NFkB (Santa Cruz Biotech. Santa Cruz, CA), rabbit anti-BACE 1 (Abcam, Cambridge, MA) antibodies at a dilution of 1:100 and rabbit anti- caspase 3 (Cell Signaling Technology) antibodies at a dilution of 1:400 and were subsequently stained at room temperature for one hour with highly specific Alexa Fluor 488 and Alexa Fluor 594 conjugated secondary goat anti- rabbit IgG antibodies (Molecular probes) at a dilution of 1:1000. Sections were incubated with Hoechst 33342 (Sigma) for staining nuclei along with the secondary antibody incubation. Negative controls were performed by omitting primary antibodies. Images were acquired using an Olympus IX-70 fluorescence microscope (Olympus America, Melville, NY). Total of three sections from each rat brain were taken and ten microscopic fields (from areas marked in Fig. 3C) in each section were acquired and analyzed using Simple PCI version 5.3.1 imaging software (Compix, Inc., Cranberry Township, PA). Microfluorimetric analysis was conducted as described previously (Stokely et al., 2008). Background adjustment for fluorescence was calculated by subtracting total fluorescence from the appropriate secondary antibody control from the total fluorescence measured for each image to determine the primary antibody specific total fluorescence within each specific area. Primary antibody specific total fluorescence was compared among different experimental groups and tested for statistical significance by ANOVA with post-hoc Bonferroni multiple comparison test.
Statistical data analysis
Data are expressed as the mean standard error of mean. Statistical significance was determined by ANOVA with post-hoc Newman Keuls and Bonferroni multiple comparison tests, using GraphPad Prism 4 statistical software and a p value of less than 0.05 was considered significant.
RESULTS
NAE 16:0 treatment is neuroprotective against and facilitates functional recovery from ischemia/reperfusion injury
We performed MCAO followed by 24 hr reperfusion in rats, using an intraluminal filament model. Vehicle-treated rats displayed extensive infarction (38.4 ± 5.7%) in the cortical and subcortical areas, as determined using TTC-stained coronal brain slices (Fig. 1A,B), and consistent with previous reports (Xu et al., 2003; Tsubokawa et al., 2007).
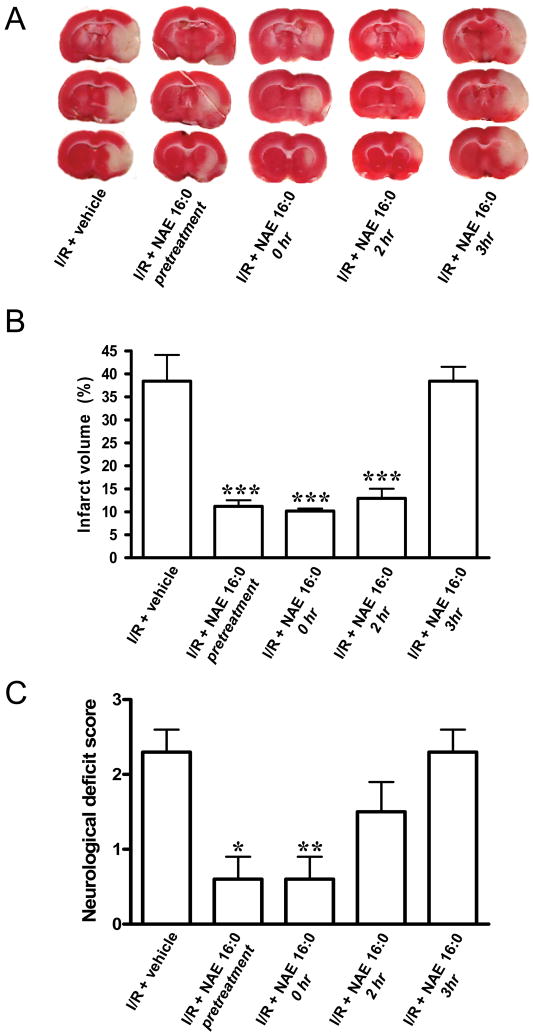
Figure 1. NAE 16:0 is neuroprotective against ischemic tissue damage and improves neurological outcome following I/R.
(A) Representative TTC-stained sections of rat brain following 90 min. MCAO/24 hr reperfusion (ischemia/reperfusion; I/R), treated with vehicle or NAE 16:0 at various time points. Viable tissue stains red, whereas damaged ischemic brain tissue appears unstained/white. (B) Quantification of the volume of the ischemic lesion (infarct volume) revealed that NAE 16:0 was neuroprotective when administered from 30 min. before MCAO (pretreatment), up to two hrs after occlusion. Administration of NAE 16:0 at 3 hrs post-MCAO had no effect on the size of the ischemic lesion. (C) I/R causes a moderate to severe neurological phenotype, as determined by the standardized neurological deficit score. NAE 16:0 reduced I/R-induced neurological deficits significantly (none to mild neurological phenotype) when administered before or at the time of occlusion. Data are shown as mean ± s.e.m. * p<0.05, ** p<0.01, *** p<0.001, compared with vehicle treated control group as determined using one-way ANOVA with Newman Keuls multiple comparison post-hoc test.
Administration of NAE 16:0 (10 mg/kg i.p.) 30 min. before, during or 2 hrs after occlusion resulted in a significant reduction in of infarct volume compared with vehicle treatment (11.2 ± 1.3%, 10.2 ± 0.5% and 12.9 ± 2.0%, respectively, n=3, P<0.001, Fig. 1A,B). This corresponds to 70.7%, 73.3%, and 66.3% less infarction compared with vehicle, respectively. In contrast, NAE 16:0 treatment 3 hrs post occlusion did not significantly affect infarct size, which was 38.4 ± 3.1% (Fig. 1A,B). The neuroprotective effect NAE 16:0 was also reflected by an improvement of the ischemia/reperfusion injury-induced neurological deficits, assessed following MCAO and 24 hr reperfusion using the standardized neurological deficit score (Fig. 1C). The mean neurological deficit score was 2.3 in vehicle-treated rats, corresponding to a medium to severe neurological phenotype. The score of NAE 16:0-treated rats improved significantly to scores of 0.6 when NAE 16:0 administered 30 min. prior to (n=3, P<0.05) or at the same time as MCAO occurred (n=3, P<0.01) (Fig. 1C). When we administered NAE 16:0 two hrs following MCAO, we observed a mild improvement of neurological deficits, however, this did not reach statistical significance. At three hrs post-MCAO, NAE 16:0 treatment had no effect on the neurological outcome (n=3, Fig. 1C).
NAE 16:0 neuroprotection is independent of CB1 and VR1 receptors, but dependent on uptake into cells
In order to gain mechanistic insight into NAE 16:0-mediated neuroprotection, we performed MCAO and reperfusion experiments in the presence of inhibitors of CB1 and VR1 endocannabinoid receptors. To this end, AM251 (a CB1 antagonist) and CPZ (a VR1 antagonist) were co-administered with NAE 16:0 at the time of MCAO. Blocking CB1 and VR1 receptors did not alter the neuroprotective effects of NAE 16:0 treatment: infarction volume was 15.2 ± 5.7% in comparison to 10.2 ± 0.5% of NAE 16:0 treatment alone (n=3, Fig. 2A,B). Similarly, there was no difference in the neurological outcome of rats treated with NAE 16:0 without or in the presence of AM251 and CPZ (n=3, Fig. 2C). These data suggest that neuroprotection by NAE 16:0 is not mediated via the classical endocannabinoid receptors CB1 and VR1.
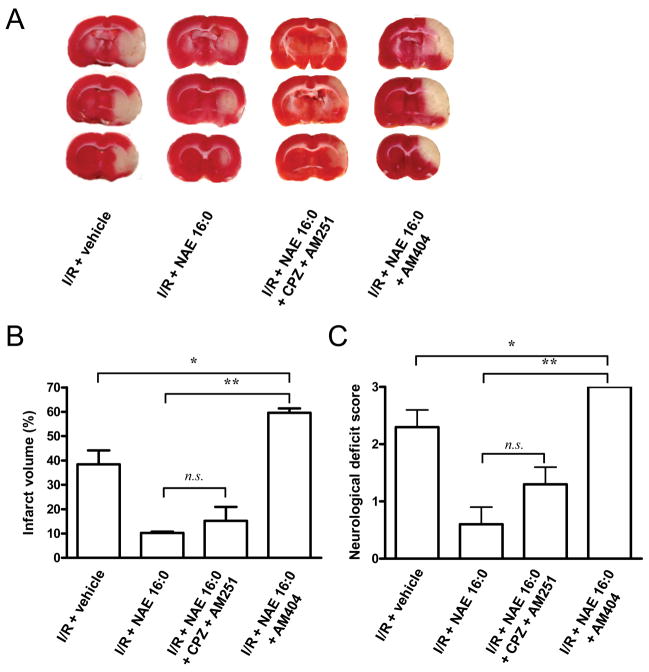
Figure 2. NAE 16:0-mediated neuroprotection is independent of activation of CB1 and VR1 receptors.
(A) Representative TTC-stained sections of rat brain following 90 min. MCAO/24 hr reperfusion (ischemia/reperfusion; I/R). NAE 16:0 was administered at the time of occlusion. In one experimental group capsazepine (CPZ) and AM251 were co-administered in order to inhibit cannabinoid (CB1) and vanilloid (VR1) receptors; in another, the endocannabinoid uptake inhibitor AM404 was co-administered with NAE 16:0. (B) Neuroprotection by NAE 16:0, as assessed by quantification of the infarct volume, was unaltered in the presence of the CB1 and VR1 receptor antagonists CPZ and AM251. In contrast, blocking endocannabinoid uptake using AM404 significantly increased the size of ischemic lesion by 500%, compared to NAE 16:0 treatment, and by 50% when compared to I/R in the presence of vehicle control. (C) The size of the infarct volume correlated with the neurological deficit score: blocking CB1 and VR1 receptors using CPZ and AM251, respectively, did not significantly affect the neurological deficit score. AM404 treatment induced more severe neurological deficits than MCAO in the presence of vehicle alone. Data are presented as mean ± s.e.m. for each group. * p<0.05, ** p<0.01, n.s. non-significant, using one-way ANOVA with Newman Keuls multiple comparison post-hoc test.
Using AM404, an NAE uptake inhibitor, allowed us to assess whether uptake into cells was required for NAE 16:0’s neuroprotective effects. When we co-administered AM404 with NAE 16:0 at the time of MCAO, we found a significant increase of brain infarction volume exceeding that of vehicle-treated rat (59.6 ± 1.8% and 38.4 ± 5.7%, respectively; n=3, P<0.05; Fig. 2A,B). In accordance, AM404-treated rats exhibited the maximum neurological deficit score, which was significantly more severe than that of vehicle-treated rats (n=3, P<0.05, Fig. 2C).
NAE 16:0 protects against ischemia-induced cell death and apoptosis
One of the hallmarks of ischemia/reperfusion injury is the induction of apoptotic and cell death pathways. We here used quantification of pyknotic nuclei in HE staining and positive cells in TUNEL labeling studies to determine the effects of NAE 16:0 treatment on apoptosis and cell death. Following ischemia/reperfusion, 68.2 ± 0.7% of nuclei in cortical and subcortical areas (Fig. 3C) on the side ipsilateral to the injury were pyknotic, as indicative of undergoing DNA fragmentation, compared with only 11.7 ± 0.5% in the sham-operated group. NAE 16:0 treatment reduced the number of pyknotic nuclei by half to 32.0 ± 2.1% (n=3, P<0.001; Fig. 3A,D). Similarly, the number of TUNEL-positive cells was reduced by 60%, from 38.3 ± 8.8% to 15.4 ± 0.2%, when NAE 16:0 was administered (n=3, P<0.05, Fig. 3B,E). The percentage of TUNEL-positive cells in the sham-operated group was 0.8 ± 0.6% (Fig. 3E).
NAE 16:0 decreases ischemia reperfusion induced levels of immunoreactivity for proteins mediating cell death and cellular defense
There is a large number of proteins that are up-regulated in response to ischemia/reperfusion injury and that have been proposed or shown to mediate cell death and cellular defenses. We here chose five proteins and investigated using microfluorometry, whether NAE 16:0 treatment was effective in preventing up-regulation of these proteins.
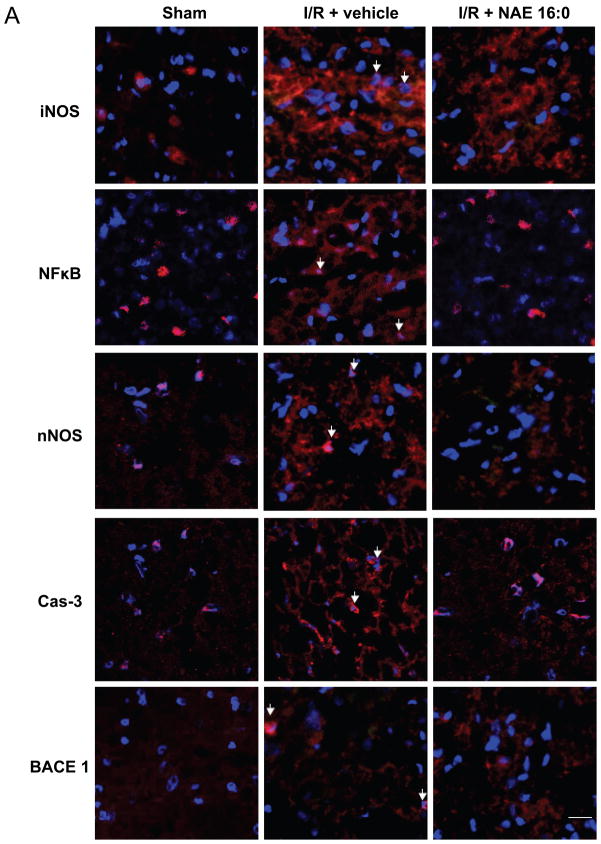
Qualitative assessment showed that iNOS, NFκB, nNOS, Cas-3 immunoreactivities appeared up-regulated in ischemia/reperfusion-treated rat brain, compared with sham-operated control (Fig. 4A). Furthermore, NAE 16:0 treatment at the time of MCAO prevented this up-regulation. BACE 1 immunoreactivity was similar in all three groups.
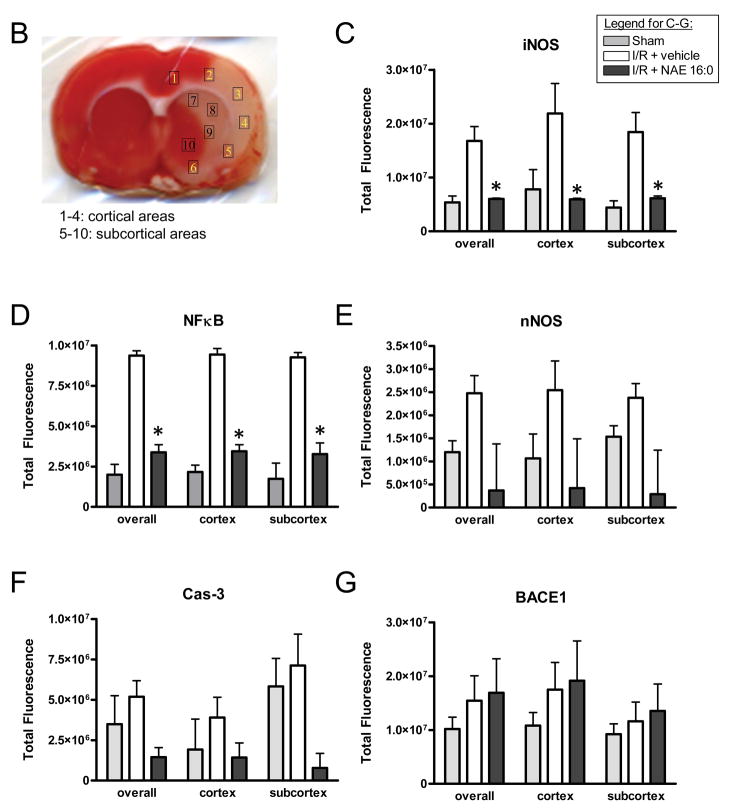
Figure 4. NAE 16:0-mediated neuroprotection involves iNOS and NFκB signaling pathways.
(A) Representative photomicrographs of fluorescence of Hoechst, a nuclear counterstain (blue), and immunofluorescence for inducible nitric oxide synthase (iNOS), nuclear factor kappa B (NFκB), neuronal nitric oxide synthase (nNOS), caspase-3 (Cas-3) and beta secretase 1 (BACE1) from cortical area 4 described in below. Immunoreactivites for iNOS, NFkB, nNOS and Cas-3 appeared increased ipsilaterally in the I/R group when compared to sham-operated animals. That increase was attenuated in the NAE 16:0-treated group. In contrast, expression of BACE1 was similar across all three experimental groups. Arrows indicate examples of positive immunoreactivity. Scale bar = 50 μm. (B) TTC-stained section vehicle-treated I/R brain, indicating the areas selected for quantitative microfluorimetric analysis. Areas 1–6 are cortical areas, comprising the ischemic core and penumbral areas, whereas areas 7–10 represent subcortical areas mainly including ischemic penumbral area. (C–G) Microfluorimetric analysis of immunoreactivities of iNOS, NFκB, nNOS, Cas-3 and BACE1 in sham-operated, vehicle-treated and NAE 16:0-treated brain sections. Overall immunoreactivities of all proteins increased in the vehicle-treated I/R group compared to sham-operated animals. NAE 16:0-treatment at the time of I/R prevented the ischemia-mediated increase of iNOS, NFκB, nNOS and Cas-3. This effect reached statistical significance for iNOS and NFκB (p<0.05). Microfluorimetric analysis of BACE1 immunoreactivity revealed no statistically significant change following I/R without or in the presence of NAE 16:0. Data are presented as mean ± s.e.m. for each group. *p < 0.05 as determined by two-way ANOVA with Bonferroni post-hoc test.
In order to quantify changes in immunoreactivities, we selected a total of 10 areas in cortex and subcortex (Fig. 4B) and performed microfluorometry. NAE 16:0 had a significant effect of preventing up-regulation of iNOS and NFκB, both in ipsilateral cortical and subcortical areas (Fig. 4C,D). The apparent lack of up-regulation of nNOS and Cas-3 did not reach statistical significance in our microfluorometric quantification (Fig. 4E,F). Quantification of BACE 1 confirmed that neither ischemia/reperfusion nor NAE 16:0 treatment significantly affected protein levels (Fig. 4G). The quantification of the individual cortical and subcortical areas is provided in Supplemental Figure 1.
DISCUSSION
Here we investigated the mechanism of NAE 16:0-mediated neuroprotection against ischemia/reperfusion injury, as a model for stroke. We show that administration of NAE 16:0 reduced infarct volume in both cortical and subcortical areas and improved neurological outcome when administered up to two hrs following MCAO. The mechanism of neuroprotection by NAE 16:0 is intracellular, and independent of activation of endocannabinoid receptors. Rather, NAE 16:0 appears to prevent the induction of apoptotic and neuroinflammatory pathways.
The extent of NAE 16:0 neuroprotection in our rat model of MCAO and reperfusion is similar to that reported recently by Schomacher and colleagues (2008). However, the previous study failed to address the therapeutic window of NAE 16:0 as well as to investigate the mechanism by which neuroprotection is mediated. We here could show that NAE 16:0 was effectively neuroprotective when administered between 30 min. prior to MCAO (pre-treatment) up to two hrs following MCAO. When administered at the same time as MCAO, neuroprotection was maximal, as assessed by the largest reduction of ipsilateral infarct volume and best neurological outcome.
NAE 16:0 has been shown not to be a ligand for cannabinoid (CB1 and CB2) or vanilloid (VR1) receptors (Lambert and Di Marzo, 1999; Sugiura et al., 2000), hence excluding these targets as likely mediators of NAE 16:0 neuroprotection. Accordingly, blocking CB1 and VR1 receptors using the specific inhibitors AM251 and CPZ did not significantly affect NAE 16:0 neuroprotection in our rat model of ischemia/reperfusion injury.
However, the role of CB1 and VR1 in neuroprotection is controversial: some reports showed that short-term ischemia reduced CB1 expression, and inhibition of CB1 lead to protection against ischemia, suggesting that CB1 activation is detrimental (Schomacher et al., 2006; Sommer et al., 2006). Others have also shown that activation of VR1 leads to neuronal death (Maccarrone et al., 2000; Shirakawa et al., 2008). If this were the case, then CB1 and VR1 antagonists as used in the present study should either have had no effect on NAE 16:0 neuroprotection, or even offered more protection than NAE 16:0 alone.
It has also been suggested that some non-cannabinoid NAEs can serve as ‘entourage’ compounds, which enhance the activity of cannabinoid receptor ligands, such as AEA and 2-arachidonylglycerol, thereby contributing to cytoprotection (De Petrocellis et al., 2001; Jonsson et al., 2001; Smart et al., 2002). However, our data showing that the CB1 and VR1 inhibitors, AM251 and CPZ, respectively, do not affect NAE 16:0-mediated neuroprotection rule out any potential entourage effect of NAE 16:0. Furthermore, our data strongly support the notion that neuroprotection must occur via an intracellular mechanism.
We tested this hypothesis by performing MCAO in the additional presence of the NAE uptake inhibitor, AM404. This completely reversed the neuroprotective effects in our model, further suggesting that NAE 16:0 must be taken up into the cell via the NAE transporter in order to act as a neuroprotectant. Interestingly, AM404 has been shown to activate VR1 leading to cell death in cancer cell lines (Zygmunt et al., 2000; Chang et al., 2008). It is thus possible that AM404 administration caused VR1 activation and subsequent neuronal death regardless of any inhibitory effects on the NAE transporter. The fact that AM404 actually significantly increased infarct volume compared to vehicle-treated controls may support that hypothesis. It was beyond the scope of the present study, however, to address whether AM404 interfered prevented NAE 16:0-mediated neuroprotection by inhibiting NAE uptake or by activation of VR1.
Furthermore, NAE 16:0 has been shown to be a poor agonist of the ‘peripheral’ CB2 receptor, thereby making it an unlikely target for NAE 16:0 in our studies (Lambert and Di Marzo, 1999; Sugiura et al., 2000). The nature and extent of CB2 distribution in the brain further adds to the controversy of CB2 as a potential receptor target eliciting NAE 16:0-mediated neuroprotective effects (Galiegue et al., 1995; Schatz et al., 1997; Brusco et al., 2008a; 2008b).
In our model, NAE 16:0 reduced the number of pyknotic and TUNEL-labeled nuclei suggesting that NAE 16:0 reduced apoptotic cell death in the brain. Furthermore, NAE 16:0 administration reduced the expression of NFκB and iNOS, both contributing to neurodegeneration in models of stroke (Huang et al., 1994; Moro et al., 2004; Wen et al., 2004a).
Our microfluorimetric quantification from multiple cortical and sub-cortical areas clearly demonstrates that NAE 16:0 prevents the up-regulation of both NFκB and iNOS in the ischemia/reperfusion model. We interpret such reduction in the immunoreactivities of iNOS and NFκB as an improvement in the outcome in the MCAO stroke model.
Whilst the involvement and contributions of nNOS and iNOS in ischemia have been well characterized (Huang et al., 1994; Moro et al., 2004; Perez-Asensio et al., 2005), the role of NFκB in ischemia and reperfusion injury remains somewhat elusive and controversial (Crack and Taylor, 2005; Fraser, 2006; Nijboer et al., 2008). Numerous studies have shown that activation of NFκB in neurons leads to neuroprotection by increasing the expression of genes encoding anti-apoptotic proteins (Mattson, 2005). Others show that NFκB activation is associated with neuroinflammation or neurodegeneration (Nichols, 2004; Wen et al., 2004b; Yenari and Han, 2006). In some studies, inhibition of NFκB also lead to neuroprotection (Nichols, 2004; Yenari and Han, 2006). The duration of NFκB activation following stroke may determine, whether NFκB contributes to neurodegeneration or initiates neuroprotection (Crack and Taylor, 2005).
The present study shows that non-cannabinoid NAEs, specifically NAE 16:0, are neuroprotective in experimental models for stroke, and that the mechanism of neuroprotection is independent of CB1 or VR1 receptor activation, but rather mediated through an intracellular, anti-apoptotic pathway. The data presented here provide the basis for more detailed mechanistic analyses of how NAE 16:0 and other saturated NAEs affect intracellular signaling pathways involved in neurodegeneration and neuroprotection with the potential to ultimately provide new targets for therapeutic intervention in stroke.
Supplementary Material
(A–E) Microfluorimetric analysis per area (as defined in Figure 4B), showing similar immunoreactivities and I/R and NAE 16:0 treatment-associated changes in all areas investigated. × denotes that the fluorescence signal was below the detection threshold. Data are presented as mean ± s.e.m.
Acknowledgments
We thank Dr. James Simpkins at the University of North Texas Health Science Center at Fort Worth and Dr. Kent Chapman at the University of North Texas for their support. This study was supported in part by P20-MD001633 from NCMHD (R.S.D.), grants EY014227 from NIH/NEI, RR022570 from NIH/NCRR and AG010485, AG022550 and AG027956 from NIH/NIA (P.K.) as well as by The Garvey Texas Foundation (P.K.). We thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Abbreviations
- AEA
arachidonylethanolamine
- CB1/2
cannabinoid receptor type 1/2
- COX-2
cyclooxygenase-2
- CPZ
capsazepine
- HE
hematoxylin and eosin
- ICA
internal carotid artery
- I/R
ischemia/reperfusion
- iNOS
inducible metric oxide synthase
- MCAO
middle cerebral artery occlusion
- NAE
N-acylethanolamine
- NAPE
N-acylethanolamine-hydrolyzing phospholipase D
- NFκB
nuclear factor kappa B
- nNOS
neuronal nitric oxide synthase
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- tPA
tissue plasminogen activator
- TdT
terminal deoxynucleotidyl transferase
- TTC
2, 3, 5 - triphenyltetrazolium chloride
- TUNEL
TdT-mediated dUTP Nick-End Labeling
- VR1
vanilloid receptor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008a;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci. 2008b;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- Chang HT, Huang CC, Cheng HH, Wang JL, Lin KL, Hsu PT, Tsai JY, Liao WC, Lu YC, Huang JK, Jan CR. Mechanisms of AM404-induced [Ca(2+)](i) rise and death in human osteosarcoma cells. Toxicol Lett. 2008;179:53–58. doi: 10.1016/j.toxlet.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Epps DE, Natarajan V, Schmid PC, Schmid HO. Accumulation of N-acylethanolamine glycerophospholipids in infarcted myocardium. Biochim Biophys Acta. 1980;618:420–430. doi: 10.1016/0005-2760(80)90260-x. [DOI] [PubMed] [Google Scholar]
- Epps DE, Schmid PC, Natarajan V, Schmid HH. N-Acylethanolamine accumulation in infarcted myocardium. Biochem Biophys Res Commun. 1979;90:628–633. doi: 10.1016/0006-291x(79)91281-6. [DOI] [PubMed] [Google Scholar]
- Fraser CC. Exploring the positive and negative consequences of NF-kappaB inhibition for the treatment of human disease. Cell Cycle. 2006;5:1160–1163. doi: 10.4161/cc.5.11.2773. [DOI] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system--an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- Green AR, Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord. 2005;4(2):109–18. doi: 10.2174/1568007053544156. [DOI] [PubMed] [Google Scholar]
- Hu XM, Zhang Y, Zeng FD. Effects of beta-aescin on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol Sin. 2004;25:1267–1275. [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Gorelick PB. The Janus face of cyclooxygenase-2 in ischemic stroke: shifting toward downstream targets. Stroke. 2005;36:182–185. doi: 10.1161/01.STR.0000153797.33611.d8. [DOI] [PubMed] [Google Scholar]
- Jonsson KO, Vandevoorde S, Lambert DM, Tiger G, Fowler CJ. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol. 2001;133:1263–1275. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. J Pharmacol Exp Ther. 2005;313:88–94. doi: 10.1124/jpet.104.079509. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol. 2006;69:691–696. doi: 10.1124/mol.105.016428. [DOI] [PubMed] [Google Scholar]
- Koulen P, Chapman K. Modulation of intracellular calcium signaling by N-acylethanolamines. 2006-0142395. US patent publication number. 2006:A1.
- Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic? Curr Med Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agro A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J Biol Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- Moesgaard B, Jaroszewski JW, Hansen HS. Accumulation of N-acyl-ethanolamine phospholipids in rat brains during post-decapitative ischemia: a 31p NMR study. J Lipid Res. 1999;40:515–521. [PubMed] [Google Scholar]
- Moesgaard B, Petersen G, Jaroszewski JW, Hansen HS. Age dependent accumulation of N-acyl-ethanolamine phospholipids in ischemic rat brain. A (31)P NMR and enzyme activity study. J Lipid Res. 2000;41:985–990. [PubMed] [Google Scholar]
- Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Schmid PC, Schmid HH. N-acylethanolamine phospholipid metabolism in normal and ischemic rat brain. Biochim Biophys Acta. 1986;878:32–41. doi: 10.1016/0005-2760(86)90341-3. [DOI] [PubMed] [Google Scholar]
- Nichols TC. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- Perez-Asensio FJ, Hurtado O, Burguete MC, Moro MA, Salom JB, Lizasoain I, Torregrosa G, Leza JC, Alborch E, Castillo J, Knowles RG, Lorenzo P. Inhibition of iNOS activity by 1400W decreases glutamate release and ameliorates stroke outcome after experimental ischemia. Neurobiol Dis. 2005;18:375–384. doi: 10.1016/j.nbd.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Schomacher M, Muller HD, Sommer C. Short-term ischemia usually used for ischemic preconditioning down-regulates central cannabinoid receptors in the gerbil hippocampus. Acta Neuropathol. 2006;111:8–14. doi: 10.1007/s00401-005-1109-2. [DOI] [PubMed] [Google Scholar]
- Schomacher M, Muller HD, Sommer C, Schwab S, Schabitz WR. Endocannabinoids mediate neuroprotection after transient focal cerebral ischemia. Brain Res. 2008;1240:213–220. doi: 10.1016/j.brainres.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005;50:340–350. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- Shirakawa H, Yamaoka T, Sanpei K, Sasaoka H, Nakagawa T, Kaneko S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem Biophys Res Commun. 2008;377:1211–1215. doi: 10.1016/j.bbrc.2008.10.152. [DOI] [PubMed] [Google Scholar]
- Shouman B, Fontaine RH, Baud O, Schwendimann L, Keller M, Spedding M, Lelievre V, Gressens P. Endocannabinoids potently protect the newborn brain against AMPA-kainate receptor-mediated excitotoxic damage. Br J Pharmacol. 2006;148:442–451. doi: 10.1038/sj.bjp.0706755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor AD, Irvin SM, Greenberg DA. Endocannabinoids protect cerebral cortical neurons from in vitro ischemia in rats. Neurosci Lett. 2000;278:157–160. doi: 10.1016/s0304-3940(99)00922-2. [DOI] [PubMed] [Google Scholar]
- Smart D, Jonsson KO, Vandevoorde S, Lambert DM, Fowler CJ. ‘Entourage’ effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br J Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schomacher M, Berger C, Kuhnert K, Muller HD, Schwab S, Schabitz WR. Neuroprotective cannabinoid receptor antagonist SR141716A prevents downregulation of excitotoxic NMDA receptors in the ischemic penumbra. Acta Neuropathol. 2006;112:277–286. doi: 10.1007/s00401-006-0110-8. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Garg P, Bhat MA, Koulen P. Transient 5-(4-phenylbutoxy)psoralen (PAP-1) treatment dissociates developing pathologies in autoimmune optic neuritis into two distinct pathology profiles. J Neurosci Res. 2008;86:2111–2124. doi: 10.1002/jnr.21645. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Jadhav V, Solaroglu I, Shiokawa Y, Konishi Y, Zhang JH. Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke. 2007;38:1057–1062. doi: 10.1161/01.STR.0000257978.70312.1d. [DOI] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004a;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004b;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta. 2007;1772:473–483. doi: 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–E) Microfluorimetric analysis per area (as defined in Figure 4B), showing similar immunoreactivities and I/R and NAE 16:0 treatment-associated changes in all areas investigated. × denotes that the fluorescence signal was below the detection threshold. Data are presented as mean ± s.e.m.