Abstract
Imbalanced protein load within cells is a critical aspect for most diseases of aging. In particular, the accumulation of proteins into neurotoxic aggregates is a common thread for a host of neurodegenerative diseases. Our previous work demonstrated that age-related changes to the cellular chaperone repertoire contributes to abnormal buildup of the microtubule-associated protein tau that accumulates in a group of diseases termed tauopathies, the most common being Alzheimer's disease. Here, we show that the Hsp90 cochaperone, FK506-binding protein 51 (FKBP51), which possesses both an Hsp90-interacting tetratricopeptide domain and a peptidyl-prolyl cis-trans isomerase (PPIase) domain, prevents tau clearance and regulates its phosphorylation status. Regulation of the latter is dependent on the PPIase activity of FKBP51. FKB51 enhances the association of tau with Hsp90, but the FKBP51/tau interaction is not dependent on Hsp90. In vitro FKBP51 stabilizes microtubules with tau in a reaction depending on the PPIase activity of FKBP51. Based on these new findings, we propose that FKBP51 can use the Hsp90 complex to isomerize tau, altering its phosphorylation pattern and stabilizing microtubules.
Introduction
Protein quality control by the chaperone network is essential for cell survival. In long-lived neurons, this system is critically important to maintain cellular homeostasis. In the past, the mechanisms contributing to the decisions made by the cellular chaperone system, either degradation or folding of a substrate/client, were generalized into a single system with very little substrate/client specificity. However, with the discovery of well over 100 gene products that encode for chaperone proteins in the mammalian genome, this notion has become much less pervasive. While the chaperones Hsp70 and Hsp90 possess the ATPase function that ultimately leads to protection or destruction of a client, there are a number of accessory proteins termed cochaperones that can alter both ATPase activities of these proteins as well as client interactions (Pearl and Prodromou, 2006; Wandinger et al., 2008; Hessling et al., 2009; Meimaridou et al., 2009).
While a number of studies have suggested that modulating Hsp90 directly may be clinically relevant for protein misfolding disorders (Sittler et al., 2001; Auluck et al., 2002; Waza et al., 2005; Dickey et al., 2007), identifying cochaperones that specifically affect protein subclasses (i.e., transmembrane receptors or unstructured proteins) could provide more specific drug targets with fewer adverse consequences. For example, the Hsp90 cochaperone, Aha1, was shown to rescue mutant cystic fibrosis transmembrane receptor (CFTR) from the degradation pathway, preserving its levels in a partially active state (Wang et al., 2006), a desired outcome in cystic fibrosis. We previously found that knocking down the Hsp90 cochaperone p23, which is known to regulate Hsp90 ATPase and chaperoning function (Richter et al., 2004), caused significant decreases in tau levels (Dickey et al., 2007). This led us to speculate that other Hsp90 cochaperones might also lead to preservation of abnormal proteins, which in some instances would be the desired outcome, as with CFTR, or in other instances, may be an unwanted mechanism leading to accumulation of unstructured proteins, as with hyperphosphorylated tau (phospho-tau). Kraemer et al. (2006) showed that knockdown of several cochaperones with siRNA could prevent or accelerate tau pathology in a Caenorhabditis elegans model of tauopathy. From a genome-wide screen, they identified the ortholog of CHIP, an ubiquitin ligase previously linked to tau accumulation, Hsp70, a major ATPase of the chaperone network, and FKBP52, a PPIase that can interact directly with Hsp90 and Hsp70 through a tetratricopeptide repeat (TPR) domain (Radanyi et al., 1994; Höhfeld et al., 1995; Blatch and Lässle, 1999; Pirkl and Buchner, 2001; Pirkl et al., 2001). Interestingly, mammals also carry the FKBP51 gene, which is 70% similar to FKBP52, but is not present in C. elegans (Richardson et al., 2007). Therefore, we endeavored to elucidate how these two proteins might impact tau stability in mammalian systems with the goal of possibly identifying a novel target for therapeutic development.
Materials and Methods
Tissue samples.
Nontransgenic mouse brain tissues were prepared as previously described (Dickey et al., 2009). Alzheimer's disease and normal (control) human brain tissue samples (medial temporal gyrus) were provided by Dr. Tom Beach (Sun Health, Phoenix, AZ). Postmortem interval was between 2.5 and 3 h, and samples were gender and age matched.
Antibodies, siRNAs, and chemicals.
12E8 (anti-S262/S356 p-tau) was provided by P. Seubert (Elan Pharmaceuticals, San Francisco, CA). PHF1 (anti-S396/S404 p-tau) was provided by P. Davies (Albert Einstein College of Medicine, Yeshiva University, New York, NY). Anti-FKBP51 and anti-FKBP52 were provided by Drs. David F. Smith and Marc Cox (Mayo Clinic, Scottsdale, AZ). JJ3 (anti-p23) was provided by Dr. David O. Toft (Mayo Clinic, Rochester, MN). Anti-V5 and anti-HA were obtained from Invitrogen. Anti-Hsp90α was obtained from Stressgen Biotechnologies. pT231 tau antibody was from Abcam. pS199-202, pS396, and pS212 antibodies were from Anaspec; anti-GAPDH was obtained from BIODESIGN International. Anti-tau (total tau) was obtained from Santa Cruz Biotechnology. Anti-tubulin was obtained from Sigma-Aldrich. Secondary antibodies were obtained from Southern Biotech. All antibodies were used at a 1:1000 dilution with the exception of PHF1, which was used at a dilution of 1:200. All siRNAs were obtained from Qiagen, and their sequences are listed in Table 1. siRNA efficiency for protein knockdown was validated by Western blot (Fig. 1C). Chymotrypsin was obtained from Sigma-Aldrich.
Table 1.
siRNA sequences used in present study
| Gene name | Common name | Target sequence |
|---|---|---|
| PTGES3 | p23 | CAGCTTAGGGAAAGAGAATAA |
| FKBP4 | FKBP52 | AGGGATGAGGTCTGATAAGAA |
| FKBP5 | FKBP51 | TCGGCTGGCAGTCTCCCTAAA |
| Nonsilencing control | — | Proprietary—from Qiagen |
Figure 1.
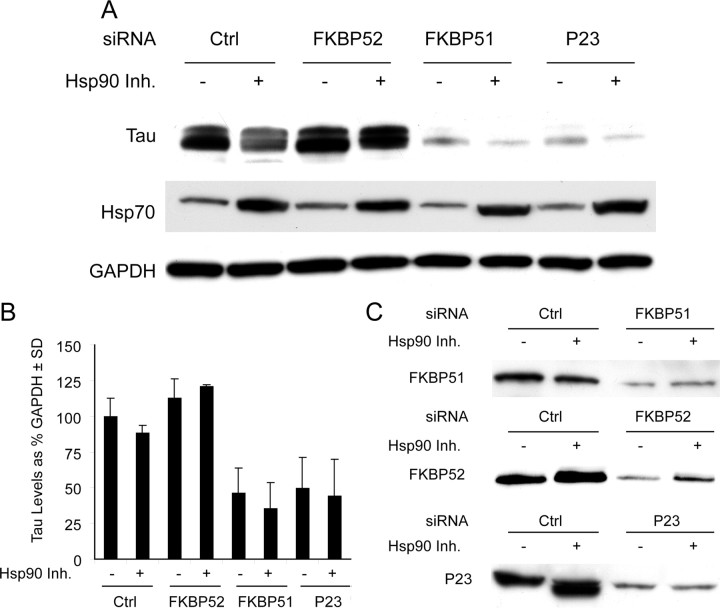
The similar Hsp90 immunophilins, FKBP51 and FKBP52, have divergent effects on tau stability. A, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with indicated siRNAs or scrambled negative control siRNA (Ctrl) for 48 h and treated with 1 μm Hsp90 inhibitor 17AAG for 24 h and analyzed by Western. P23 siRNA was used as a positive tau-reducing control. B, Western blot quantification by densitometry from repeated experiments. Levels of tau were calculated from A after GAPDH normalization ± SD. C, Knockdown for each gene was confirmed by Western blot. Ctrl indicates cells transfected with scrambled negative control.
Plasmids.
Flag-Hsp90 was provided by Dr. Len Neckers (National Institutes of Health, Rockville, MD). Wild-type (WT) 4R tau and HA-ubiquitin were provided by Dr. Michael Hutton (Mayo Clinic, Jacksonville, FL). FKBP51 and FKBP52 constructs were generated by our lab. FKBP51 PPIase mutants were generated by our lab using site-directed mutagenesis (Stratagene).
Cell culture and transfection.
HeLa and HEK293 cells were grown in Opti-Mem plus 10% FBS (Invitrogen) and passaged every 3–5 d based on 90% confluence. IMR32 cells were maintained in Opti-Mem plus 10% FBS and 2% of 200 mm l-glutamine (Cellgro, Mediatech).
siRNA experiments were performed using human gene-specific validated and genome-wide siRNAs (Table 1). Final concentration of siRNAs was 20 nm in Opti-Mem, with 2 μl of siLentFect transfection reagent (Bio-Rad) used per well. This mixture was incubated in a final volume of 500 μl for 20 min and then added to 40–50% confluent HeLa cells stably overexpressing V5-tagged wild-type human tau (HeLa C3) in six-well plates for a final in-well volume of 2.5 ml. Seventy-two hours after transfection, cells were washed with cold PBS and harvested in M-PER buffer (Pierce) containing 1× protease inhibitor cocktail (Calbiochem) and 1 mm phenylmethylsulfonyl fluoride and 1× phosphatase inhibitor I and II cocktails (Sigma). siRNA transfection in IMR32 cells were done as described above.
For plasmid transfections, we used Lipofectamine 2000 reagent from Invitrogen. For most experiments, HeLa cells stably transfected with V5-tagged 4R human tau were transfected with 3 μg of DNA. Cells were incubated for 4 h with the Lipofectamine/plasmid mixture in Opti-MEM media for 4 h, and this was replaced with fresh complete media for an additional 44–48 h. Cells were harvested as described above.
For co-IP of proteins from human brain, tissues from Alzheimer's disease patient and normal (control) human were homogenized in M-PER buffer. The tissue homogenate was centrifuged at 14,000 × g at 4°C. Supernatants were collected. Seven hundred micrograms of collected protein were used for immunoprecipitation with one of the following antibodies: anti-FKBP51 or anti-IgG. The resulting immunoprecipitates were analyzed by Western blotting.
For co-IP from cell cultures, HeLa cells stably overexpressing V5-tagged wild-type human tau, were transfected with 5 μg of each plasmid and Lipofectamine 2000. After 48 h cells were washed with cold PBS and harvested in M-PER buffer. The lysates were precleared for 1 h at 4°C with 25 μl of protein G (Pierce) and 1 μg of anti-rabbit IgG. Lysates were loaded on to spin columns and the flow through was collected. Lysates were incubated with 2 μg of antibody for 2–3 h at 4°C with rocking. Then 50 μl of protein G added and rocked at 4°C for overnight. The protein G beads were pelleted and washed five times with PBS buffer. The precipitates were subjected to Western blot analysis.
Protein purification.
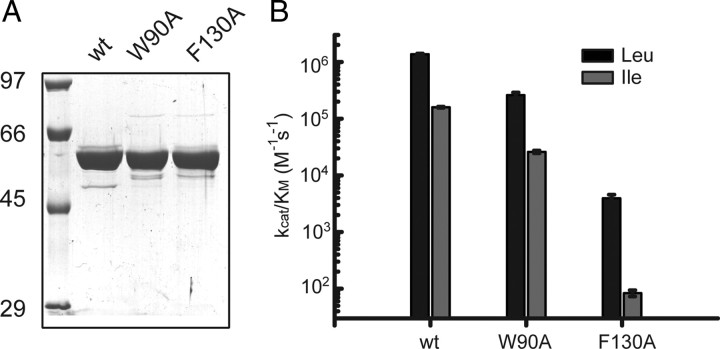
pET28 vectors carrying the genes for wild-type FKBP51, the two mutants FKBP51 W90A and FKBP51 F130A, FKBP52, and tau were transformed into the Escherichia coli strain BL21 (DE3) Codon Plus. LB medium containing kanamycin was inoculated with a respective stationary overnight culture. Cultures were grown at 30°C to an OD600 of 0.5. Protein expression was induced by addition of 1 mm isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. Cells were harvested by centrifugation at 4000 × g for 15 min at 4°C. Pelleted cells were lysed by a cell disruption system (Basic Z model, Constant Systems) at a pressure of 1.8 kbar. All proteins were purified via a succession of Ni-NTA-Sepharose chromatography (equilibrated in 50 mm NaH2PO4, 500 mm NaCl, and 30 mm imidazole, pH 7.5, elution by a step gradient to 300 mm imidazole) and gel filtration chromatography with a Superdex 200 column in 40 mm HEPES, 150 mm KCl, and 5 mm MgCl2, pH 7.5. The purity of all proteins was verified on a Coomassie Brilliant Blue-stained SDS-polyacrylamide gel. All FKBP51s were judged to be >95% pure. The proteins were shock-frozen and stored at −80°C. The concentrations of purified FKBP51, FKBP51 W90A, and FKBP51 F130A were determined using the extinction coefficients of 0.732, 0.634, and 0.733 respectively, for a 1 mg/ml solution in a 1 cm cuvette at 280 nm, calculated according to Gill and von Hippel (1989).
PPIase activity.
PPIase activity toward peptide substrates was measured by protease-coupled assay (Fischer et al., 1984) using the synthetic peptide succinyl-Ala-X-Pro-Phe-p-nitroanilide (Bachem), with X being either Leu or Ile. p-Nitroanilide can only be cleaved off by chymotrypsin when the X-Pro bond is in the trans configuration. The release of p-nitroanilide results in an increase in absorbance at 390 nm. The measurements were performed in a Cary 50 Bio UV/Vis spectrophotometer (Varian) with a thermostated cell holder at a constant temperature of 10°C. To obtain the rate constants the reaction kinetics were fitted to a monoexponential function using the program Origin 8.0 (OriginLab). The rate constants were plotted against their respective enzyme concentration, and a linear regression was performed. The activity of the FKBP51s (kcat/KM) was determined by the slope of the linear regression. All activity values are means of three independent experiments. The experimental error is indicated.
Microtubule polymerization in vitro using Xenopus egg extracts.
Cytostatic factor (CSF)-arrested Xenopus egg extracts were prepared according to a standard protocol as previously described (Desai et al., 1999) and supplemented with 20 μm rhodamine-labeled tubulin for visualization of microtubule structures. Recombinant His6-tagged WT FKBP51 or F130A mutant FKBP51 proteins were added to the extracts in the concentration of 1 μm. Recombinant His6-tagged tau was added at 2.5 μm. For control, equivalent volumes of buffer were added. Nocodazole (20 μm) was used as negative control, and 20 μm taxol was used as a positive control. Extracts were incubated on ice for 1.0 h to ensure an even distribution of the added proteins. Microtubule polymerization was studied according to a published protocol (Budde et al., 2006). In brief, to stimulate microtubule polymerization extracts were supplemented with 5% anhydrous DMSO and incubated for 30 min at room temperature. Obtained microtubule asters were analyzed by fluorescent microscopy. Lengths of polymerized microtubules were measured by using AxioVision software (Zeiss).
Microtubule pelleting from Xenopus egg extracts was performed as described by Budde et al. (2006). In brief, extracts supplemented with recombinant WT or F130A His6-FKBP51 or buffer alone and incubated as mentioned above were diluted 1:20 in buffer containing 80 mm K-PIPES, pH 6.8, 1 mm MgCl2, 1 mm EGTA, and 30% glycerol. Polymerized microtubules in the diluted extracts were precipitated by centrifugation through a 40% glycerol cushion. The levels of pelleted microtubules were analyzed by Western blotting with monoclonal mouse anti-α-tubulin antibodies (Sigma T5168, final concentration 2 μg/ml).
Chymotrypsin assay.
Recombinant tau and FKBP51 (wild-type and F130A) were diluted to a concentration of 10 ng/μl in 20 μl of reaction buffer (20 mm Tris-HCl, 40 mm CaCl2). α-Chymotrypsin (Sigma) was resuspended in 1 m HCl at a concentration of 1 U/μl. This was then diluted to 1 μU/μl in reaction buffer. One microliter of this diluted enzyme or equivalent buffer was added to the protein mixtures indicated (see Fig. 6), and samples were incubated with gentle shaking at 25°C for indicated times. An equivalent volume of SDS loading buffer was added to the tubes, and samples were boiled for 5 min. Samples were analyzed by standard Western blot.
Figure 6.
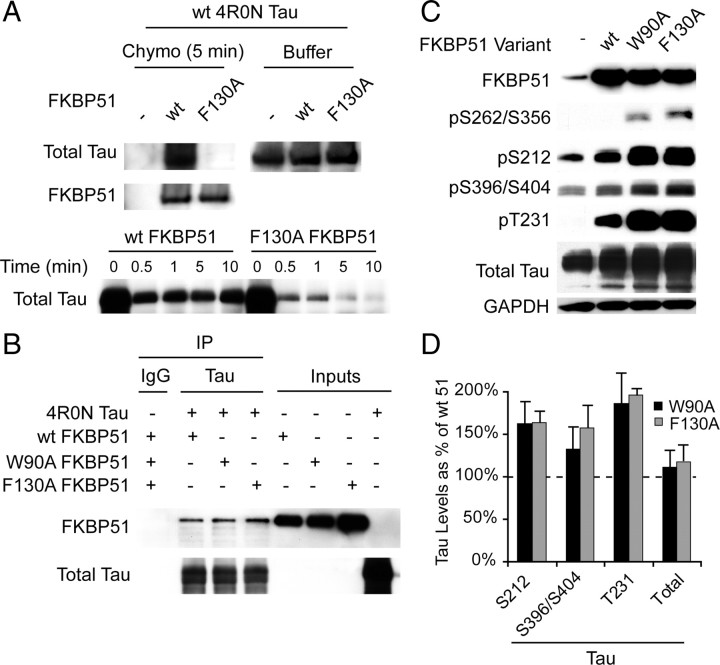
FKBP51 PPIase activity is a critical mediator of tau dephosphorylation. A, Recombinant wild type 4R0N tau was incubated with WT or PPIase-dead (F130A) FKBP51 for 5 min in the presence of α-chymotrypsin or buffer for indicated times. Western blot analysis showed that WT FKBP51 abrogated chymotrypsin-mediated tau degradation, while F130A FKBP51 did not. B, Indicated purified recombinant proteins were incubated for 30 min with agitation at 37C and co-IPed with rabbit polyclonal tau (Santa Cruz Biotechnology) antibody or nonspecific rabbit IgG for 1 h at room temperature. Protein alone is indicated as inputs. Total tau was probed for using mouse monoclonal Tau12. C, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with vector (−), WT, W90A, or F130A FKBP51 for 48 h. D, Quantitation of Western blot from C shows increases in indicated phospho-tau species following transfection with mutant FKBP51 relative to wild-type FKBP51. Error bars (SD) show variability from replicate experiments.
Immunohistochemistry.
Horizontal brain sections from 9-month-old C57BL6 were mounted onto slides and blocked for 60 min at 25°C with 5% normal goat serum in TBST. Mouse anti-FKBP51 and rabbit anti-tau (1:100) were incubated overnight at 4°C. Slides were washed 5× for 5 min in 1× TBS. Secondary antibodies [AlexaFluor 488 (anti-mouse; 1:1000) and 594 (anti-rabbit; 1:4000)] were diluted in blocking buffer and incubated with slides for 60 min at 25°C. Slides were washed and stained with Hoechst at 1:20,000 dilution for 2 min at 25°C, then washed and coverslipped. Imaging and colocalization scatter analysis were performed with the Zeiss Imager AxioVision.
Results
Distinct activities of FKBP52 and FKBP51 knockdown on tau stability
We previously showed that siRNA targeting the Hsp90 cochaperone p23 caused reductions in tau levels (Dickey et al., 2007). With this in mind, we wanted to determine whether other Hsp90 cochaperones could elicit a similar response. Based on the work of Kraemer et al. (2006), which revealed a possible role for FKBP51 and FKBP52 in tau biology, we investigated how depletion of these two proteins affect the stability of tau. HeLa cells stably transfected with tau were individually transfected with siRNAs targeting FKBP52, FKBP51, P23, or a nonsilencing control (Ctrl). Forty-eight hours after transfection, cells were treated with the Hsp90 inhibitor, 17-AAG [17-(allylamino)-17-demethoxygeldanamycin], for an additional 24 h. Cells were harvested and tau levels were analyzed by Western blot (Fig. 1A). Knockdown of p23 and FKBP51 caused dramatic reductions in total tau levels compared to control or FKBP52 siRNAs, regardless of treatment with 17-AAG (Fig. 1A,B). In fact, FKBP52 marginally increased tau levels. Hsp90 inhibitor activity was confirmed by increases in Hsp70 levels (Zou et al., 1998) and GAPDH was used to control for protein loading. Knockdown of the P23, FKBP51, and FKBP52 proteins were confirmed on separate gels (Fig. 1C). These data are representative of experiments that have been performed at least in triplicate.
Effects of FKBP51 on the regulation of tau levels in cultured cells
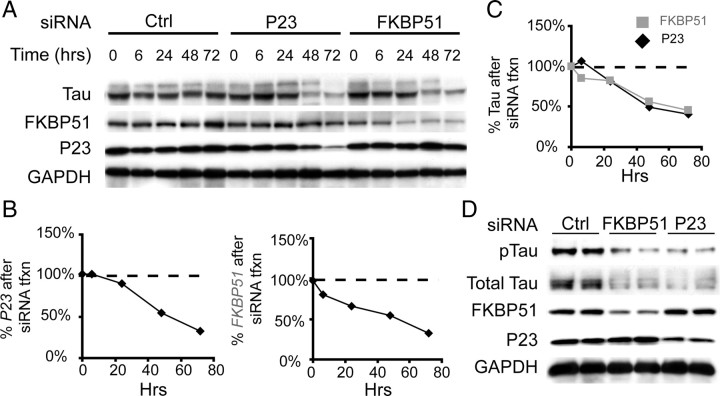
As our original goal was to identify additional cochaperone siRNAs that could facilitate tau reductions in an effort to identify novel tau drug targets, we focused our studies on FKBP51 and p23. We performed a time course study in stably transfected HeLa cells overexpressing tau, and found that decreases in tau levels mirrored FKBP51 reductions, similar to what was seen with p23 siRNA (Fig. 2A–C). To investigate the effects of p23 and FKBP51 siRNA on endogenous tau levels, we used the IMR32 cholinergic neuroblastoma cell model. siRNA for both FKBP51 and p23 facilitated reductions in endogenous tau levels in this neuronal derived cell line (Fig. 2D). Since deletion of p23 in mice results in embryonic lethality (Grad et al., 2006), whereas FKBP51 knock-out mice are viable and healthy at a young age (Cox and Smith, 2006), we focused our efforts on FKBP51.
Figure 2.
FKBP51 siRNA potently reduces tau levels in relevant cell models. A, HeLa cells stably overexpressing wild-type human tau were transfected with p23, FKBP51, or scrambled negative control siRNA (Ctrl), and harvested at 0, 6, 24, 48, and 72 h after transfection. Total tau (V5) levels appeared similarly reduced concomitant with p23 or FKBP51 decreases compared to Ctrl. B, Quantitation of p23 and FKBP51 levels after respective siRNA transfection are shown as a percentage of Ctrl siRNA transfected at each time point. C, Quantitation of total tau levels with FKBP51 siRNA (gray squares) and p23 siRNA (black diamonds) are shown to be similar as a percentage of Ctrl at corresponding each time point in hours (Hrs). D, IMR32 cells were transfected with FKBP51, p23, or Ctrl siRNA for 72 h, and lysates were analyzed by Western blot for indicated antibodies.
FKBP51–tau interactions in the brain
We wanted to investigate the profile of FKBP51 in the brain and determine whether tau and FKBP51 might interact in vivo. Using lysates from brain tissue collected at different ages from mice, we found that FKBP51 levels were not detected until 5.5 month of age and were increased further at 9 months (Fig. 3A). This suggested that FKBP51 levels increase with age in the brain and may only begin to affect tau at this point. Using immunofluorescent histochemistry for endogenous FKBP51 and tau proteins, we demonstrated a high level of colocalization between these proteins in brain tissue from the 9-month-old mice (Fig. 3B). Moreover, colocalization was prevalently seen in axonal tracts (see arrows) where there is an abundance of microtubules (Fig. 3B; higher magnification shown in Fig. 3C). While these data were suggestive of a tau/FKBP51 interaction, we wanted to determine whether these proteins were complexed in human brain tissue. To determine this, we coimmunoprecipitated FKBP51 from both Alzheimer's disease (AD) and control medial temporal gyrus tissue (n = 2 AD and 2 normal). We found that FKBP51 indeed could interact with tau from both AD patients and control cases (Fig. 3D), further suggesting a functionally relevant relationship between FKBP51 and tau. We then investigated whether FKBP51 would preferentially interact with phosphorylated tau species. We increased the number of samples per group (4 for AD and 4 for normal) and again coimmunoprecipitated FKBP51. After gel electrophoresis, immunoblotting showed increased association of pS396 and pS199-S202 tau species with FKBP51 in AD tissue (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). These findings compelled us to further investigate the mechanism by which FKBP51 was regulating tau biology.
Figure 3.
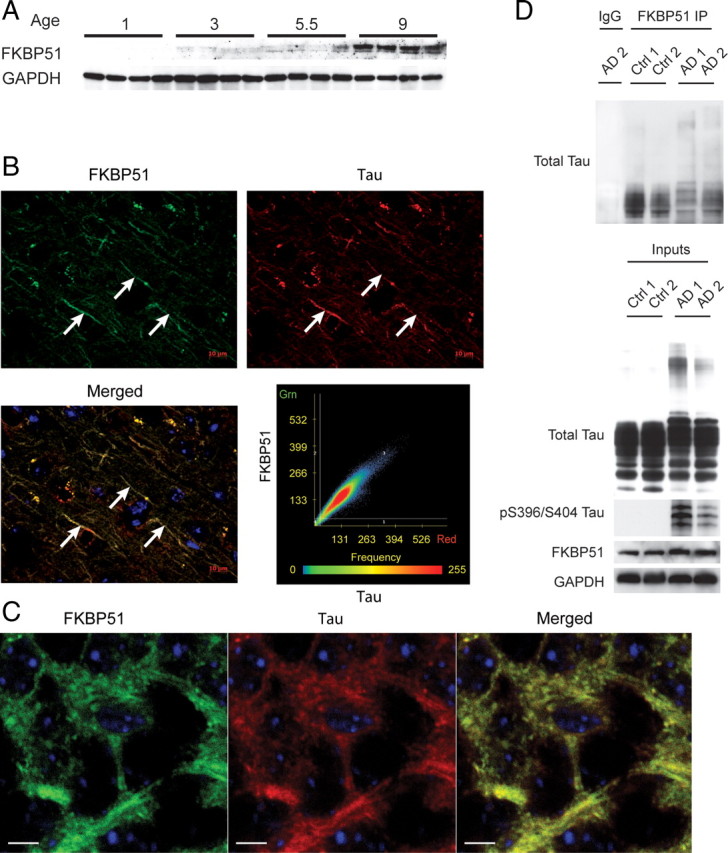
FKBP51 increases with age and associates with tau from normal and AD brain. A, Brain lysates from mice harvested at indicated age (in months) were analyzed for FKBP51 levels by Western blot. Detectable levels of FKBP51 were first seen in 5.5-month-old mice and were markedly apparent at 9 months. B, Immunofluorescent staining of FKBP51 (green) and total tau (red) in brain tissue from 9-month-old mice shows very strong colocalization of these two proteins within neuronal axon processes (arrows). C, Higher magnification (100×) of neuronal cells from hippocampus CA1 region of mouse brain, immunostained with FKBP51 antibody (green), tau antibody (red), and nuclei (blue). Merged image shows colocalization of these two proteins. Scale bars, 5 μm. D, Brain tissue lysates from Alzheimer's disease (AD 1 and 2) and age-matched normal (Ctrl 1 and 2) brain tissue lysates were immunoprecipitated with mouse anti-FKBP51 or nonspecific mouse IgG antibodies. Brain lysates (Inputs) and immunoprecipitates were analyzed by Western blot for total tau (Santa Cruz Biotechnology), pS396/S404 tau, FKBP51, and GAPDH antibodies.
Interaction of FKBP51 with tau
While we had demonstrated that FKBP51 siRNA could reduce tau levels, we wanted to determine whether the reciprocal effect was achieved via FKBP51 overexpression. Indeed, we found that FKBP51 overexpression increased phospho-tau and total tau levels (by 80%) in HeLa cells stably expressing normal human tau, while FKBP52 overexpression had no affect (Fig. 4A). These experiments were repeated multiple times, and Student's t test of these replicates demonstrated that FKBP51 significantly increased total tau levels (p = 0.0104).
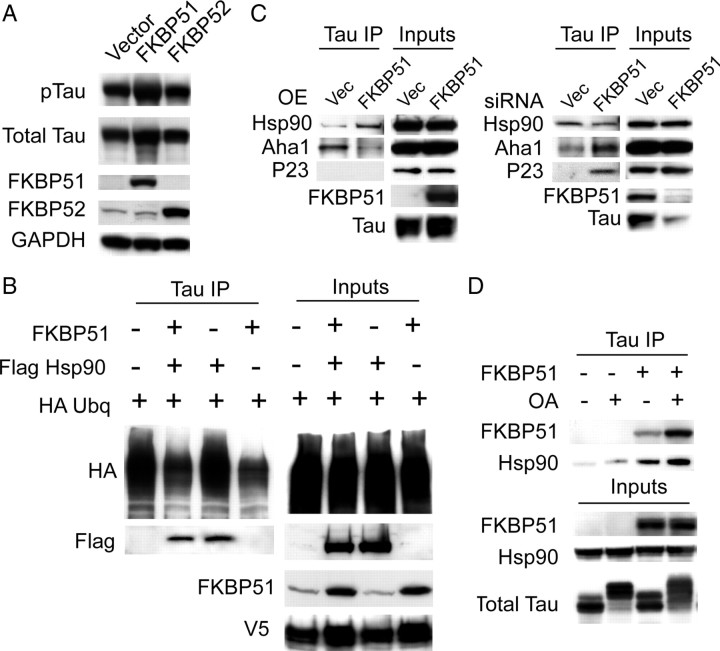
Figure 4.
FKBP51 stabilizes tau. A, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with FKBP51, FKBP52, or a vector control DNA harvested after 48 h. Western blot shows that FKBP51-transfected cells increase total tau (V5) and phospho-tau (pS212) compared to vector-containing cells. B, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with FKBP51, Flag Hsp90, and/or HA-ubiquitin. Co-IP with V5 antibody revealed that FKBP51 overexpression reduces ubiquitination of tau independent of Hsp90. C, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with FKBP51 plasmid or FKBP51 siRNA and tau was coimmunoprecipitated from these lysates after 48 h (for overexpression) or 72 h (for siRNA). Binding of endogenous Aha1 and P23, two well characterized Hsp90 cochaperones, was assessed. The association of Aha1 with tau was reduced or increased when FKBP51 was overexpressed or knocked down, respectively. P23 only associated with tau when FKBP51 was knocked down. Hsp90 binding to tau was increased by FKBP51 overexpression, and reduced slightly by FKBP51 knockdown. D, HeLa cells stably overexpressing V5-tagged wild-type human tau were transiently transfected with FKBP51 or empty vector for 48 h and treated with 100 nm okadaic acid (OA; +) or vehicle (−) for 30 min. Co-IP of these lysates with V5 antibody shows increased FKBP51 and Hsp90 binding to tau with OA treatment. Total tau levels were measured with anti-V5.
We speculated that FKBP51 might be preserving tau by impairing its ubiquitination. Stable tau transfectants overexpressing FKBP51 showed reductions in tau ubiquitination following tau immunoprecipitation, while Hsp90 overexpression had no impact (Fig. 4B). We then investigated what effect overexpression or knockdown of FKBP51 might have on the interaction of tau with other Hsp90 cochaperones that comprise a mature Hsp90 complex. Surprisingly, we found that FKBP51 overexpression decreased the endogenous association of another Hsp90 profolding cochaperone, Aha1, with tau despite increasing Hsp90 binding (Fig. 4C). Endogenous p23 binding to tau however was not detected. Conversely, knockdown of FKBP51 with siRNA increased the association of endogenous Aha1 with tau despite decreasing the number of Hsp90 tau complexes. Moreover, endogenous p23 binding to tau was only detectable when FKBP51 was knocked down (Fig. 4C). We then investigated what impact tau phosphorylation would have on its interaction with FKBP51. Interestingly, overexpression of FKBP51 in stable tau transfectants enhanced the association of tau with Hsp90, suggesting a complex assembly that could be facilitated by upregulation of FKBP51 (Fig. 4D). Moreover, treatment with the phosphatase inhibitor okadaic acid for 30 min enhanced the association of FKBP51. Hsp90 binding to tau was also enhanced slightly due to hyperphosphorylation. FKBP51 overexpression also altered the distribution of tau in the presence of okadaic acid, indicating further that FKBP51 might be directly regulating tau phosphorylation. As we began to investigate this, it became increasingly likely that the cis-trans peptidyl prolyl-isomerase (PPIase) activity of FKBP51 might be playing a major role in tau biology at the chaperone interface.
PPIase activity of WT FKBP51 and mutants
PPIase activity had previously been established as an enzymatic function that could regulate tau phosphorylation. This has been elegantly presented for Pin1 in a series of studies from the Lu laboratory (Liou et al., 2003; Pastorino et al., 2006; Lim et al., 2008). However, Pin1 and FKBP51 differ in that FKBP51 possesses a TPR domain and thus can interact directly with Hsp90, whereas Pin1 does not. Moreover, a function for this PPIase activity of FKBP51 has yet to be established. To investigate whether FKBP51 PPIase activity specifically could affect tau biology, we generated PPIase-deficient mutants based on similar mutations previously described for FKBP52 (Riggs et al., 2007) and assayed them for PPIase activity. Using site-directed mutagenesis we replaced W90 or F130 with alanine. The proteins were expressed in E. coli and purified to >95% homogeneity for both mutants and wild-type FKBP51 (Fig. 5A). PPIase activity was measured for each protein using succinyl-Ala-Leu-Pro-Phe-pNA (Leu) and succinyl-Ala-Ile-Pro-Phe-pNA (Ile) as substrates. The F130A mutant was essentially determined to be enzymatically dead, with >99% reduction in isomerase activity, whereas the W90A mutant maintained some activity (∼25% activity for one substrate and ∼40% for the other) (Fig. 5B). With the enzymatically dead enzyme variant, we were then able to determine the direct impact of FKBP51's PPIase activity on tau phosphorylation.
Figure 5.
Characterization of PPIase-deficient FKBP51 mutant proteins. A, Coomassie Blue staining of recombinant WT, W90A, and F130A FKBP51 purified by Ni-NTA affinity and gel filtration chromatography. B, Column diagram depicting the catalytic efficiency of WT, W90A, and F130A FKBP51 as determined with a protease-coupled enzyme assay. Absolute values are shown.
Effects of FKBP51 PPIase mutants on tau
We tested whether tau could be isomerized by FKBP51. We incubated purified tau with α-chymotrypsin, which can cleave 90% of peptides in the trans-conformation, but <10% when the same peptide is in the cis-conformation. This enabled us to directly test whether FKBP51 was able to isomerize tau. Indeed we found that wild-type FKBP51 abrogated tau degradation by chymotrypsin, while the dead F130A mutant did not (Fig. 6A). We then wanted to investigate whether FKBP51 mutants could still bind to tau despite enzyme deficiency. Indeed we found that all three FKBP51 variants were capable of binding to tau in vitro (Fig. 6B). Moreover, this demonstrated that FKBP51 could bind to tau independently, similar to what was previously demonstrated with CHIP (Grelle et al., 2006). We then evaluated the effects of the PPIase-deficient mutants on tau. We transfected WT and both mutant forms of FKBP51 into stable tau transfectants, and then performed Western analysis with various phospho-tau antibodies. This revealed that the mutants further increased phospho tau compared to WT FKBP51. In particular, tau phosphorylated at S396/S404, T231, and S212 was dramatically elevated relative to wild-type (Fig. 6C,D). Most intriguing was our finding that wild-type FKBP51 produced no detectable levels of pS262/S356 tau, whereas the PPIase-deficient mutants did cause production of pS262/S356 tau (Fig. 6C). These data demonstrated unequivocally that FKBP51's PPIase activity was critically involved in tau processing.
FKBP51 facilitates microtubule formation
Recently Chambraud et al. (2007) showed that FKBP52 and FKBP51 slowed microtubule polymerization using a microtubule assembly assay reconstituted with recombinant proteins in vitro; however, FKBP52 had a much more potent effect. Now in light of our findings suggesting that the role of FKBP51 on microtubule dynamics might be quite different based on its repertoire of interaction partners such as tau (Fig. 4), we turned to a more physiological system to measure microtubule kinetics in the presence of FKBP51, the Xenopus oocyte extract system (Desai et al., 1999; Wang et al., 2008). This system endogenously contains many of the known interaction partners for FKBP51 (data not shown), allowing us to determine whether FKBP51 might have distinct effects on microtubule assembly when associated with these partners. We supplemented the oocyte extracts with rhodamine-labeled tubulin and either wild-type FKBP51, mutant FKBP51, or FKBP52 recombinant proteins. As controls, we also supplemented the extracts with nocodazole or taxol. Fluorescent imaging of these extracts revealed that wild-type FKBP51 promoted microtubule polymerization relative to extracts treated with buffer only (Fig. 7A; supplemental Fig. S2, available at www.jneurosci.org as supplemental material). Conversely, neither mutant F130A FKBP51 nor FKBP52 stimulated microtubule formation. Quantification of the radii of these microtubule clusters (n is shown in Fig. 7B) showed that microtubule length in the presence of wild-type FKBP51 was greater than microtubule length in extracts treated with buffer only, F130A FKBP51, or FKBP52 (Fig. 7B). Nocodazole treatment disrupted endogenous microtubule formation, while taxol treatment produced densely packed microtubule bundles that were immeasurable due to their structure. A replicate experiment was then performed to biochemically assess tubulin migration through a 40% glycerol cushion in the presence of either wild-type or mutant FKBP51. Western blot for tubulin following cushioning revealed a significant increase in the amount of tubulin polymerization in the presence of WT FKBP51 relative to buffer, but not in the presence of the F130A mutant (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). We then evaluated the effects of FKBP51 on microtubule structure when these extracts were also supplemented with recombinant tau. In the presence of tau alone, long networks of microtubules were apparent (Fig. 7C). Interestingly, however, when FKBP51 was also added, these networks became even more complex and arboreal, suggesting that FKBP51 can use tau to modulate microtubule dynamics. Quantification of these microtubule asters was not possible given the dramatic effects of tau and the tau/FKBP51 combination on microtubule network formation. Therefore, FKBP51, which can regulate tau phosphorylation via the chaperone complex, is also able to stabilize microtubules. This provides a new function for FKBP51 that has not previously been reported.
Figure 7.
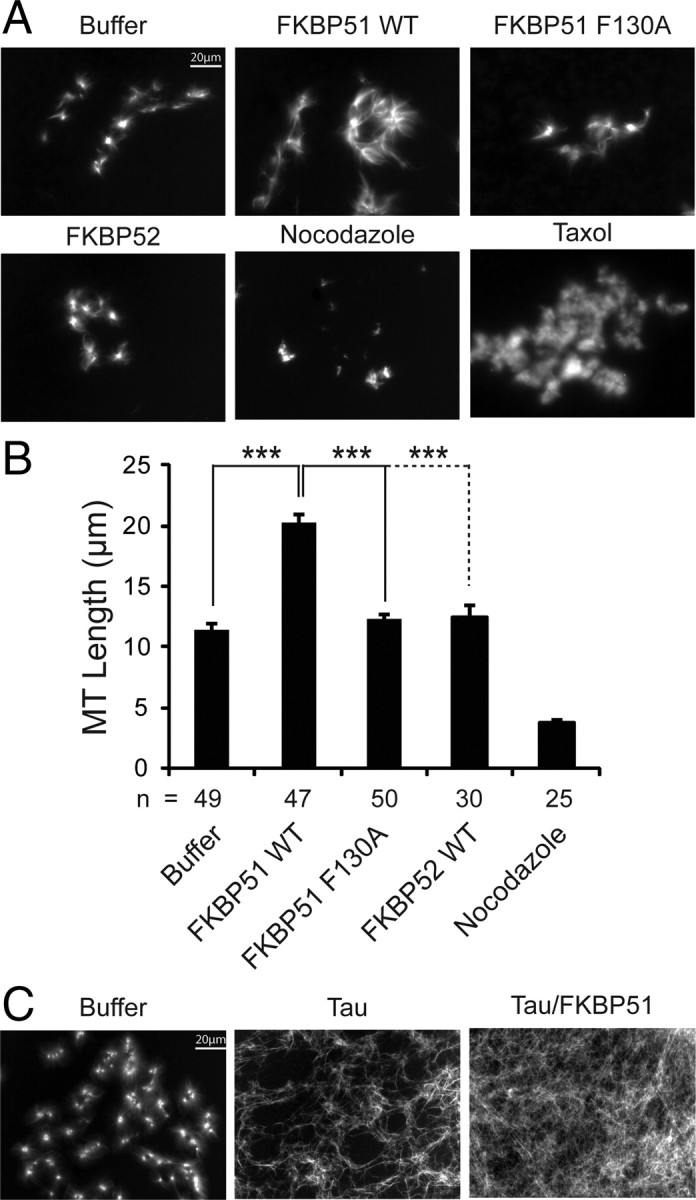
PPIase activity is essential for FKBP51 to facilitate microtubule formation and tau-mediated microtubule polymerization is enhanced by FKBP51. In vitro microtubule assembly assays were performed using Xenopus oocyte extracts supplemented with rhodamine-labeled tubulin. A, Panels show tubulin structures in sample buffer only or supplemented with 1 μm recombinant WT FKBP51, 1 μm F130A FKBP51, 1 μm FKBP52, nocodazole, or taxol. B, Microtubule (MT) lengths were measured for each condition (n = number of asters measured per condition) using AxioVision software from Zeiss, and values are indicated in micrometers ± SD. ***p < 0.001. C, Panels show tubulin structures in sample buffer only or supplemented with 2.5 μm tau alone or 2.5 μm tau plus 1 μm FKBP51. Tau alone caused dramatic morphologic changes to microtubules, which were accentuated in the presence of FKBP51.
Discussion
In these studies, we were interested in following up on a previous finding showing that knockdown of the obligate Hsp90 cochaperone p23, which is essential for Hsp90 ATPase function, reduced tau levels in cells (Dickey et al., 2007). We speculated that if we disrupted the activity of Hsp90 by either inhibiting its ATPase activity with compounds, as previously shown (Dickey et al., 2007), or reducing levels of cochaperones that typically stimulate Hsp90 refolding activity, we could force tau to be degraded.
We surveyed the literature for the most well defined profolding Hsp90 cochaperones that might be linked to tau, and selected FKBP52, FKBP51, and P23 as our primary candidates (Stepanova et al., 1996; Blatch and Lässle, 1999; Mayer et al., 2002). All three of these proteins contain TPR domains that allow them to associate with Hsp90 and Hsp70. In addition, both FKBP51 and FKBP52 also possess PPIase activity, which facilitates cis-trans isomerization around a prolyl-peptide bond (Pirkl and Buchner, 2001). Interestingly, despite the similarities between these two immunophilins, we found that knockdown of FKBP51 dramatically reduced tau levels while knockdown of FKBP52 did not. This notion of distinct cochaperones affecting Hsp90 function differentially was further confirmed by our results in Figure 4C showing that removing FKBP51 from the cell actually enhances the association of other profolding cochaperones, Aha1 and P23, with tau. Therefore, perhaps immunophilins, such as FKBP52 and FKBP51, contextually regulate the dynamics of the Hsp90 complex specifically based on the type of client that is bound. It suggests that there are many distinct Hsp90 complexes residing within cells that can be modulated by both the type of client that is bound and the interacting repertoire of cochaperones.
Looking more specifically at the relationship of FKBP51 with tau, we found that FKBP51 was not expressed at detectable levels in the mouse brain until 5.5 months. Moreover, we found endogenous FKBP51 associated with tau from both normal and AD human brain tissues. These data suggest an interesting relationship between tau and FKBP51, a protein that has not been assigned a clear biochemical role in the mammalian brain until now. FKBP51 overexpression preserves tau in cells and protects it from ubiquitination, perhaps by twisting tau in such a way as to prevent access to ubiquitin ligases. We also show that phosphorylation of tau drives the association of FKBP51 with tau, suggesting that as tau falls from the microtubules, it is recognized by the chaperone machinery and primed for dephosphorylation (Fig. 8). Therefore, we hypothesized that this could indicate a mechanism of energetic conservation that neurons have adopted, whereby recycling of tau is much less demanding than producing de novo tau for every plastic event in the brain.
Figure 8.
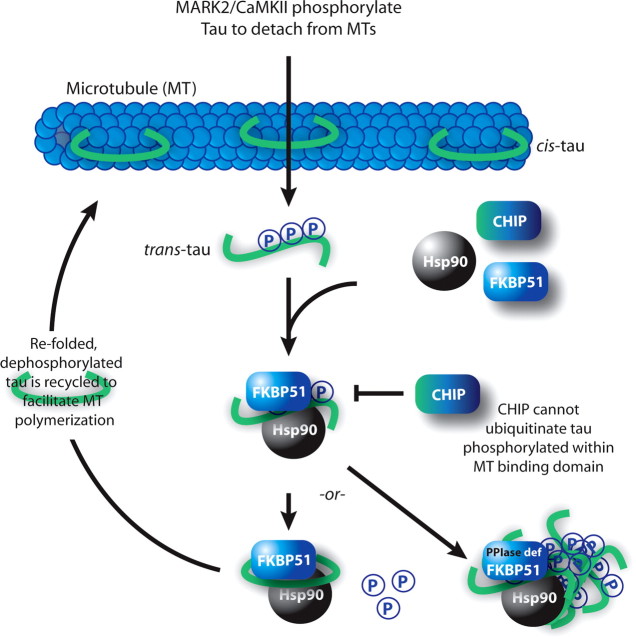
FKBP51 works with the Hsp90 complex to recycle tau for microtubule (MT) stabilization. Tau in the cis-conformation stabilizes MTs. Phosphorylation within the MT binding domain by MT affinity regulating kinase 2 or calcium/calmodulin kinase II detaches tau from the microtubules, which favors trans conversion. Hsp90 recognizes the misfolded tau; however, CHIP is unable to ubiquitinate this particular phospho-tau species. FKBP51 is then sequestered to Hsp90, where it isomerizes tau to the cis-conformation, allowing for dephosphorylation and recycling to the microtubule. When PPIase activity is blocked (PPIase def), FKBP51 perpetuates the association of phospho-tau with Hsp90, promoting tau accumulation.
With this in mind, we investigated the role of the PPIase activity in regulating tau biology. Certainly, it was previously shown that the PPIase, Pin1, has potent activity toward tau, leading to tau dephosphorylation in cooperation with protein phosphatases. It was also shown recently that a disease-causing tau mutant was processed very differently by Pin1 relative to wild-type tau (Lim et al., 2008). Thus, the notion that FKBP51 PPIase activity could be affecting tau processing and accumulation would not be unexpected. Indeed FKBP51 PPIase-deficient mutants led to pronounced increases in several important phospho-tau epitopes. These included pT231, pS262/S356, and pS212. The pT231 site is an essential priming site for GSK3β-mediated phosphorylation and is also linked to the isomerase activity of Pin1 (Lin et al., 2007). Phosphorylation at this site alters the ability of Pin1 to process tau correctly (Lim et al., 2008). The pS262/S356 site, which can be mediated by MARK2/PAR1 and CaMKIIα, stimulates the ordered release of tau from microtubules, and is also resistant to CHIP-mediated ubiquitination. Thus, PPIase deficiency of FKBP51 increases the amount of this particular species as well as other phospho-tau species, perhaps making it resistant to degradation (Fig. 8). The pS212 site is phosphorylated by Akt, and we recently demonstrated that Akt and MARK2/PAR1 may coordinate with each other to regulate tau phosphorylation and degradation (Dickey et al., 2008). In context with our results from the microtubule assays in Figure 7, these data suggest that FKBP51 facilitates microtubule stabilization by facilitating the dephosphorylation of tau. Indeed, when considering the recent discovery that FKBP52 actually destabilizes microtubules (Chambraud et al., 2007), this complimentary data may provide a dichotic relationship for these two similar proteins at the microtubule scaffold.
In summary, we propose that the Hsp90 complex may serve as a harbor for multiple proteins to interface. While a number of other Hsp90 cochaperones could use this interface to regulate tau in novel ways, we have focused our efforts on the Hsp90 cochaperone FKBP51. We show that the PPIase activity of FKBP51 critically balances tau phosphorylation state, leading to microtubule stabilization. Thus, FKBP51 spares tau from degradation by regulating its phosphorylation within the Hsp90 complex, and perhaps leads to its recycling along the axonal tracts to avoid derivation of new tau (Fig. 8). We also suggest that inhibitors targeting FKBP51 PPIase activity would likely have adverse consequences, leading to tau hyperphosphorylation. Instead, a successful therapeutic approach might be disrupting the FKBP51/Hsp90 interface, attenuating the interaction all together and allowing prodegradation cochaperones like CHIP to facilitate tau removal. However, as an increasing number of Hsp90 cochaperones are being shown to interact directly with tau, it poses an interesting possibility; that increasing levels of tau can facilitate an imbalance in the cell, such that tau may sequester critical cochaperones from their normal functions, leading to deleterious consequences for the cell. By continuing to identify the mechanisms involved in the regulation of this protein and perhaps other disease-related proteins that may inadvertently possess a chaperone-binding signature, we can continue to develop our understanding of the pathogenesis of neurodegenerative diseases.
Footnotes
This work was supported by the Rosalinde and Arthur Gilbert Foundation/American Federation for Aging Research, CurePSP, and National Institute on Aging Grant R00AG031291. Andreas Schmid was supported by the CompInt program of the Elitenetzwerk Bayern. We thank Dr. Tom Beach for brain tissue samples. We also thank Dr. David F. Smith for his support with this project, both in the form of reagents and advice. Dr. Huntington Potter also provided invaluable insights through discussions. We thank Dr. Peter Davies for PHF1 (pS396/S404) tau antibody. We would also like to thank Dr. Peter Seubert for 12E8 antibody.
References
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Blatch GL, Lässle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Budde PP, Desai A, Heald R. Analysis of microtubule polymerization in vitro and during the cell cycle in Xenopus egg extracts. Methods. 2006;38:29–34. doi: 10.1016/j.ymeth.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Chambraud B, Belabes H, Fontaine-Lenoir V, Fellous A, Baulieu EE. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. FASEB J. 2007;21:2787–2797. doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- Cox MB, Smith DF. Functions of the Hsp90-binding FKBP immunophilin. In: Blatch GL, editor. Networking of chaperones by co-chaperones. Austin, TX: Landes Bioscience/Eurekah.com; 2006. pp. 13–23. [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Jr, Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Koren J, Zhang YJ, Xu YF, Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C, Bailey RM, Dunmore J, Soresh S, Leon C, Morgan D, Petrucelli L. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci U S A. 2008;105:3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, Koren J, Johnson A, Anderson L, Lebson L, Lee D, Dickson D, de Silva R, Binder LI, Morgan D, Lewis J. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am J Pathol. 2009;174:228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Bang H, Mech C. [Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides] Biomed Biochim Acta. 1984;43:1101–1111. [PubMed] [Google Scholar]
- Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, Floss T, Miller CA, 3rd, Picard D. The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol. 2006;26:8976–8983. doi: 10.1128/MCB.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelle G, Kostka S, Otto A, Kersten B, Genser KF, Müller EC, Wälter S, Böddrich A, Stelzl U, Hänig C, Volkmer-Engert R, Landgraf C, Alberti S, Höhfeld J, Strödicke M, Wanker EE. Identification of VCP/p97, carboxyl terminus of Hsp70-interacting protein (CHIP), and amphiphysin II interaction partners using membrane-based human proteome arrays. Mol Cell Proteomics. 2006;5:234–244. doi: 10.1074/mcp.M500198-MCP200. [DOI] [PubMed] [Google Scholar]
- Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16:287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- Höhfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Kraemer BC, Burgess JK, Chen JH, Thomas JH, Schellenberg GD. Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Hum Mol Genet. 2006;15:1483–1496. doi: 10.1093/hmg/ddl067. [DOI] [PubMed] [Google Scholar]
- Lim J, Balastik M, Lee TH, Nakamura K, Liou YC, Sun A, Finn G, Pastorino L, Lee VM, Lu KP. Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J Clin Invest. 2008;118:1877–1889. doi: 10.1172/JCI34308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Cheng JT, Liang LC, Ko CY, Lo YK, Lu PJ. The binding and phosphorylation of Thr231 is critical for Tau's hyperphosphorylation and functional regulation by glycogen synthase kinase 3beta. J Neurochem. 2007;103:802–813. doi: 10.1111/j.1471-4159.2007.04792.x. [DOI] [PubMed] [Google Scholar]
- Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003;424:556–561. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Nikolay R, Bukau B. Aha, another regulator for hsp90 chaperones. Mol Cell. 2002;10:1255–1256. doi: 10.1016/s1097-2765(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol. 2009;42:1–9. doi: 10.1677/JME-08-0116. [DOI] [PubMed] [Google Scholar]
- Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. 2001;308:795–806. doi: 10.1006/jmbi.2001.4595. [DOI] [PubMed] [Google Scholar]
- Pirkl F, Fischer E, Modrow S, Buchner J. Localization of the chaperone domain of FKBP52. J Biol Chem. 2001;276:37034–37041. doi: 10.1074/jbc.M102595200. [DOI] [PubMed] [Google Scholar]
- Radanyi C, Chambraud B, Baulieu EE. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JM, Dornan J, Opamawutthikul M, Bruce S, Page AP, Walkinshaw MD. Cloning, expression and characterisation of FKB-6, the sole large TPR-containing immunophilin from C. elegans. Biochem Biophys Res Commun. 2007;360:566–572. doi: 10.1016/j.bbrc.2007.06.080. [DOI] [PubMed] [Google Scholar]
- Richter K, Walter S, Buchner J. The Co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J Mol Biol. 2004;342:1403–1413. doi: 10.1016/j.jmb.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittler A, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, Hartl FU, Wanker EE. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum Mol Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- Wang LH, Yan M, Xu DZ, Cao JX, Zhu XF, Zeng YX, Liu Q. Requirement of aurora-A kinase in astral microtubule polymerization and spindle microtubule flux. Cell Cycle. 2008;7:1104–1111. doi: 10.4161/cc.7.8.5738. [DOI] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]