Abstract
Treatment of unadenylylated glutamine synthetase from Escherichia coli with tetranitromethane or with N-acetylimidazole produces alterations in catalytic parameters that are similar to alterations caused by the physiologically important process of adenylylation. All three modification reactions lead to a change in divalent ion requirement for biosynthetic activity; the unmodified enzyme requires Mg2+ for activity, whereas the modified enzymes exhibit increased activity with Mn2+. The γ-glutamyl transferase activity of the modified enzyme is more sensitive to feedback inhibition by tryptophan, histidine, CTP, and AMP, and to inhibition by Mg2+ or to inactivation by 5 M urea. Finally, the pH optimum for the unmodified enzyme is 7.9, while the modified enzymes are more active at pH 6.8.
Since treatment of the enzyme with N-acetylimidazole results in a decrease in absorbancy at 278 mμ and treatment with tetranitromethane causes an increase in absorbancy at 428 mμ, the effects of these reagents are probably due to modification of certain tyrosyl groups on the enzyme. However, other evidence indicates that the tyrosyl residues which are susceptible to adenylylation in the adenylyltransferase-catalyzed reaction are not involved in the acetylation or nitration reactions.
Full text
PDF

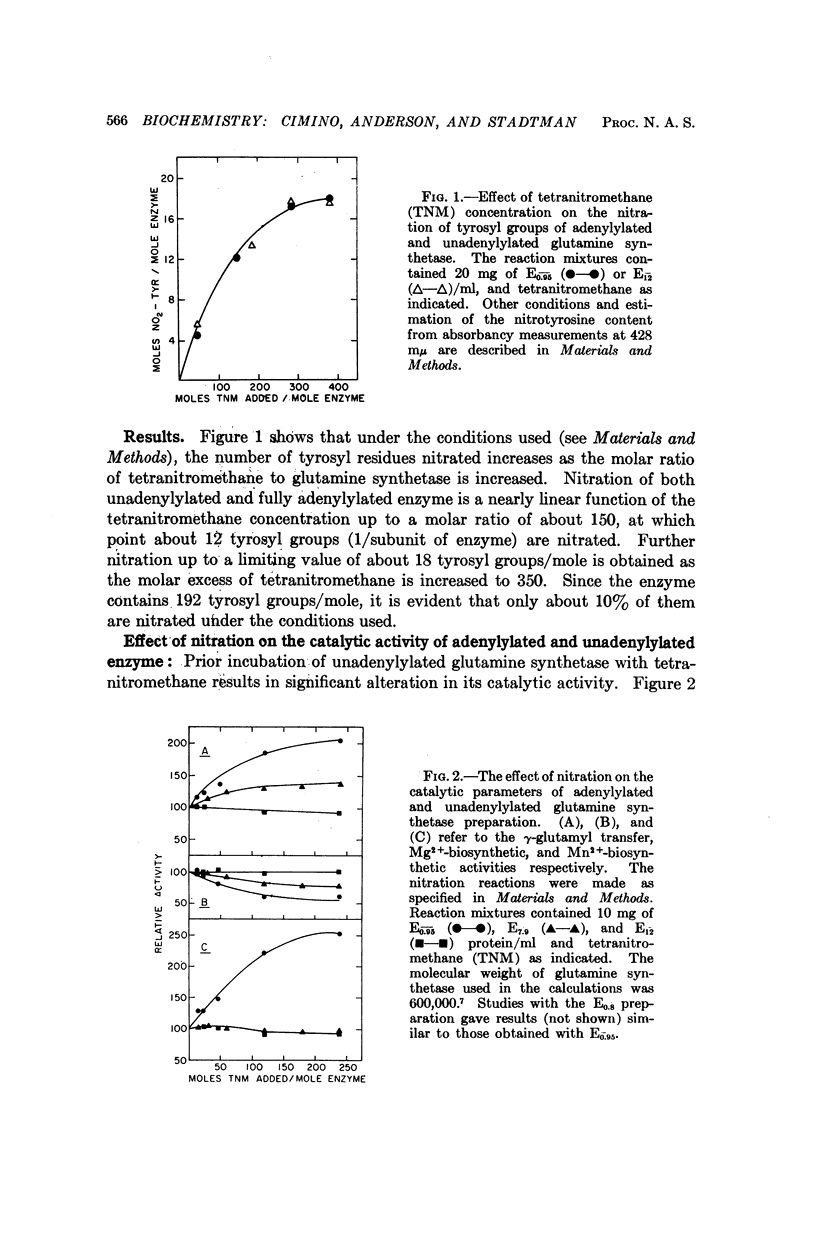

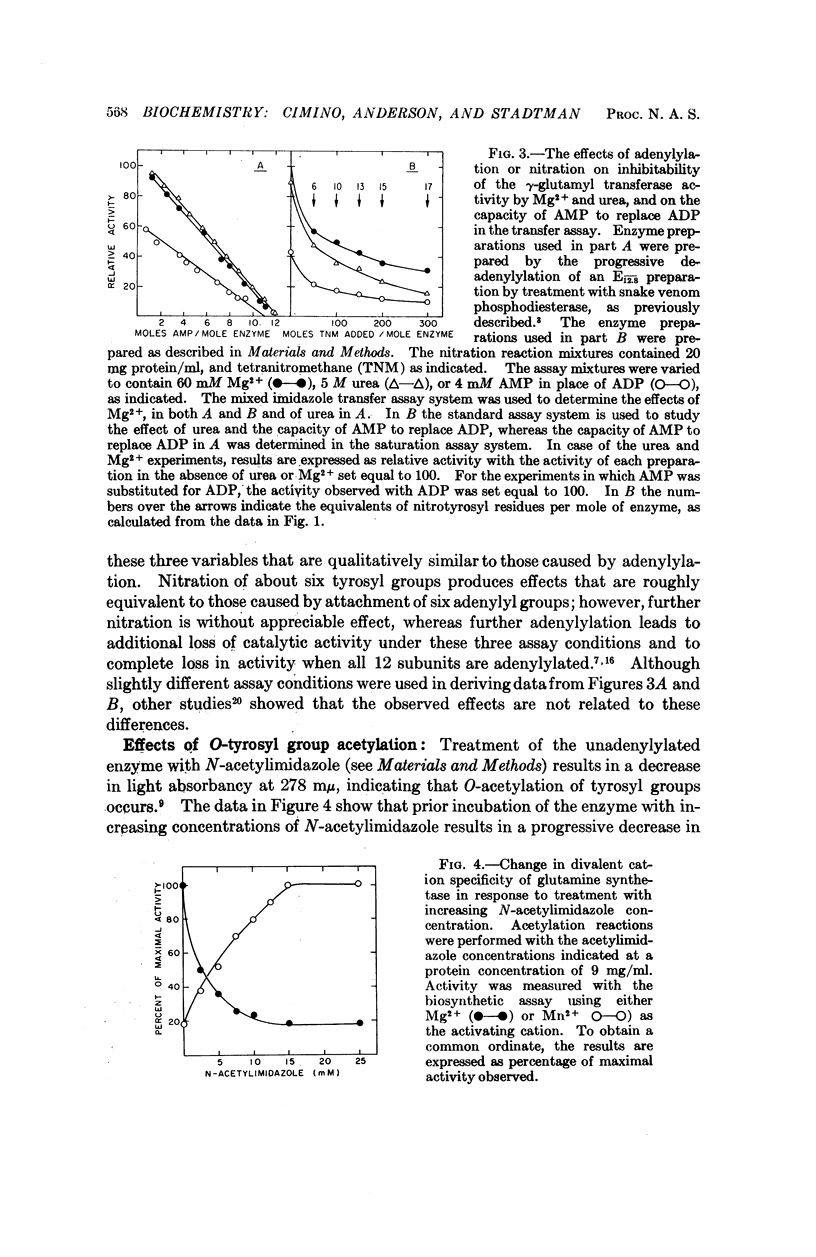



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. X. Effect of growth conditions on the susceptibility of Escherichia coli glutamine synthetase to feedback inhibition. J Bacteriol. 1967 Oct;94(4):949–957. doi: 10.1128/jb.94.4.949-957.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry. 1967 Jan;6(1):358–361. doi: 10.1021/bi00853a053. [DOI] [PubMed] [Google Scholar]
- SIMPSON R. T., RIORDAN J. F., VALLEE B. L. FUNCTIONAL TYROSYL RESIDUES IN THE ACTIVE CENTER OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. Biochemistry. 1963 May-Jun;2:616–622. doi: 10.1021/bi00903a039. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. Glutamine synthetase deadenylylating enzyme. Biochem Biophys Res Commun. 1968 Jan 11;30(1):32–37. doi: 10.1016/0006-291x(68)90708-0. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. IX. Reactivity of the sulfhydryl groups of the enzyme from Escherichia coli. J Biol Chem. 1967 Nov 10;242(21):5069–5079. [PubMed] [Google Scholar]
- Shapiro B. M. The glutamine synthetase deadenylylating enzyme system from Escherichia coli. Resolution into two components, specific nucleotide stimulation, and cofactor requirements. Biochemistry. 1969 Feb;8(2):659–670. doi: 10.1021/bi00830a030. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Harell D., Riordan J. F. Reaction of tetranitromethane with sulfhydryl groups in proteins. Biochemistry. 1969 Dec;8(12):4740–4745. doi: 10.1021/bi00840a013. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Shapiro B. M., Ginsburg A., Kingdon H. S., Denton M. D. Regulation of glutamine synthetase activity in Escherichia coli. Brookhaven Symp Biol. 1968 Jun;21(2):378–396. [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the properties of active sites of enzymes. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Stadtman E. R. Regulation of glutamine synthetase. 3. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1967 Mar 20;118(3):736–755. doi: 10.1016/0003-9861(67)90412-2. [DOI] [PubMed] [Google Scholar]
- Wulff K., Mecke D., Holzer H. Mechanism of the enzymatic inactivation of glutamine synthetase from E. coli. Biochem Biophys Res Commun. 1967 Sep 7;28(5):740–745. doi: 10.1016/0006-291x(67)90378-6. [DOI] [PubMed] [Google Scholar]



